Aerobic granular sludge (AGS) is a kind of special biofilm formed by microbial self-immobilization under aerobic conditions, and was considered one of the most promising technologies for the treatment of domestic sewage [1]. Compared with traditional activated sludge, AGS has been paid increasing attention due to its strong resistance to shock loading rates, outstanding settleability and superior decontamination performance [2, 3]. However, the defects of unstable operation to treat sewage still restricted its practical application [4, 5]. Besides, the mechanism of sludge granulation and keeping granular stabilization is still unclear now, which has greatly restricted its engineering application.
Quorum sensing (QS) is the main information-exchanging processes in microbial communities [6]. Microorganisms secrete one or more kinds of signal molecules according to their density and environmental factors. Homologous receptors bind to signal and trigger signal transduction, and this induces gene expression that related to bacterial aggregation and growth, promoting group aggregation of single bacteria [7]. AGS has compact structure and high biomass, which can meet the basic conditions of forming microbial QS system. With the help of biosensor in situ detection, it was found that the concentration of N-acyl-homoserine lactones (AHLs) in mature AGS was significantly higher than that in aerobic activated sludge (AAS). This observation could be attributed to the compact structure and dense biomass of mature AGS that are conducive to the accumulation of AHLs. The structure of activated sludge is loose and the sludge density is relatively low, which is not beneficial for stimulating effective QS. Tan et al. pointed out that the sludge granulation process was not random, and was highly related to positive expression of QS system based on AHLs [8]. With the continuous attachment, aggregation and growth of activated sludge, the content of AHLs named N-(3-oxooctanoyl)-L-homoserine lactone (3-oxo-C8-HSL) increased about 100 times during the granulation process [9].
Recently, the exogenous selective enhancement of AHLs-medicated QS is considered to be an innovative method to improve decontamination performance [10, 11]. As described by Huizhi et al., exogenous 5 nmol/L AHLs were beneficial to the pollutant removal performance, whereas exogenous 50 nmol/L and 500 nmol/L AHLs limited decontamination performance [12]. Li et al. found that the best-performing AHL in terms of ammonia degradation rate was N-hexanoyl-L-homoserine lactone (C6-HSL), and N-(3-oxodecanoyl)-L-homoserine lactone (3-oxo-C10-HSL) could accelerate the formation of nitrifying granules [13]. Maddela et al. had reported that AHLs-mediated QS exhibited a constructive effect on the rapid granulation and particle stability of activated sludge [14]. The AHLs-manipulation could induce the secretion of extractive polymeric substances (EPS) that act as the main skeleton for the formation of AGS, and affect the microbial compositions as well [15, 16]. However, up to now, studies mainly focused on the succession of the QS-related activities and communities during the start-up period, while the granulation process of AGS cultivation in sequencing batch reactor (SBR) reactor with long-term addition of exogenous AHLs remains unrevealed [17]. Furthermore, research about the quorum quenching (QQ)-related activities and communities is scarce. As described by Maddela et al., the regulatory relationship between AHLs and functional microbes responsible for nutrient removal was not completely clear, and the long-term dynamic response between QS and functional microbial compositions remained to be understood [14]. Therefore, it is of certain significance to investigate the functional microorganisms with AHLs added in SBR systems. The purpose of this study is to explore the role and mechanism of AHL-manipulation in the formation of AGS via adding appropriate AHLs into SBR reactor.
Batch experiments were performed to investigate the most suitable AHL type and concentration for decontaminating. Specifically, 250 mL aerobic activated sludge mixture and 250 mL synthetic wastewater were added to 1 L shaking flask. The reactors were magnetically stirred at the full stage. They were operated in a 6 h cycle, consisted of 120 min of anaerobic, 90 min of oxic, 140 min of anoxic and 10 min of setting. The aeration rate in aerobic stage was controlled to 200 mL/min. The experimental scheme of batch experiments was shown in Table S1 (Supporting information). In order to enhance the reliability of data, we conducted two parallel experiments for each group of batch experiments.
Two identical sequencing batch reactors (SBRs) (R1 and R2) were used to conduct the experiments. The inner diameter of the SBR was 110 mm, the height was 300 mm, and the working volume was 2.4 L. The exchange volumetric rate was 50% (Fig. S1 in Supporting information). The SBRs were operated in 6 h cycle, including 2 min of feeding, 120 min of anaerobic, 90 min of oxic, 130 min of anoxic, 15 min of sedimentation and 5 min of discharging. Increasing hydrodynamic shear force by shortening settling time of the reactor gradually achieved rapid granulation [14, 18] (Table S2 in Supporting information). The sludge with fast settling speed can be screened to realize granulation [19]. The temperature was relatively stable (20–25 ℃).
The seed sludge was collected from the Shahu WWTP in Wuhan, China. Table S3 (Supporting information) displayed the index of seed sludge. The synthetic wastewater was composed of (per liter): NaAc 323.7 mg, NH4Cl 76.5 mg, KH2PO4 14.6 mg, CaCl2 10.6 mg and MgSO4·7H2O 10 mg and 1 mL of trace solution as described by He et al. [20]. The most suitable AHL type and concentration obtained from batch experiments were used and added into R2 for 121 days.
The concentration of chemical oxygen demand (COD), total phosphorus (TP), NH4+-N, NO2--N, NO3--N, mixed liquor suspended solids (MLSS), mixed liquid volatile suspended solids (MLVSS) and sludge volume index (SVI) were analyzed using standard methods [21]. Sludge particle sizes was measured by a laser particle analyzer (Mastersizer 2000, Malvern, UK). A HACH HQ30D meter was used to measure the dissolved oxygen (DO). A scanning electron microscope (SEM, VEGA 3, Tescank, Czech Republic) was used to observe the microscopic morphology of the AGS.
The tightly bound extracellular polymeric substances (TB-EPS) of AGS in these systems were extracted by an improved thermal extraction method [22]. Tightly bound protein (TB-PN) content was determined by a modified Lowry method with bovine serum albumin as the standard. Tightly bound polysaccharides (TB-PS) content was analyzed using a sulfuric acid-anthrone colorimetric method [23].
TB-EPS was characterized by three-dimension excitation emission matrix fluorescence spectroscopy (3D-EEM) fluorescence spectrometry using a spectrophotometer (HITACHI, F-7000, Japan). In this study, EEM scans were performed at the emission wavelengths (Em) from 250 nm to 550 nm at 5 nm increments and the excitation wavelength (Ex) from 200 nm to 400 nm at 5 nm intervals. The excitation and emission silts were kept at 10 nm, and the scan rate of spectra were recorded at 1200 nm/min. Subtracting an ultrapure water blank from the fluorescence spectra of the samples to reduce the Raleigh scattering effects.
The AGS samples of R1 and R2 were collected on the 83rd and 121st days, respectively. Subsequently, the AGS samples were sequenced by Illumina MiSeq platform (PE300, CA, U. S. A.), and the primer sets were 338F (5′-ACTCCTACGGGAGGCAGG-3′), 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The data were analyzed on the free online platform of Majorbio Cloud Platform (www.majorbio.com). Detailed bioinformatics analysis was based on the previous report of He et al. [24].
SPSS 22.0 was used for the analysis of variance (ANOVA) to determine whether there were differences between datasets. The relation between the datasets was considered to be significant if P < 0.05.
Since the addition of different types of AHLs (long-chain, short-chain, etc.) may exhibit different effects on QS of AGS system, it is of certain necessity to investigate the effects of different AHLs on the decontamination performance of the system. In this study, the signal molecules with a short N-group side chain (C6-HSL) and a long N-group side chain (3-oxo-C10-HSL) were selected from the two groups (with or without β-position substituent groups) according to the research by Li et al. [13]. Herein, 5 nmol/L C6-HSL and 3-oxo-C10-HSL were respectively added into two batch reactors, which were numbered as A2, A3. The blank reactor without AHLs addition, named A1, was used as the control group.
Time profiles of C, N and P concentrations in batch reactors under different AHLs addition were shown in Fig. 1a. At the end of anaerobic stage (120 min), COD were largely removed, the COD concentration in A2-A3 (about 29 mg/L) was relatively lower than A1 (45.54 mg/L). It could be seen that the maximum TP concentration were lower in A1 and A3 (about 9 mg/L) than that in A2 (10.71 mg/L). At the end of aerobic stage (210 min), the concentration of TP in A2 was the lowest (2.07 mg/L), while that in A1 and A3 were both about 4.5 mg/L. The NH4+-N oxidization rate in A2 (0.07 mg L-1 min-1) was higher than that in A1 and A3 (both were about 0.04 mg L-1 min-1). In the anoxic stage, the decreasing concentrations of NO3--N and NO2--N were attributed to the denitrification process. The removal rate of total inorganic nitrogen (TIN) in A2 (96.11% ± 1.84%) was higher than that in A1 (90.94% ± 3.54%) and A3 (92.05% ± 3.96%). These results showed that the addition of C6-HSL could promote the performance of phosphorus release and uptake, NH4+-N oxidization and denitrification. While the effect of 3-oxo-C10-HSL on aerobic sludge was not obvious. According to Li et al., the degradation efficiency of NH4+-N was closely related to the chemical structure of AHL added to the bioreactor [13]. The promotion of NH4+-N degradation increased with the decrease of AHL N-side chain length, which confirmed our observation.
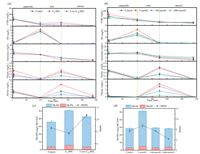
|
Download:
|
| Fig. 1. Time profiles of C, N and P concentrations in batch reactors: (a) Under different AHLs addition, (b) under different concentrations of C6-HSL addition. Contents of TB-EPS secreted by aerobic sludge: (c) Under different types of AHLs addition, (d) under different concentrations of C6-HSL addition. | |
It was reported that the addition of 10-9 mol/L (nmol/L) levels of AHLs could significantly change the operating state of the sequencing biofilm batch reactor (SBBR) system [12]. Therefore, to further investigate the effect of AHL dosage on activated sludge system, different concentrations (0, 5, 100, 1000 nmol/L) of C6-HSLwere added in aerobic activated sludge system, respectively named as B1, B2, B3, B4.
Fig. 1b presented the time profiles of C, N and P concentrations in batch reactors under different doses of C6-HSL addition. At the end of anaerobic stage (120 min), the phosphorus release in B2 was the highest (11.50 mg/L), while that in B3 was the lowest (6.57 mg/L). During the aerobic phase, TP in B2 had the highest removal efficiency. At the end of aerobic stage (210 min), the NH4+-N oxidization rate in B1–B3 (about 0.08 mg/L·min) were higher than that in B4 (0.05 mg/L·min). The removal efficiency of TIN in B2 (91.57% ± 2.26%) was the highest. As it could be seen, the removal efficiency of C, N and P primarily increased and then decreased over the increasing C6-HSL concentration range of 0–1000 nmol/L, with the maximum TP, NH4+-N and TIN removal obtained at the C6-HSL concentration of 5 nmol/L. When the concentration of AHLs was more than 100 nmol/L, the promotion of AHLs on the system's performance was not significantly enhanced, and some indicators even showed that the system was slightly inhibited. Song et al. found that QS and QQ coexisted in the bioreactor, and the QQ would affect the occurrence of QS and the balance between them was conducive to the operation of the bioreactor [25]. The high concentration of exogenous AHLs in this study might destroy the balance between QQ and QS in the system, and extra time was needed to reach a new equilibrium. Therefore, the impact on the operation of the system will lead to a decrease in pollutant removal efficiency.
According to the related research, AHLs were likely to regulate the secretion of EPS, especially the secretion of PS, and realize the communication between cells [12]. It can be seen from Fig. 1c that the concentration of TB-PS and TB-PNn the activated sludge with 5 nmol/L C6-HSL addition was significantly higher than that of the control group and the activated sludge with 5 nmol/L 3-oxo-C10-HSL addition, indicating that C6-HSL could induce the most secretion of TB-EPS. Notably, the concentration of C6-HSL had a significant effect on TB-PS secretion, the sludge with 5 nmol/L C6-HSL addition secreted the most TB-PS, and the addition of 100 nmol/L, 1000 nmol/L C6-HSL had little effect on TB-PS secretion (Fig. 1d). The results showed that adding 5 nmol/L C6-HSL was most beneficial to the secretion of TB-EPS.
According to the granule morphology (Fig. S2 in Supporting information), the first 83 days were considered as the start-up period (from flocculent sludge to aerobic granular sludge), and the last 38 days were considered as the stable period (no flocculent sludge).
Exogenous 5 nmol/L C6-HSL could directly affect the performance of pollutant removal and might play different roles in different stages. During the whole operation process, the good removal performances of COD and NH4+-N were all achieved in R1 and R2, indicating that the 5 nmol/L C6-HSL had little influence on them. The average removal efficiencies of TIN in R1 and R2 varied significantly in the start-up period (P < 0.05), and the removal rate of TIN in R2 was higher than R1 (Fig. 2a). This result indicated that the exogenous 5 nmol/L C 6-HSL contributed to denitrification during the AGS's formation period. In the stable period, the removal efficiency of TP in R2 was about 15% higher than that of R1 (Fig. 2b), suggesting that C6-HSL facilitates the enrichment of phosphate accumulating organisms (PAOs) or can enhance the activity of PAOs. The reason might be that the addition of C6-HSL increased the particle sizes of sludge and enlarged the anoxic/anaerobic zone in sludge, which could provide an optimum niche for the growth of PAOs and enhanced the removal efficiency of TP in the system [25].

|
Download:
|
| Fig. 2. Concentration and removal efficiency of (a) TIN, (b) TP in R1 and R2. Concentration changes of (c) NO2--N and (d) NO3--N in R1 and R2. | |
As the systems had experienced one adaptive phase, the concentration of NO3--N and NO2--N fluctuated greatly from day 40 to day 80 (start-up period). In the stable period, the concentration of NO3--N increased in both R1 and R2 (Fig. 2d), which was 5.57 ± 2.51 mg/L and 6.18 ± 1.17 mg/L, respectively (Table S5 in Supporting information). With regard to the concentration of NO2--N, there were significant differences between R1 (0.51 ± 0.72 mg/L) and R2 (0.09 ± 0.14 mg/L) (Fig. 2c). It should be attributed to that in the start-up period, there was more flocculent sludge, dissolved oxygen was easy to be used by microorganisms. With the increase of particle size, the particle was limited by mass transfer resistance [26]. Previous studies found that large size of granules help it dividing anaerobic and anoxic zone in its inner structure [27], indicating that anaerobes such as denitrification bacteria (DNB) could multiply greatly and NO3--N was reduced to NO2--N. Moreover, as the half-saturation constant value of oxygen for nitrite oxidizing bacteria (NOB) is higher than ammonia oxidizing bacteria (AOB), the adaption ability of AOB to lower DO concentration was much stronger than that of NOB [28], and if NO2--N could not be utilized by NOB in time inside the particles, the NO2--N would accumulate.
The concentration of NO2--N in R1 was significantly higher than that in R2, indicated that the addition of 5 nmol/L C6-HSL might enhance the activity of NOB or promote the growth of NOB [29]. At the same time, the relatively smaller fluctuations of concentration of NO2--N and NO3--N in R2 than R1, indicated that the C6-HSL-manipulation improved the stability of system denitrification.
A comparison of SEM observation of the microstructures at the end of the start-up period exhibited that there were more filamentous bacteria on the surface of R1, and the sludge structure was relatively loose (Fig. S3 in Supporting information). Accordingly, the settle ability of the sludge in R1 was worse than that in R2 (Fig. 3a). The size distribution showed that there were more large granules in R2 at the end of start-up period (Fig. S5 in Supporting information), indicating the addition of AHLs facilitated the formation of the AGS. At the start-up stage, the flocculent sludge with poor settling performance was gradually discharged out of the SBRs by continuously reducing the settling time from 15 min to 2 min, and 80% of biomass had been loosed (the MLVSS of R1 decreased from 2456 mg/L to 400 mg/L; the MLVSS of R2 decreased from 2872 mg/L to 442 mg/L) (Fig. S6 in Supporting information). Besides, the MLVSS/MLSS of R2 was higher than that in R1 (P < 0.05). It should be attributed to that C6-HSL promote the growth of microorganisms and increase the organic matter, which was consistent with EDS analysis (Fig. S4 in Supporting information).

|
Download:
|
| Fig. 3. (a) Variations in sludge settleability during the operation. (b) Variations in TB-EPS during overall operation. (TB-PS and TB-PN refer to tightly bound polysaccharides and tightly bound protein, respectively). The EEM spectra of TB-EPS in two SBRs: (c) R1 and (d) R2 at the start-up period (80th day); (e) R1 and (f) R2 at the stable period (120th day). | |
EPS were typically reported to benefit cell adhesion and integration, and help maintain the stability of the overall structure of the microbial community [30]. Although some studies suggested that hydraulic shear may cause EPS formation, it is not yet fully understood the main factor for increasing EPS formation [30]. It was observed that both TB-PS and TB-PN of the two SBRs exhibited an overall increase with some fluctuation in the start-up period as the floccular biomass began to transform into granules. In the start-up period, the average TB-PN and TB-PS of R1 were lower than those of R2 (TB-PN of 4.99 ± 0.45 mg/g MLVSS and TB-PS of 24.65 ± 0.71 mg/g MLVSS for R1; TB-PN of 7.48 ± 0.32 mg/g MLVSS and TB-PS of 26.05 ± 0.92 mg/g MLVSS for R2). It was concluded that C6-HSL could induce the production of TB-PN and TB-PS in the start-up period, which confirmed that C6-HSL could participate in the QS, accelerate the formation of biofilm, and guide the formation of PN and PS in the early stage [11]. PN had been reported as the core EPS component of AGS, and was of vital importance for the internal structure of granules. Therefore, particles size could become larger when C6-HSL was added (Fig. S5). While in the stable period, as the AGS granulation had been completely finished, the TB-PN and TB-PS in R1 and R2 maintained at a high level (TB-PN were about 7 mg/g MLVSS, TB-PS were more than 50 mg/g MLVSS), which revealed that the addition of C6-HSL might have little influences on the TB-EPS of mature AGS.
The increase of PS/PN can reduce the binding force between bacteria and water molecules, thus shorten the process of sludge granulation. Morgan et al. found that the PS/PN ratio determined the surface charge of EPS [31]. The surface of PN contained negative charges, while the surface of PS carried positive charges [32]. According to the research of Kun et al., EPS would affect the sedimentation of sludge by affecting the electronegativity of the sludge surface [33]. Therefore, as the PS/PN ratio increased, the electronegativity decreased, and the settleability of the sludge became better. It was found that PS/PN of the two SBRs had a significant difference only in the stable period (P < 0.05) (Fig. 3b). It indicated that C6-HSL could improve the sludge settle ability in the stable period. This was consistent with the results that the SVI5 was relatively higher in the sludge in R1 in the stable period (Fig. 3a) [34].
To explore the influence of exogenous C6-HSL on the composition of organic substances in TB-EPS, the extracted TB-EPS were subjected to 3D-EEM fluorescence spectroscopy. It was found that there were more peaks on the region Ⅲ and Ⅴ of the spectrum in the start-up period, indicating that in the process of granulation, some organic matter was biodegraded and the biodegradability was improved [35]. According to the previous research, the EEM diagram is divided into five areas: Region Ⅰ (Ex: 200–250 nm, Em: 200–330 nm) was related to tyrosine-like protein, region Ⅱ (Ex: 200–250 nm, Em: 330–380 nm) could be assigned to tryptophan-like protein, region Ⅲ (Ex: 200–250 nm, Em: 380–500 nm) referred to fulvic acid-like organics, region Ⅳ (Ex: 250–280 nm, Em: 200–380 nm) belonged to soluble microbial by-product-like materials and region Ⅴ (Ex: 250–280 nm, Em: 380–500 nm) was related to the humic acid-like organics [36, 37]. Proteins and humic acids were the main components of EPS in the sodium acetate-fed sludge [38]. Notably, there were more humic acid-like organics in R2 than R1 (Figs. 3c and d). As described by He et al., hydrophobic humic acid-like organics might facilitate the granulation of sludge, inferring that C6-HSL might promote sludge granulation [39]. Accordingly, the concentration of aromatic protein and tryptophan-like protein in R2 were higher than that in R1 in the stable period (Figs. 3e and f), illustrated that exogenous C6-HSL can promote the microbial secretion of protein substances in EPS. This is consistent with the findings of Chen et al. that the addition of exogenous AHLs supernatant stimulated the secretion of tryptophan-like and aromatic proteins [36]. Although the composition and structure of the EPS didnot change, the fluorescence intensity of various components increased via adding exogenous C6-HSL. This was in accordance with the results obtained by Wang et al. [40].
Four separate samples were taken from each reactor at the end of the start-up period (R1_1, R2_1) and stable period (R1_2, R2_2) for microbial community analysis by 16S rRNA Illumina MiSeq sequencing methodology. The coverage index all reached more than 97%, indicating that the sequencing results were reliable (Table S6 in Supporting information) [41].
From the phylum result in Fig. S8 (Supporting information), it was worth noticing that the abundance of Chloroflexi and Acidobacteria in R2 with C6-HSL addition was significantly higher than that in R1. Tan et al. studied the relationship between AHLs expression and granulation, and found that C6-HSL could significantly affect the abundance of Chloroflexi, which was consistent with our results [8]. Moreover, studies have shown that Acidobacteria could secrete EPS (mainly PS) to protect themselves [42]. This explained the increase of PS content when C6-HSL was added in the start-up period (Fig. 3b).
A heatmap from the top 50 predominant genera within four samples was given in Fig. 4a. It should be noted that in the four samples, the dominant genus was all known glycogen accumulating organisms (GAOs), the Candidatus_Competibacter, and the relative abundance were 25.56%, 16.65%, 17.23% and 24.52%, respectively (R1_1 > R2_2 > R2_1 > R1_2). Thomas et al. showed that the bacteria could secrete special colloidal PS to make EPS more adhesive [43], which was conducive to the aggregation of microorganisms in aerobic sludge and the formation of dense particles. The filamentous bacteria Thiothrix was the dominant genus in R1 that accounted for the top 4%. The abundance of Thiothrix in R1 was significantly higher than that in R2 (R1 accounted for 9.95% and 10.95% vs. R2 accounted for 2.22% and 2.20%), which was consistent with the results of SEM analysis that confirmed more filamentous bacteria on the sludge surface of R1 than R2 (Fig. S3). The overgrowth of filamentous bacteria was typically reported as the culprit for the sludge bulking, which meant that the R1 system was about to lose stability. Therefore, it can be inferred that the deteriorating settleability of granular sludge in R1 under long-term operating should be attributed to the overgrowth of Thiothrix (Fig. 3a). Bing et al. found that exogenous AHLs supernatant could induce the sustained release of AHLs in the system and effectively inhibit the overgrowth of filamentous bacteria [44]. The abundance of norank_f_Saprospiraceae in R2_1 was higher than R1_1 (R2_1 accounted for 6.12% vs. R1_1 accounted for 2.69%), illustrated that the addition of C6-HSL might promote its growth during the start-up period. Previous studies have reported that norank_f_Saprospiraceae accelerated the hydrolysis of organic substrates and facilitate the denitrification process by providing carbon sources [45, 46]. Therefore, norank_f_Saprospiraceae might enhance the carbon metabolism and denitrification process in some content. The abundance of Nitrospira in R2_2 was significantly higher than that in R1_2 (R2_2 accounted for 7.52% vs. R1_2 accounted for 3.77%) and was the dominant genus in R2_2 that accounted for the top 4%.

|
Download:
|
| Fig. 4. (a) The heatmap of microbes at genus level. (b) The heatmap of key functional microbes at genus level. (c) The abundance of key functional microbes at genus level. (d) AHL-related genera in the microbial aggregates. +, containing QS/QQ members; -, no QS/QQ members were reported yet. | |
In order to clarify the internal relationship between the microbial community and the decontamination performance of SBRs, a phylogenetic classification was carried out from key functional groups [47]. Figs. 4b, c exhibited the distribution pattern of key functional groups involved in biological nitrogen and phosphorus removal based on sequencing results at genus level.
Functional bacteria had different responses to AHL in different periods. In the start-up period, the abundance of the GAO Candidatus_Competibacter in R1 was significantly higher than that in R2 (25.59% vs. 17.23%), and the relative abundance of PAO Candidatus_Accumulibacter in R1 was significantly lower than that of R2 (0.76% vs. 1.61%). GAOs are the main competitor of PAOs in the biological phosphorus removal process. GAOs and PAOs both have the ability to store acetate as polyhydroxyalkanoates (PHA) under anaerobic conditions. In aerobic condition, both GAOs and PAOs obtain energy by decomposing PHA, but GAOs don't absorb P, while PAOs absorb P and synthesize polyphosphate. Therefore, AHL favored the accumulation of PAOs and suppress the growth of GAOs in the start-up period, interpreting that the higher removal rate of TP in R2 than R1 (Fig. 2b). In the stable operation period, AOB and NOB responded most positively to C6-HSL, with a relative abundance of R2_2 significantly higher than R1_1 (AOB: 0.28%vs. 0.11%; NOB: 7.52% vs. 3.77%). The AOB Nitrosomonas can nitrify NH4+-N to NO2--N and possessed great efficiency. In contrast to the percent of AOB, the NOB Nitrospira genus enriched more significantly. NOB can oxidize NO2--N into NO3--N. Gao et al. found that there was a AHLs synthetase in Nitrospira multiformis [48]. Combined with the above result, it could be confirmed that NOB could synthesize C6-HSL [39]. The abundant NOB with the efficient AOB ensured nearly complete nitrification with AHL addition. The ratio variation of the relative abundances of AOB and NOB interpreted the differences of the concentrations of NO and NO in R1 and R2. The denitrifying bacteria (DNB) can denitrify NOx--N to N2. Fig. 4c illustrated that the abundance of DNB in R2 was higher than R1 in the start-up period (R2_1 > R1_1), which was corresponding with the higher removal rete of TIN in R2 in the start-up period (Fig. 2a). In the stable period, the abundance of DNB in R1 rise sharply (R1_2 > R2_2), while the removal rete of TIN was not increase. This should be attributed to the lack of available carbon sources. Therefore, with a certain carbon source, the TIN removal rate was considerably comparable to that of the activated sludge even with higher DNB abundance [47]. The relative abundance varied with the addition of C6-HSL, illustrating that AHL-manipulation could regulate the composition of functional bacteria and enhance the stability of sludge system.
Some studies have screened the bacteria in the sewage treatment reactor or soil environment, and tested the secretion and degradation ability of AHLs of the strains, which provides a detailed reference for future research [17, 45, 49, 50]. It was found that AHLs producing bacteria and AHLs co-exist in AGS system were conducive to maintaining the balance of AHLs in the system [25]. The succession of AHLs related genera explains the apparent change in the operating of the reactor from the mechanism. Fig. 4d and Table S8 (Supporting information) showed the succession of microbiol community related to QQ and QS genera in the microbiol aggregates. Notably, the genera without QS- related activity was more than that with QS- related activity. The diversity and activities of QS- and QQ- related communities implied that QQ and QS activities coexisted in the SBRs and keep in a dynamic balance [13].
For the relative abundance of QS-related genera, the main QS-related genera Candidatus_Competibacter in R1 decreased during the stable period, but the opposite trends were observed in R2. Not only that, Nitrosomonas and Nitrospira in R2 became greatly enriched in the stable period, while slightly increased in R1. Accordingly, the relative abundance of Nitrosomonas and Nitrospira in R2 were significantly higher than in R1 at the end of the stable period (Nitrosomonas: 0.28% vs. 0.11%; Nitrospira: 7.52% vs. 3.77%). This is consistent with the study by Li et al. that Nitrosomonas Europaea and AOB biofilm activities were regulated by the AHL-based communication system [13]. These results showed that C6-HSL-manipulation could promote the growth of QS-related genera (i.e., AOB, NOB) in the long-term operation of SBR, therefore regulate the nitrogen transformation of the SBR.
In terms of QQ-related genera, the observations throughout the SBRs start-up and the successive stable period showed an increase in R1, indicating that QQ related bacteria will accumulate in the stable period in the R1 without C6-HSL addition [17]. Opposite trends were observed in R2, only the abundances of Acinetobacter and Chryseobacterium ascended. The other QQ-related genera in R2 became greatly declined after the start-up and long-term operation (Table S8 in Supporting information). Hence it could be inferred that the long-term addition of C6-HSL will inhibit QQ activities by inhibiting QQ related genera after granular sludge matures.
These results showed that exogenous C6-HSL-manipulation had vital influence on the QS activities during the stable period rather than the start-up period. Moreover, exogenous C6-HSL-manipulation inhibited QQ-related activities and enhanced QS-related activities during the stable period, which enhanced the stability and optimized the decontamination performance of AGS system.
Batch experiments showed that 5 nmol/L C6-HSL could contribute to the NH4+-N oxidation rate and EPS secretion. Further SBR experiments implied the complicated role of C6-HSL on the long-term operation of AGS. Specifically, significant differences were acquired in COD and TIN removal, and the ratio of PS/PN significantly increased, which could improve the stability of AGS. Microbial compositions related to QQ and QS considerably shifted with the addition of C6-HSL. Results demonstrated that C6-HSL-medicated QS was beneficial to improve the activity of AOB and NOB, therefore regulated the nitrogen transformation of the SBR. In summary, the process of graduation, simultaneous nitrogen and phosphorus removal performance and structural stability of AGS can be enhanced by exogenous C 6-HSL-manipulation.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.04.061.
| [1] |
Q. He, J. Song, W. Zhang, et al., J. Hazard. Mater. 382 (2020) 121043. DOI:10.1016/j.jhazmat.2019.121043 |
| [2] |
A.M. Maszenan, Y. Liu, W.J. Ng, Biot. Adv. 29 (2011) 111-123. DOI:10.1016/j.biotechadv.2010.09.004 |
| [3] |
J. Zhou, Q. Sun, Bioproc. Biosyst. Eng. 43 (2020) 663-672. DOI:10.1007/s00449-019-02264-w |
| [4] |
F. Cecconi, M. Garrido-Baserba, R. Eschborn, J. Damerel, D. Rosso, Environ. Sci. Water Res. Technol. 6 (2020) 679-690. DOI:10.1039/C9EW00784A |
| [5] |
M. Pijuan, U. Werner, Z. Yuan, Water Res 45 (2011) 5075-5083. DOI:10.1016/j.watres.2011.07.009 |
| [6] |
W.C. Fuqua, S.C. Winans, E.P. Greemberg, J. Bacteriol. 176 (1994) 269-275. DOI:10.1128/jb.176.2.269-275.1994 |
| [7] |
P.D.T. Nguyen, N.A. Mustapha, K. Kadokami, et al., Appl. Microb. Biot. 103 (2019) 1485-1495. DOI:10.1007/s00253-018-9553-9 |
| [8] |
C.H. Tan, K.S. Koh, C. Xie, et al., ISME J 8 (2014) 1186-1197. DOI:10.1038/ismej.2013.240 |
| [9] |
B. Zhang, P.N.L. Lens, W. Shi, et al., Chem. Eng. J. 334 (2018) 2373-2382. DOI:10.1016/j.cej.2017.11.151 |
| [10] |
J. Wang, Q. Liu, B. Wu, et al., Sci. Total Environ. 685 (2019) 28-36. DOI:10.1016/j.scitotenv.2019.05.249 |
| [11] |
H. Chen, A. Li, C. Cui, et al., Environ. Int. 130 (2019) 104946. DOI:10.1016/j.envint.2019.104946 |
| [12] |
H. Huizhi, H. Junguo, L. Jian, Y. Huarong, Z. Jie, Bioresour. Technol. 211 (2016) 339-347. DOI:10.1016/j.biortech.2016.03.068 |
| [13] |
A. Li, B. Hou, M. Li, Bioresour. Technol. 196 (2015) 550-558. DOI:10.1016/j.biortech.2015.08.022 |
| [14] |
N.R. Maddela, B. Sheng, S. Yuan, et al., Chemosphere 221 (2019) 616-629. DOI:10.1016/j.chemosphere.2019.01.064 |
| [15] |
L. Jun, L. Jun, X. Kai, S. Balasubramanian, Bioresour. Techno. Rep. 5 (2019) 51-58. DOI:10.1016/j.biteb.2018.12.002 |
| [16] |
M. Gao, Y. Liu, Z. Liu, H. Li, A. Zhang, Biochem. Eng. J. 151 (2019) 107329. DOI:10.1016/j.bej.2019.107329 |
| [17] |
Y. Li, T. Tian, B. Li, H. Yu, Water Res. 179 (2020) 115904. DOI:10.1016/j.watres.2020.115904 |
| [18] |
S.A. Sunil, L. Duu-Jong, L. Juin-Yih, Bioresour. Technol. 100 (2009) 5359-5361. DOI:10.1016/j.biortech.2009.05.058 |
| [19] |
E. Szabo, M. Hermansson, O. Modin, F. Persson, B. Wilen, Water 8 (5) (2016) 172(Switzerland).
|
| [20] |
Q. He, S. Zhang, Z. Zou, L. Zheng, H. Wang, Bioresour. Technol. 220 (2016) 651-655. DOI:10.1016/j.biortech.2016.08.105 |
| [21] |
A W W A. APHA, WEF. Standard Methods for Examination of Water and Wastewater, American Public Health Association, Washington, 2012, p. 1360.
|
| [22] |
S. Comte, G. Guibaud, M. Baudu, Enzym. Microb. Technol. 38 (2005) 237-245. |
| [23] |
Q. He, Z. Yuan, J. Zhang, et al., Chemosphere 173 (2017) 411-416. DOI:10.1016/j.chemosphere.2017.01.085 |
| [24] |
Q. He, J. Zhang, S. Gao, et al., Bioresour. Technol. 294 (2019) 122151. DOI:10.1016/j.biortech.2019.122151 |
| [25] |
X. Song, Y. Cheng, W. Li, et al., Bioresour. Technol. 171 (2014) 472-476. DOI:10.1016/j.biortech.2014.08.027 |
| [26] |
Y. Chen, S. Liu, F. Fang, et al., Environ. Sci. Technol. 42 (2008) 8465-8470. DOI:10.1021/es8010157 |
| [27] |
Z.C. Chiu, M.Y. Chen, D.J. Lee, C.H. Wang, J.Y. Lai, Appl. Microb. Biot. 75 (2007) 685-691. DOI:10.1007/s00253-007-0847-6 |
| [28] |
L. Wang, B. Li, Y. Li, J. Wang, Chemosphere 263 (2021) 123184. |
| [29] |
S. Yuepeng, G. Yuntao, W. Dan, L. Kai, W. Guangxue, Bioresour. Technol. 259 (2018) 136-145. DOI:10.1016/j.biortech.2018.03.025 |
| [30] |
J.H. Tay, Q.S. Liu, Y. Liu, Lett. Appl. Microb. 33 (2001) 222-226. DOI:10.1046/j.1472-765x.2001.00986.x |
| [31] |
J.W. Morgan, C.F. Forster, L. Evison, Water Res. 24 (1990) 743-750. DOI:10.1016/0043-1354(90)90030-A |
| [32] |
G. Sheng, H. Yu, X. Li, Biot. Adv. 28 (2010) 882-894. DOI:10.1016/j.biotechadv.2010.08.001 |
| [33] |
Y. Kun, Di Wang, L. Jun, S.Y. Ya, Adv. Mater. Res. 3473 (2014) 262-265. |
| [34] |
S. Li, X. Fei, L. Cao, Y. Chi, Sci. Total Environ. 691 (2019) 799-809. DOI:10.1016/j.scitotenv.2019.07.191 |
| [35] |
J. Jimenez, E. Gonidec, J.A.C. Rivero, et al., Water Res. 50 (2014) 359-372. DOI:10.1016/j.watres.2013.10.048 |
| [36] |
W. Chen, P. Westerhoff, J.A. Leenheer, K. Booksh, Environ. Sci. Technol. 37 (2003) 5701-5710. DOI:10.1021/es034354c |
| [37] |
J. Sun, L. Guo, Q. Li, et al., Environ. Sci. Pollut. Res. Int. 23 (2016) 24061-24067. DOI:10.1007/s11356-016-7610-4 |
| [38] |
L. Wei, X. Xia, F. Zhu, et al., Water Res. 181 (2020) 115903. DOI:10.1016/j.watres.2020.115903 |
| [39] |
E.O. Burton, H.W. Read, M.C. Pellitteri, W.J. Hickey, Appl. Environ. Microb. 71 (2005) 4906-4909. DOI:10.1128/AEM.71.8.4906-4909.2005 |
| [40] |
X. Wang, W. Wang, Y. Li, et al., Rsc Adv. 8 (2018) 30783-30793. DOI:10.1039/C8RA05545A |
| [41] |
P. Antwi, J. Li, P.O. Boadi, et al., Bioresour. Technol. 235 (2017) 348-357. DOI:10.1016/j.biortech.2017.03.141 |
| [42] |
A.M. Kielak, T.C.L. Castellane, J.C. Campanharo, et al., Sci. Rep. 7 (2017) 41193. DOI:10.1038/srep41193 |
| [43] |
W.S. Thomas, K.L. Lynette, P. Maite, Y. Zhiguo, Appl. Microb. Biot. 92 (2011) 1297-1305. DOI:10.1007/s00253-011-3385-1 |
| [44] |
Z. Bing, L. Wei, G. Yuan, et al., Water Res. 169 (2020) 115193. DOI:10.1016/j.watres.2019.115193 |
| [45] |
J. Liu, Y. Yuan, B. Li, et al., Bioresour. Technol. 244 (2017) 1158-1165. DOI:10.1016/j.biortech.2017.08.055 |
| [46] |
T. Zhang, B. Wang, X. Li, et al., Chem. Eng. J. 335 (2018) 330-337. DOI:10.1016/j.cej.2017.09.188 |
| [47] |
Q. He, L. Chen, S. Zhang, et al., Bioresour. Technol. 263 (2018) 214-222. DOI:10.1016/j.biortech.2018.05.007 |
| [48] |
J. Gao, A. Ma, X. Zhuang, G. Zhuang, Appl. Environ. Microb. 80 (2014) 951-958. DOI:10.1128/AEM.03361-13 |
| [49] |
C. D'Angelo-Picard, D. Faure, I. Penot, Y. Dessaux, Environ. Microb. 7 (2005) 1796-1808. DOI:10.1111/j.1462-2920.2005.00886.x |
| [50] |
A. Kim, S. Park, C. Lee, C. Lee, J. Lee, J. Microb. Biot. 24 (2014) 1574-1582. DOI:10.4014/jmb.1407.07009 |
 2021, Vol. 32
2021, Vol. 32 

