b State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao 066004, China
With the development of industrialization, the traditional physical, biological and chemical methods cannot meet the requirements of efficient wastewater treatment of refractory organics [1-3]. Advanced oxidation processes (AOPs) are expected to reach high requirements and strict regulations in organic effluent disposal [4]. AOPs include photocatalysis [5-7], catalytic ozonation [8], catalytic wet air oxidation [9, 10], electrochemical oxidation [11, 12], and Fenton reaction [13, 14]. One of the most widely used is Fenton oxidation, that is, ferrous ion (Fe2+) activates hydrogen peroxide (H2O2) to produce hydroxyl radical (•OH) [15]. The •OH has high oxidation ability, which can quickly and non-selectively degrade various refractory organics in water [16].
Fenton system has some of the advantages, such as mild reaction conditions, simple equipment, convenient operation, and high oxidation rate [17]. However, the pH range of Fenton reaction is narrow (pH 3-5) [18]. H2O2 is easy to be decomposed, leading to the decrease of utilization [19]. Besides, the liquid H2O2 is inconvenient for storage and transportation. Moreover, Fe2+ is hard to recover and liable to produce the precipitation of ferric ion (Fe3+) [20]. These factors limit the Fenton reaction efficiency and its further application.
To overcome the bottlenecks of conventional Fenton reaction, the improved Fenton and Fenton-like processes have been well developed recently [21]. Introducing complexing agent into Fenton reaction is the most common way, which can effectively prolong the existence time of dissolved Fe2+ and Fe3+, broaden the application range of pH value, promote electron transfer, and prevent iron precipitation [22]. The usual chelation agents are ethylenediaminetetraacetic acid and hydroxylamine, but they would generate toxic products during reactions and secondary pollution [23-25]. So diverse small molecule organic acids have attracted great attention, such as ascorbic acid (AA) [26], citric acid [27] and oxalic acid [28]. These carboxylates could not only have the favorable complexing capability, but also be nontoxic and degradable.
Calcium peroxide (CaO2) is a stable solid peroxide, which is easy to transport and store. CaO2 could slowly and stably produce H2O2 over a broad range of pH, reducing the disproportionation of H2O2. Hence, CaO2 has been extensively researched as the substitution of H2O2 [29]. On the other side, Fe3+ is more stable than Fe2+, and Fe3+ could also catalyze H2O2 by initiative superoxide and per hydroxyl actuated reactions to generate a series of reactive oxygen species [30], such as •OH and superoxide anion free radical (O2•−). But the direct application of Fe3+ would easily form iron sludge, exhausting the Fenton reactivity. Also, the reduction of Fe3+ to Fe2+ is slowly.
To sum up, using the AA complexed Fe3+ to react with CaO2 is a prospective improved method to surmount the application barriers of traditional Fenton reaction, which is seldom reported. In addition, response surface method (RSM) combines math and statistics means to model and optimize the influence of a few independent variables, which is widely and efficiently applied for the modelling and optimization in the various engineering issues. Therefore, we established a Fe3+-AA complex catalyzed CaO2 (Fe3+/AA/CaO2) Fenton-like process to promote the removal of multiple organic dyes in water, and the operation parameters (Fe3+ concentration, AA dosage, and CaO2 amount) were optimized by the RSM for the methyl orange (MO) decolorization. Furthermore, the H2O2 decomposition, Fe ions change, and active species identification were studied to reveal the synergistic reaction mechanisms.
The sources properties of chemicals applied in this study were introduced in Text S1 (Supporting Information). The tests were implemented in flask (100 mL) at room temperature on magnetic stir. The desired concertation of simulated wastewater was added in the beaker, and its initial pH was regulated by sulfuric acid and sodium hydroxide. Then, the reaction was initiated through adding Fe3+, AA, and CaO2 in order. Sample aliquots (1 mL) was extracted at the given time intervals, and added 1 mL sodium thiosulfate to quench oxidation process, then filtered by 0.22 µm filter membrane for determination of concentration. The dye concentration was measured by the UV-spectrophotometer at different specific wavenumbers. The total organic carbon (TOC) was monitored through TOC analysis meter. The H2O2 amount was determined by titanium potassium oxalate method [31]. The concentrations of Fe3+ and total Fe were detected by 1, 10-phenanthroline monohydrate color method [32]. The determination of Fe4+ was used by dimethyl sulfoxide (DMSO) oxidation method [33]. Tert-butyl alcohol (TBA), p-benzoquinone (BQ) and L-histidine were used to quench •OH, O2•−, and singlet oxygen (1O2), respectively. The qualitative test for active species was determined through electron spin resonance (ESR) spectrometer. The detailed analyses parameters were described in Text S2 (Supporting information).
The RSM from Box-Behnken was designed on the basis of Design-Expert 8.0.6 Trial software. The experimental design of three factors (Fe3+ dose, AA concentration, and CaO2 amount) and three levels was adopted to investigate the MO removal. The detailed design and analysis of RSM described in Text S3 (Supporting information), and the numbered and actual values for the variables were presented in Table S1 (Supporting information).
Fig. 1a compares the MO removal by the Fe3+/AA/CaO2 and other control processes. There were no MO decolorization by the Fe3+ alone, AA alone, CaO2 alone, AA/CaO2, and Fe3+/AA systems, and only 28.90% of MO was decolored in the Fe3+/CaO2 after 15 min treatment. Nevertheless, 93.40% of MO was removed by the Fe3+/AA/CaO2, indicating the significant improvement in the synergistic process. In contrast, the MO removals were 25.40% and 15.30% for the Fe3+/H2O2 and Fe3+/AA/H2O2, respectively, which was clearly inferior to the CaO2 relevant processes. Above phenomena could be explained through the following reasons: firstly, the dissolved CaO2 could stably supply H2O2 for the Fenton-like system and release O2 simultaneously (R1 and R2) [34], avoiding the disproportionation of H2O2. Secondly, after adding the AA, the complexation of Fe3+ and AA could be formed (R3) [34, 35], which could accelerate the electron transfer, improving the Fe2+ formation from the Fe3+ reduction and decreasing the precipitation of Fe3+. Thirdly, except for the conventional Fenton reactions for the generations of •OH and O2•− (R4 and R5), the AA mediated Fe3+/CaO2 process could produce more reactive species, such as O2•− and 1O2 (R6 and R7) [36, 37], which are the more selective species for oxidizing the electron-rich groups in the organic compounds.
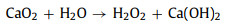
|
(1) |
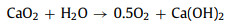
|
(2) |
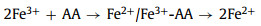
|
(3) |

|
(4) |
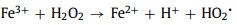
|
(5) |
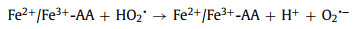
|
(6) |

|
(7) |
|
Download:
|
| Fig. 1. (a) MO removal in different processes. (b) UV-vis spectroscopy for MO decolorization. (c) TOC removal of MO. (d) Different dyes removal in Fe3+/AA/CaO2 system. Conditions: [MO] = [other dyes] = 0.15 mmol/L, [Fe3+] = 2 mmol/L, [AA] = 1 mmol/L, [CaO2] = [H2O2] = 3 mmol/L, initial pH 6.50. | |
Fig. 1b shows the change of MO absorption peak (465 nm) with treatment time. The characteristic peak intensity decreased with the increase of time, which indicated that the azo bond of MO was attacked and cleaved by the active substances [38, 39]. At the same time, the peak intensity at less than 300 nm increased gradually, proving that more small molecules were formed during the MO removal [40]. The mineralization of MO was verified by the TOC analysis. As show in Fig. 1c, the TOC removal enhanced with the treatment time, and reaches 19.40% after 120 min, proving that the dye molecular structure was decomposed into lesser organic matters and mineralized into H2O and CO2 under the synergistic process. The comparatively low TOC removal could be attributed to the addition of organic acid complexing agent, which would compete with the reactive species generated in the synergy and enhance the organic load of wastewater. Besides, the oxidation performance of Fe3+/AA/CaO2 for different organic dyes were determined (Fig. 1d). Other four dyes (Congo red (CGR), acid fuchsin (AF), methylene blue (MB), and reactive brilliant blue (KN-R)) were treated under the Fe3+/AA/CaO2 system with the identical conditions, and their decolorization efficiencies all were higher than 84.00% after 15 min reaction, certifying this coupling system has a good ability to treat various dye wastewaters.
Table S2 (Supporting information) lists the three levels and three variables full factor analysis of each experiment based on RSM results, including the experimental and predicted values of MO removal. According to the results and data analysis, the quadratic linear regression equation obtained by running Design-Expert 8.0.6 trial software was as follows [41, 42]:
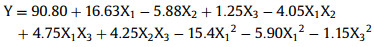
|
(10) |
Table S3 (Supporting information) displays the results of variance analysis. The P-value was less than 0.0001, indicating that the result was significant, and the 51.23 of F-value was a further proof of the significant model [43]. Meanwhile, due to the influence of interference error, only 0.01% chance would appear such a large F-value. The R2 value indicates that the model is to what extent impact the variability of data. In this study, the R2 value of quadratic linear regression model was 0.9850, demonstrating that 98.50% of the MO removal efficiency was affected by the independent variables, and only 1.50% of influence was unaffected by the model. Radj2 is optimized for R2 by considering the covariates or predictors, which is more suitable to compare those models with various of arguments. The value of Radj2 (0.9658) was close to that of R2, ensuring the reliability of quadratic model. In addition, the signal to noise ratio of 20.199 showed that the result was feasible for this model [44].
Verification of model feasibility is a vital process, which is the indispensable way to evaluate whether the result is suitable. The linear distribution points of normal probability plots can prove the residuals obey normal distribution. As shown in Fig. S1a (Supporting information) the points were distributed on both sides of the straight line, proving it obeyed normal distribution. Also, the result suggested that the quadratic formula given by the model could well predict the effect of Fe3+, AA and CaO2 on the MO decolorization. Fig. S1b (Supporting information) presents the comparison of MO removal ratio for the predicted and experimental values. All the spots were dispersed around the regression curve, testifying that the simulated outcomes were consistent with the actual data. From Fig. S1c (Supporting information), the residuals were randomly distributed around 0 with the change of ± 3.0, indicating that the response of model was identical to the normal distribution. Fig. S1d (Supporting information) reveals the matching correlation for the residual and simulative values. The residual was a random distribution, manifesting that the change of original value was constant for the response value [44].
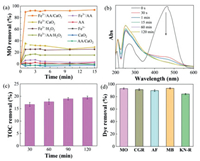
The response surface and contour could manifest the interaction of various factors on the decolorization of MO. Fig. 2a shows the effect of Fe3+ and AA concentrations on the MO removal. The dose of CaO2 was fixed at 3 mmol/L. The decolorization of MO underwent an increase and decrease with the increscent AA amount (0.25 to 1.75 mmol/L). This is because the excessive AA would react with active substances. With the increase of Fe3+ concentration from 1 mmol/L to 3 mmol/L, the removal of MO also enhanced, proving that the Fe3+ was a crucial factor as a catalyst in this synergistic method. Fig. 2b confirmed that there was interaction between Fe3+ and AA, then the generated functions of complexation and reduction could improve the Fenton-like reaction and MO removal. Fig. 2c reveals the effect of Fe3+ and CaO2 concentrations on the decolorization of MO. The concentration of AA kept constant. When AA was 1 mmol/L, the MO removal increased with the enhancing CaO2 amount. This is due to the fact that the more oxidant added, the more active species would be produced in the system, benefiting the dye removal. Fig. 2d also verified the interrelationship between Fe3+ and CaO2. Figs. 2e and f present the effect of AA and CaO2 quantities on the MO elimination. The Fe3+ concentration remained at 2 mmol/L. With the decrease of AA dosage and the increase of CaO2 amount, the MO decolorization was augmented. The result suggested that there was an optimum for the AA addition.

|
Download:
|
| Fig. 2. Response surface plots of interaction for two independent variables: Fe3+ and AA (a and b), CaO2 and Fe3+ (c and d), and CaO2 and AA (e and f). | |
As the predesigned experiments were completed, Design-Expert 8.0.6 was applied to optimize parameters for the Fe3+/AA/CaO2 system. The simulation result indicated that the best MO decolorization was 99.30% under the optimal conditions of 2.76 mmol/L Fe3+, 0.68 mmol/L AA and 4 mmol/L CaO2. Furthermore, the proof test demonstrated that the elimination of MO could reach 98.90% under the same conditions. The experimental outcome was agreed well with the predicted value, attesting that the RSM model was reliable and its optimization was successful.
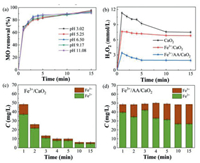
Owing to the significant influence of solution pH on the Fenton process, the MO removal in the Fe3+/AA/CaO2 was investigated at the different initial pH. Fig. 3a displays that the MO eliminations varied little when the wastewater pH changed from 3 to 11, and the decolorizations were all higher than 90%. The result demonstrated that the presented collaborative Fenton-like system could perform well in a wide range of pH.
|
Download:
|
| Fig. 3. (a) Effect of pH on MO removal in Fe3+/AA/CaO2. (b) H2O2 concentrations in different processes. Fe2+ and Fe3+ concentrations in Fe3+/CaO2 (c) and Fe3+/AA/CaO2 (d). Conditions: [MO] = 0.15 mmol/L, [Fe3+] = 2 mmol/L, [AA] = 1 mmol/L, [CaO2] = 3 mmol/L, initial pH 6.50. | |
Fig. 3b presents change of H2O2 concentration during the diverse processes. The H2O2 amounts all rose at the beginning and then declined with the time for the three experiments, and their sequence was: CaO2 > Fe3+/CaO2 > Fe3+/AA/CaO2. The phenomena suggested that the addition of AA could be beneficial to improve the H2O2 decomposition.
The variations of Fe3+ and Fe2+ concentrations during the Fe3+/CaO2 and Fe3+/AA/CaO2 systems are shown in Figs. 3c and d, respectively. The total Fe ions content of Fe3+/AA/CaO2 remained constant, and which was decreased sharply with time in the Fe3+/CaO2. Besides, the Fe2+ concentration of the complexation system was obviously higher than that of Fe3+/CaO2. Above result verified the complexing function of AA, which could not only maintain the dissolved state for Fe3+ and Fe2+, but also improve the remarkably promote the reduction of Fe3+ and reduce the iron sludge generation.
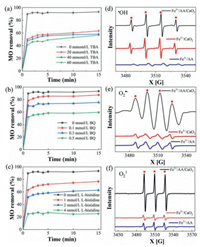
The reactive oxygen species masking experiment was employed to investigated the functions of various active species in the Fe3+/AA/CaO2 system (Fig. 4). TBA can effectively quench •OH, and the reaction rate constant is 6.0 × 108 L mol-1 s-1 [45, 46]. BQ is commonly used as a scavenger of O2•−, (9.6 × 108 L mol-1 s-1), and L-histidine can mask 1O2 and •OH [47-49]. In Fig. 4a, the addition of TBA significantly inhibited the removal of MO, and the decolorization rate decreased from 93% to 55% as the TBA increased from 0 to 60 mmol/L. However, when the concentration of TBA was enhanced to 60 mmol/L, the suppression effect was obviously weakened. It could be concluded that excessive TBA could not completely confine the MO decolorization, and other active substances also were responsible for the elimination of MO. From Fig. 4b, while the amount of BQ was augmented from 0.1 mmol/L to 0.5 mmol/L, the MO decolorization was declined from 87% to 59%, showing that O2•− was also played a non-ignorable role for the dye decontamination. Fig. 4c displays that the scavenging effect was augmented with the increase of L-histidine. When the concentration of L-histidine was enhanced to 4 mmol/L, the MO decolorization declined to 25%, demonstrating that 1O2 was also an essential reactive species in this Fenton-like system. In addition, the ESR analysis was employed to further confirm the existences of these active species. The typical signals for •OH, O2•− and 1O2 were detected in the spectrum of Figs. 4d-f, respectively. Obviously, these peak intensities for the Fe3+/AA/CaO2 was higher than those of Fe3+/CaO2 and Fe3+/AA, corroborating that the generations of these reactive species in the collaborative system. More importantly, the ESR results were in accordance with the scavenging tests.
|
Download:
|
| Fig. 4. Effect of different scavengers on MO removal in Fe3+/AA/CaO2: (a) TBA, (b) BQ, (c) L-histidine. ESR spectra for •OH (d), 1O2 (e), and O2•− (f). | |
Moreover, to explore the role of Fe4+ in this oxidative process, the Fe4+ amounts were measured by DMSO oxidation method. From Fig. S2 (Supporting information), the difference for the consumptions of DMSO was not significant in the Fe3+/CaO2 and Fe3+/AA/CaO2 systems. Furthermore, the formations of dimethyl sulfone (DMSO2) were quite small and comparative, indicating that the Fe4+ productions were tiny during the two processes. Besides, the little decrease of the DMSO could be attributed to the attack of •OH radical [33]. These results proved that the Fe4+ was not the dominant active species in the Fe3+/AA/CaO2.
In this work, a Fenton-like system using CaO2 as the oxidant coupled with AA chelated Fe3+ was proposed to effectively remove various organic dyes in water. The Fe3+/AA/CaO2 process could significantly improve the MO decolorization compared with other control systems. By the RSM optimization, the mutual effect of operation conditions (concentrations of Fe3+, AA and CaO2) was explored, and the optimal parameters were obtained (2.76 mmol/L Fe3+, 0.68 mmol/L AA and 4 mmol/L CaO2). Using the optimized parameters, the elimination of MO could reach 98.90% in the proof experiment, which was agreed well with the simulated result (99.30%). The complexation process could perform well in a wide range of pH (3-11), improving the H2O2 decomposition, and promoting the dissolution and cycle of Fe3+/Fe2+. The quenching test demonstrated that •OH, O2•− and 1O2 were all responsible for the dye removal, and the ESR analysis certified the existence of these reactive species in the collaborative system. This work was fundamental to provide an improved and optimized method for Fenton-like technique.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThe authors thank the financial support from the Natural Science Foundation of China (No. 51908485), the Natural Science Foundation of Hebei province (Nos. E2020203185, B2020203033, B2018203331), and the University Science and Technology Program Project of Hebei Provincial Department of Education (No. QN2020143).
Supplementary materialsSupplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.04.050.
| [1] |
H. Zhang, Q. Ji, L. Lai, et al., Chin. Chem. Lett. 30 (2019) 1129-1132. DOI:10.1016/j.cclet.2019.01.025 |
| [2] |
Z. Wang, X. Teng, M. Xie, et al., Chin. Chem. Lett. 31 (2020) 2864-2870. DOI:10.1016/j.cclet.2020.03.051 |
| [3] |
M. Du, Q. Yi, J. Ji, et al., Chin. Chem. Lett. 31 (2020) 2803-2808. DOI:10.1016/j.cclet.2020.08.002 |
| [4] |
I.A. Ike, T. Karanfil, J. Cho, et al., Water Res. 164 (2019) 114929. DOI:10.1016/j.watres.2019.114929 |
| [5] |
D. Yuan, M. Sun, S. Tang, et al., Chin. Chem. Lett. 31 (2020) 547-550. DOI:10.1016/j.cclet.2019.09.051 |
| [6] |
J. Chen, J. Zhan, Y. Zhang, et al., Chin. Chem. Lett. 30 (2019) 735-738. DOI:10.1016/j.cclet.2018.08.020 |
| [7] |
Y. Li, W. Xiang, T. Zhou, et al., Chin. Chem. Lett. 31 (2020) 2757-2761. DOI:10.1016/j.cclet.2020.01.032 |
| [8] |
K. Wei, X. Cao, W. Gu, et al., Environ. Sci. Technol. 53 (2019) 6917-6926. DOI:10.1021/acs.est.8b07132 |
| [9] |
I. Benhamed, L. Barthe, R. Kessas, et al., Appl. Catal. B: Environ. 187 (2016) 228-237. DOI:10.1016/j.apcatb.2016.01.016 |
| [10] |
H. Guo, D. Li, Z. Li, et al., Sep. Purif. Technol. (2021) 118543. |
| [11] |
J. Li, Y. Li, Z. Xiong, et al., Chin. Chem. Lett. 30 (2019) 2139-2146. DOI:10.1016/j.cclet.2019.04.057 |
| [12] |
S. Tang, M. Zhao, D. Yuan, et al., Sep. Purif. Technol. 255 (2021) 117690. DOI:10.1016/j.seppur.2020.117690 |
| [13] |
D. Yuan, C. Zhang, S. Tang, et al., Water Res. 163 (2019) 114861. DOI:10.1016/j.watres.2019.114861 |
| [14] |
H. Guo, Z. Li, Z. Xie, et al., Vacuum 185 (2021) 110022. DOI:10.1016/j.vacuum.2020.110022 |
| [15] |
C. Shan, H. Liu, M. Hua, et al., Environ. Sci. Technol. 54 (2020) 5893-5901. DOI:10.1021/acs.est.0c00159 |
| [16] |
M. Gągol, A. Przyjazny, G. Boczkaj, Chem. Eng. J. 338 (2018) 599-627. DOI:10.1016/j.cej.2018.01.049 |
| [17] |
Y. Zhu, R. Zhu, Y. Xi, et al., Appl. Catal. B: Environ. 255 (2019) 117739. DOI:10.1016/j.apcatb.2019.05.041 |
| [18] |
X. Xu, W. Chen, S. Zong, et al., Chem. Eng. J. 373 (2019) 140-149. DOI:10.1016/j.cej.2019.05.030 |
| [19] |
H. Luo, Y. Cheng, Y. Zeng, et al., Sci. Total Environ. 732 (2020) 139335. DOI:10.1016/j.scitotenv.2020.139335 |
| [20] |
B. Shen, C. Dong, J. Ji, et al., Chin. Chem. Lett. 30 (2019) 2205-2210. DOI:10.1016/j.cclet.2019.09.052 |
| [21] |
D. Yuan, C. Zhang, S. Tang, et al., Sci. Total Environ. 727 (2020) 138773. DOI:10.1016/j.scitotenv.2020.138773 |
| [22] |
Z. Ouyang, C. Yang, J. He, et al., Chem. Eng. J. 402 (2020) 126122. DOI:10.1016/j.cej.2020.126122 |
| [23] |
Z. Li, L. Wang, Y. Liu, et al., Water Res. 168 (2020) 115093. DOI:10.1016/j.watres.2019.115093 |
| [24] |
Amina, X. Si, K. Wu, et al., Chem. Eng. J. 353 (2018) 80-91. DOI:10.1016/j.cej.2018.07.078 |
| [25] |
W. Wang, Y. Li, L. Li, et al., Int J. Electrochem. Sci. (2020) 11709-11722. DOI:10.20964/2020.12.49 |
| [26] |
M. Cao, Y. Hou, E. Zhang, et al., Chemosphere 229 (2019) 200-205. DOI:10.1016/j.chemosphere.2019.04.135 |
| [27] |
N. Dulova, E. Kattel, M. Trapido, Chem. Eng. J. 318 (2017) 254-263. DOI:10.1016/j.cej.2016.07.006 |
| [28] |
S. Hadi, E. Taheri, M.M. Amin, et al., J. Environ. Manage. 268 (2020) 110678. DOI:10.1016/j.jenvman.2020.110678 |
| [29] |
Y. Zhou, M. Huang, X. Wang, et al., Chemosphere 253 (2020) 126662. DOI:10.1016/j.chemosphere.2020.126662 |
| [30] |
Z. Sun, S. Li, H. Ding, et al., Chemosphere 241 (2020) 125125. DOI:10.1016/j.chemosphere.2019.125125 |
| [31] |
S. Tang, D. Yuan, Y. Rao, et al., J. Hazard. Mater. 366 (2019) 669-676. DOI:10.1016/j.jhazmat.2018.12.056 |
| [32] |
S. Tang, Z. Wang, D. Yuan, et al., J. Clean. Prod. (2020) 122253. |
| [33] |
H. Bataineh, O. Pestovsky, A. Bakac, Chem. Sci. 3 (2012) 1594-1599. DOI:10.1039/c2sc20099f |
| [34] |
C. Wang, Y. Liu, T. Zhou, et al., Chin. Chem. Lett. 30 (2019) 2231-2235. DOI:10.1016/j.cclet.2019.08.055 |
| [35] |
M. Huang, W. Xiang, C. Wang, et al., Chin. Chem. Lett. 31 (2020) 2769-2773. DOI:10.1016/j.cclet.2020.06.040 |
| [36] |
X. Wang, Y. Du, H. Liu, et al., RSC Adv. 8 (2018) 12791-12798. DOI:10.1039/C8RA01506F |
| [37] |
W. Xiang, M. Huang, Y. Wang, et al., Chin. Chem. Lett. 31 (2020) 2831-2834. DOI:10.1016/j.cclet.2020.08.006 |
| [38] |
W. Szeto, J. Li, H. Huang, et al., Chem. Eng. Sci. 177 (2018) 380-390. DOI:10.1016/j.ces.2017.10.008 |
| [39] |
X. Feng, Q. Li, K. Wang, ACS Appl. Mater. Interfaces 13 (2021) 400-410. DOI:10.1021/acsami.0c16489 |
| [40] |
H. Han, C. Shi, L. Yuan, et al., Appl. Energy 204 (2017) 382-389. DOI:10.1016/j.apenergy.2017.07.032 |
| [41] |
K. Wang, C. Liu, J. Sun, et al., Complexity 2021 (2021) 8816250. |
| [42] |
K. Wang, W. Wang, L. Wang, et al., Energies 13 (2020) 5297. DOI:10.3390/en13205297 |
| [43] |
T. Wang, Y. Zhou, S. Cao, et al., Ecotoxcol. Environ. Saf. 172 (2019) 334-340. DOI:10.1016/j.ecoenv.2019.01.106 |
| [44] |
H. Li, Y. Gong, Q. Huang, et al., Ind. Eng. Chem. Res. 52 (2013) 15560-15567. DOI:10.1021/ie401503u |
| [45] |
S. Tang, M. Zhao, D. Yuan, et al., Chemosphere 268 (2021) 129315. DOI:10.1016/j.chemosphere.2020.129315 |
| [46] |
D. Yuan, M. Sun, M. Zhao, et al., Int. J. Electrochem. Sci. 15 (2020) 8761-8770. |
| [47] |
J. Wang, S. Wang, Chem. Eng. J. 401 (2020) 126158. DOI:10.1016/j.cej.2020.126158 |
| [48] |
Z. Wu, K. Yin, J. Wu, et al., Nanoscale 13 (2021) 2209-2226. DOI:10.1039/D0NR06639G |
| [49] |
J. Wu, K. Yin, S. Xiao, et al., Adv. Mater. Interfaces 8 (2021) 2001610. DOI:10.1002/admi.202001610 |
 2021, Vol. 32
2021, Vol. 32 

