b School of Nanoscience and Technology, University of Chinese Academy of Sciences, Beijing 100049, China
Since the discovery of mechanically exfoliated graphene with extraordinary physical and chemical properties by Andre Geim and Kostya Novoselov [1], two-dimensional (2D) nanomaterials have been attracting tremendous interest in the materials science and condensed-matter physics [2], because they are characteristic of exceptional conductivity, tensile strength, flexibility, transparency, exposed sites and facile mass transfer and diffusion of substrates and products. Until now, a series of 2D nanomaterials have been developed rapidly [3], such as transition metal dichalcogenides (TMDCs) [4, 5], 2D-Xenes (X=Si, Ge, Sn, etc.) [6-9], graphitic carbon nitride (g-C3N4) [10], double layer metal hydroxides [11, 12] and hexagonal boron nitride [13]. However, despite of the potential advantages of amazing 2D nanomaterials, the simplicity of their structures often limits their applications. Therefore, it is highly desirable to pursuit diverse 2D nanomaterials with abundant design and adjustability for satisfying different applications.
Different from above 2D nanomaterials, metal-organic framework nanosheets (MONs) as the emerging materials, which are synthesized by reacting organic ligands (linkers) with specific metal ions (connectors), have been arousing great attention, because they have extremely rich metal nodes, diverse organic ligands and topological structures. The combinations between linkers and connectors often allow them to be tuned through reticular substitution or post-synthetic modifications. To date, MONs have many other appellative names such as metal-organic layers (MOLs) [14], metal-organic graphene analogues (MOGs) [15], 2D coordination polymers [16], coordination nanosheets (CONASH) [17], organometallic sheets [18] and hybrid organic-inorganic nanosheets [19]. Through these names that represent different types of MONs, we can see the massive research and rapid development of MONs.
The MONs possess the basic structure of MOFs, and they could be obtained by limiting the growth of MOFs in a certain direction or by physical and chemical stripping, and finally, the overall thickness of the MONs is at nanoscale level. Towards the application in the field of catalysis [20], the accessibility of surface sites of the MONs makes them easy to be functionalized via coordination chemistry. Moreover, the MONs are suitable for combining with other active centers [21-23], such as single metal sites, metal nanoparticles (NPs), metal oxides, metal sulfides and carbon nanotubes, and thus, they can achieve more functions and realize heterogeneous catalysis with high performance.
To date, various MONs have been successfully prepared and applied for heterogeneous catalysis [20, 24, 25], however, it is still difficult for synthesis of MONs with tunable composition and thickness, thus leading to the difficulties in tuning their catalytic performances and revealing the structure-property relationship. In this review, we aim to give a brief summary for the research progress on the rising MONs based nanocatalysts as well as their thermal catalytic performances. Firstly, different synthesis strategies are introduced for synthesizing various MONs. Secondly, synthesis of different composites of MONs with other components is summarized. Thirdly, the thermal catalytic applications of MONs and their composites are discussed and the relationships among them are revealed. Finally, the potential challenges on the synthesis of different MONs based materials as well as their thermal catalytic applications are proposed.
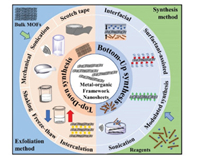
2. Synthesis strategies for MONsGenerally, there are two distinct ways to synthesize different MONs, one is called "Top-down synthesis", in which the nanosheets are isolated from bulk layered materials, and the other is called "Bottom-up synthesis", which means synthesis of the nanosheets directly from 2D entities via arresting the crystallization process (Fig. 1) [20, 24-26]. By using above two strategies, various MONs have been successfully synthesized. They are strongly correlated with the metal ions, organic ligands and reaction parameters such as solvent, temperature and pH value. Thus, the obtained MONs are often different with each other, such as the average thickness, and the lateral size. Herein, we will summarize the recent progress on synthesis of MONs by using the above two synthesis strategies.
|
Download:
|
| Fig. 1. Synthesis of MONs through "Top-down" and "Bottom-up" strategies. "Top-down" strategy refers to synthesis of MONs from bulk MOFs via various exfoliation methods. "Bottom-up" strategy refers to synthesis of MONs from reagents via interfacial synthesis, surfactant-assisted synthesis, modulated synthesis or sonication synthesis. | |
The top-down synthesis strategy involving exfoliation of bulk MOF materials has been demonstrated to be a simple and efficient approach for preparing MONs, but the exfoliation process often requires multistep approaches via mechanical or solvent-mediated strategy for weakening interlayer interactions in MOF crystals, leading to the nanosheets with multitudinous thicknesses and low yields (typically < 15%) [27].
Just like the top-down exfoliation of graphene, we can get MONs from layered MOFs, which are formed by stacking 2D layers vertically through weak interactions such as van der Waals force [28], hydrogen bonding [29, 30] and π-π stacking [31]. The key step of the top-down strategy is to break the relatively weak interaction between the molecular layers, for which the bulk MOFs can be separated by external force or inserting molecules into the layers [32]. We roughly divide these methods into two categories, including physical exfoliation such as sonication exfoliation, mechanical exfoliation, shaking exfoliation, freeze-thaw exfoliation, and chemical exfoliation such as intercalation method.
2.1.1. Physical exfoliationPhysical exfoliation is similar with the famous method called "Scotch tape" [1], which was originally used to isolate graphene nanosheets. Abhervé et al. prepared the MONs with heights down to 2 nm from a layered cationic framework by using the "Scotch tape" method [33]. Typically, the 2D nanomaterial is first peeled off from its bulk crystals by using adhesive Scotch tape. As the Scotch tape removed, MONs were left on the substrate. Using this micromechanical method can obtain the thinner layers compared with flakes (heights of ~5 nm) obtained by ultrasonic method in acetone, ethanol or acetonitrile solution, but it is difficult in controlling the thickness distribution of the tearing process, and the obtained MONs with thickness of sub-2 nm or 10~20 nm were found during the exfoliation progress.
Sonication exfoliation is an effective way to produce MONs, and the energy input provided by the ultrasonication can overcome the inter-layer interactions, facilitate solvent penetration, and serve as a stimulus for layer separation. For example, Zamora and co-workers reported the exfoliation of a layered MOF, [Cu2Br(IN)2]n (IN=isonicotinato), by sonication treatment [31]. Casting deposition of sonicated solution of [Cu2Br(IN)2]n (10-10 mg/mL) on highly oriented pyrolitic graphite (HOPG) allows to isolate the monolayers. Atomic force microscopy (AFM) topography image showed a typical thickness of 5±0.15Å based on 15 randomly isolated flakes (Fig. 2a), and they were very dense and homogenous on HOPG. Also, solvent plays an important role in obtaining MONs via the sonication exfoliation. Some typical solvents including water, methanol, ethanol, propanol, hexane and tetrahydrofuran (THF) were used for the sonication exfoliation [34], among which ethanol was found to be the most efficient solvent to exfoliate these layered MOFs and prevent the exfoliated nanosheets from restacking. Typically, the ethanol-exfoliated samples have a typical thickness ranging from about 10–100 nm, and their lateral dimensions can reach about 10 μm. The fragments of the ethanol-exfoliated sample generally have sharper corners, while as for hexane exfoliation process, it often causes the nanosheets with rounding-off of corners and widening of surface cracks. Zhang and co-workers reported the successful exfoliation of 2D monolayer aluminum tetra-(4-carboxyphenyl) porphyrin MONs by sonication exfoliation in ethanol solution [35]. The exfoliation can reach ~90% yield of MONs with the average thickness of ~1.9 nm and a lateral size from 200 nm to 2 μm. Foster et al. designed a new layered Zn-MOFs with bulky 3-methoxypropoxy groups on the terephthalic ligands, which make its dispersion and exfoliation easy in different solvents, such as N, N-dimethylformamide (DMF), water, acetonitrile, acetone and ethanol [36]. Typically, MONs with thicknesses of 5-30 nm and lateral sizes of 100-500 nm were generated via sonication in DMF. When exfoliated in acetonitrile, acetone and ethanol, MONs were obtained with shorter interlayer distance and smaller unit cell volume.

|
Download:
|
| Fig. 2. (a) AFM topography image of [Cu2Br(IN)2]n MONs deposited on HOPG. Reproduced with permission [31]. Copyright 2010, Royal Society of Chemistry. (b) Scanning electron microscopy (SEM) image of as-synthesized Zn2(bim)4crystals. The inset image shows the typical flake-like morphology of Zn2(bim)4 crystals. (c) Transmission electron microscopy (TEM) image of Zn2(bim)4 MONs. The inset shows the Tyndall effect of a colloidal suspension. Reproduced with permission [29]. Copyright 2014, American Association for the Advancement of Science. (d) The freeze-thaw exfoliation of MAMS-1 crystals into dispersed nanosheets. (e) Thickness and lateral size distribution of exfoliated MAMS-1 nanosheets after 10-cycle freeze-thaw exfoliation in hexane (horizontal line indicates the theoretical thickness of a bilayer MAMS-1 nanosheet). Reproduced with permission [37]. Copyright 2017, Nature Publishing Group. | |
In addition, the layer stacking by weak van der Waals forces among dynamically coordinated DMF molecules enables exfoliation and morphology transformation of [Ca3(HL)2(DMF)5]n MOFs (H4L=2′-amino-[1, 1′: 4′, 1′′-terphenyl]-3, 3′′, 5, 5′′-tetracarboxylic acid) [28]. Moreover, if H2O is introduced into the DMF solution, the interlayer forces is further weakened for obtaining the ultrathin Ca-MONs with single or few coordination layers.
Simple mechanical synthesis methods tend to cause the discontinuity of MONs, but combination of several methods will get unexpected results. Yang and co-workers developed a soft-physical ball milling process to exfoliate Zn2(bim)4 (bim=benzimidazole) with the sonication process [29]. Pristine Zn2(bim)4 crystals were first wet ball-milled at very low speed (60 rpm), followed by exfoliation in the mixed volatile solvents of methanol and propanol (Figs. 2b and c). The Zn ions coordinating with four benzimidazole ligands in a distorted tetrahedral geometry could form a single Zn2(bim)4 layer. These 2D layers further stack along the c axis through the van der Waals interaction and form the MOF crystal with an interlayer distance of 0.988 nm.
Shaking exfoliation is another simple physical exfoliation method. Using this method, Maeda and co-workers exfoliated La(BTP) (BTP=1, 3, 5-benzenetriphosphonic acid) to produce the nanosheets with thickness of approximately 1.3 nm [30]. In detail, the bulk MOF was shaken in the DMF solution at 40 ℃ for 3 h, which can weak the hydrogen bonds between phosphonate groups. And finally, the MONs were collected on cleaned Si substrates.
Freeze-thaw exfoliation method has also been applied for the exfoliation of layered MOFs. The exfoliation of Ni8(5-BBDC)6(μ-OH)4 (denoted as MAMS-1, BBDC=5-tert-butyl-1, 3-benzenedicarboxylic acid) was successfully achieved by using this method [37]. In detail, the MAMS-1 crystals were dispersed in hexane and heated in hot water bath at 80 ℃ for 3-5 min. Then, immediately frozen in liquid nitrogen bath at -196 ℃ until complete freeze, followed by thawing them into hot water bath (80 ℃) again. By repeating the freeze-thaw cycle, the shear force, which is produced during the volumetric change of hexane between solid state and liquid state, is exerted on the suspended MAMS-1 crystals, leading to the production of freestanding nanosheets. The obtained MONs were characteristic with about 4 nm in thickness and the average lateral size of 10.7 mm (Figs. 2d and e), proving the feasibility of freeze-thaw strategy for producing MONs.
2.1.2. Chemical exfoliationIntercalation method often involves introducing new ions or chemical bonds between the layers to increase the spacing between layers, and then, separating the layered MOFs into nanosheets. As a typical example, Xia and co-workers reported the exfoliation of La2(TDA)3 (TDA=220-thiodiacetic acid) using a sonication/Li-intercalation approach [38]. First, MOFs with fewer layers were obtained by partial shedding, then n-butyl lithium was inserted between MOFs layers. Lithium ions could increase the spacing between layers, and reduce the interlayer forces, and finally, monolayered MONs are easily obtained with a thickness of 2.0 nm. Furthermore, Zhou and co-workers developed an intercalation/chemical exfoliation method to synthesize ultrathin MONs [39]. First, the layered MOF crystals are intercalated with 4, 4′-dipyridyl disulfides through coordination bonding with the metal nodes; subsequently, selective cleavage of the disulfide bond induces exfoliation of the intercalated MOF crystals, leading to the individual freestanding MONs. This chemical exfoliation process can produce the ultrathin MONs (~1 nm) with the overall yield of ~57% at room temperature.
Altogether, the top-down synthesis strategy is a simple and effective way to obtain various MONs, and it is more suitable for synthesis of M-bim and M-BDC MONs; however, the scalability is relatively poor [25]. It is worth noting that the yield of MONs is generally low and it is difficult to solve the uniformity and adhesion of peeling thickness via top-down strategy, and moreover, there still remains some challenges to achieve, such as decentralized size distribution and re-aggregation of the laminae. Comprehensive consideration of multiple synthetic conditions will contribute to finding more effective approaches to synthesize the stable MONs.
2.2. Bottom-up synthesis of MONsThe "bottom-up" synthesis of MONs can be seen as an arrested crystallization, in which growth occurs preferentially in two dimensions from metal ions and organic linkers. The most critical step is to block growth in one direction and allow growth in the other two dimensions [20, 25]. To date, different bottom-up methods have been succeeded in synthesis of MONs from both layered and non-layered MOFs. In this section, we will introduce the synthesis of MONs via bottom-up methods, such as interfacial synthesis, surfactant-assisted synthesis, modulated synthesis and sonication synthesis.
2.2.1. Interfacial synthesis of MONsInterfacial synthesis is one of the most popular methods to obtain MONs, which limit the reaction between organic ligands and metal nodes on the interface. Therefore, MOFs cannot grow in the three demissions, leading to the formation of MONs. By considering the type of interface, this part can be divided into three parts, including liquid/liquid interface, liquid/air interface, and liquid/solid interface.
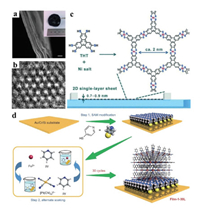
As for the liquid/liquid interface synthesis, two immiscible liquids are used to dissolve the metal ions and organic ligands, and then MONs are formed at the interface of the solvents. For example, dichloromethane-water interface was used to synthesize a thin film of Cu-BHT (BHT=benzenehexathiol) [40]. BHT was first dissolved in degassed dichloromethane to afford a saturated solution, and then, covered with degassed water to form an oil-water interface. The formation of thin films can be observed at the interface by injecting the metal ion solution into the water. N, N-dimethylacetamide (DMAC)/water interface was applied for large-scalable, bottom-up synthesis of NiFe-MONs with the thickness from 1.67 nm to 2.58 nm in the gram scale [41]. Similarly, Marinescu and co-workers reported the synthesis of Co-BHT and Co-THT (THT=1, 2, 5, 6, 9, 10-triphenylenehexathiol) MONs at the interface of water/ethyl acetate [42, 43]. The ethyl acetate solvents containing Co2+ ions allowed to evaporate over 1 h when Co2+ ions coordinated with organic ligands in water, thus leaving behind the MONs at the air-water interface. In addition, Cánovas et al. reported the synthesis of a large-area free-standing multilayer Fe3(THT)2(NH4)3 film, free of steps or cracks at the CHCl3/water interface under argon at room temperature [44]. The film thickness is tunable by adjusting the reaction time, from 20 nm as beginning to about 2 μm for 72 h (Fig. 3a). The nanosheets possessed a honeycomb structure with pore size of ~1.9 nm (Fig. 3b), which showed an ordered network with a ~1.97 nm unit cell dimension. This work indicated that such porous MONs have a high degree of chemical and structural adjustability, which will provide more possibilities for further applications.
|
Download:
|
| Fig. 3. (a) SEM image from cross-side view. Scale bar: 1 μm. Inset: photo of a large-area free-standing film. (b) High-resolution TEM (HRTEM) image of the 2D honeycomb structures. Scale bar: 2 nm. Reproduced with permission [44]. Copyright 2018, Nature Publishing Group. (c) Synthesis of a single-layer nanosheet using the Langmuir–Blodgett method at an air/water interface. Reproduced with permission [47]. Copyright 2015, Wiley-VCH. (d) Schematic illustration of the thin-film fabrication of Fe(py)2[Pt(CN)4] based on the LbL protocol. Reproduced with permission [48]. Copyright 2016, Nature Publishing Group. | |
The liquid/air interface, such as water/air interface, can be used to synthesize MONs. The MONs can be produced on the interface as the evaporation of solvent with organic ligands and then immobilized on a substrate via the Langmuir Schafer (LS) method using a Langmuir Blodgett (LB) apparatus [45, 46]. For example, Feng and co-workers reported the synthesis of large-area single layered Ni-THT MONs by using the LB method (Fig. 3c) [47]. The submonolayer of THT monomers was distributed over the water surface of the LB sink until tightly packed. 2D supramolecular polymerization was initiated by the coordination of nickel ions with dithiolene units during the diffusion of the nickel salt solution into the aqueous phase, resulting in the generation of target MONs with thickness of 0.7-0.9 nm.
The liquid/solid interfacial method is also an effective strategy for the synthesis of MONs. Generally, the MONs can be constructed through self-assembly and layer-by-layer synthesis at the interface between the metal or alloy substrate and the solution. Otsubo, Kitagawa and co-workers reported that the Fe(py)2[Pt(CN)4] (py=pyridine) nanosheets were obtained through layer-by-layer growth at the liquid/solid interface [48]. An Au/Cr/Si substrate, which covered with a self-assembled monolayer (SAM) of pyridine-terminated thiol(4-mercaptopyridine), was soaked alternately in two different ethanol solutions containing Fe2+ species or [Pt(CN)4]2–. Both could produce the MONs at room temperature for a total of 30 cycles (Fig. 3d). The thickness of MONs was increased from 16 nm to 49 nm as the number of layer-by-layer cycles from 30 to 150. Recently, Dong et al. reported a general self-dissociation-assembly strategy for in situ synthesis of the well-defined ultrathin CoNi-MON arrays [49]. In a typical process, a piece of deionized Co9Ni1 foam (2 cm×1 cm ×0.5 mm) was immersed into the mixed solution containing benzenedicarboxylic acid, DMF, ethanol and water, and then the sealed vessel was transferred into a stainless-steel autoclave and heated at 150 ℃ for 48 h. The organic ligands self-assembled with metal ions on the surface of the alloy foam, and finally, the MONs with thickness of about 8.2 nm and average length of 1 μm were obtained.
Like other 2D nanomaterials [50], chemical vapor deposition (CVD) is applied for synthesizing MONs. This method needs to deposit organic ligands and metal precursors on the metal surface, like Cu(111) [51], Au(111) [52] and Ag(111) [53]. The deposited samples are annealed at high temperature for several minutes to get the well-ordered MONs [54]. Chi et al. successfully constructed the MONs via the dehydrogenation reactions of aromatic amines on the Cu(111) surfaces [51]. Scanning tunneling microscope (STM) studies revealed that the large-scale 2D porous networks are formed by depositing TAPB (1, 3, 5-tris(4-aminophenyl) benzene) molecules on a Cu(111) surface held at 350 K, at which each amino group of TAPB has one hydrogen atom dissociated. The MONs were formed via the N-Cu-N interaction between the dehydrogenated amino groups and the copper adatoms. Au(111) was also used as metal surface to synthesize single-layer Ni3(HITP)2 (HITP=2, 3, 6, 7, 10, 11-hexaiminotriphenylene) MONs [52]. HATP (2, 3, 6, 7, 10, 11-hexaaminotriphenylene) was sublimed using an organic-beam evaporator and deposited on a clean single-crystal Au(111) (inset in Fig. 4a). The hexagonal-shaped objects are assigned as HITP molecules, and the oval-shaped objects are assigned as Ni atoms. Each HITP molecule linked head-to-head, by a Ni atom with three neighboring HITP molecules. The diamond-shaped unit cell in Fig. 4b is characteristic with a lattice constant of 2.17±0.02 nm.

|
Download:
|
| Fig. 4. (a) STM image for the single-layer Ni3(HITP)2 network self-assembled on Au(111) (inset: a molecular model of the HATP molecule, C: brown, N: blue, H: pink). Scale bar: 20 nm. (b) A HR-STM image overlaid with a molecular model (Ni: large blue balls). Scale bar: 2 nm. Reproduced with permission [52]. Copyright 2018, Royal Society of Chemistry. (c) Schematic illustration of the surfactant-assisted synthesis of Co-TCPP(Fe) MONs. Reproduced with permission [56]. Copyright 2016, Wiley-VCH. (d) Scheme of synthesis of ultrathin Ni-BDC MONs. (e) SEM and (f) TEM images of ultrathin Ni-BDC MONs. Reproduced with permission [58]. Copyright 2018, Tsinghua University Press and Springer-Verlag GmbH Germany, part of Springer Nature. | |
In a word, interfacial synthesis is a simple and powerful method to get MONs. Synthesis of MONs with different composition, thickness and even laminar size can be achieved by adjusting the type ratio of the solution and the properties of the substrates [52].
2.2.2. Surfactant-assisted methodCompared with the interfacial synthesis, surfactant-assisted synthesis has been regarded as an efficient bottom-up method to prepare different MONs, because the surfactant can not only restrict the growth of the MOFs along the layer-stacking direction but also help to stabilize the as-synthesized MONs in solution [55].
Zhang et al. prepared Co-TCPP(Fe) (TCPP=tetra(4-carboxyphenyl) porphine chloride) MONs with the assistance of polyvinyl pyrrolidone (PVP) [56]. As schematically illustrated in Fig. 4c, PVP can selectively attach on the surface of MOFs, which plays a key role in the anisotropic growth of Co-TCPP(Fe) and the formation of ultrathin 2D Co-TCPP(Fe) MONs with the thickness of 5.6±1.8 nm and the lateral size of ~2 μm. Similarly, Zn-TCPP MONs were prepared with a thickness of 90±25 nm [57]. Li and Tang et al. reported a PVP-assisted strategy to fabricate the ultrathin Ni-MONs of 1.5 nm in thickness (Figs. 4d-f) [58]. The PVP molecules firstly bind with H2BDC-NH2 ligands via formation of C=N bond, and the subsequent coordination of Ni2+ ions with carboxylic acid groups in the modified H2BDC-NH2 results in the formation of the ultrathin MONs under solvothermal condition.
Anionic surfactant sodium dodecyl sulfate (SDS) was also applied for synthesis of MONs [59, 60]. Using this surfactant, Feng et al. synthesized two kinds of MONs including Ni-HHB MONs (HHB=hexahydroxybenzene) with a thickness of 4.5±1.4 nm (~9 layers) and a lateral size of 0.25-0.56 mm2 and Cu-HHTP MONs (HHTP=2, 3, 6, 7, 10, 11-hexahydroxytriphenylene) with a thickness of 5.1±2.6 nm and a lateral size of 0.002-0.02 mm2 (~10 layers) [59]. It is noted that the negatively charged hydrophilic tail of the SDS molecules will preferentially anchor onto the surface of MOFs, which can serve as structure-directing agents to significantly alleviate the layer stacking, and thus cause the anisotropic growth of the MOFs in the 2D direction to form the ultrathin and large-sized MONs.
Bio-based surfactant sorbitol-alkylamine with polyhydroxy and amine groups in the headgroup (SAAS-Cm) was served as the competitive coordinating agents for chemically attaching onto the defects of Zr-BDC MOF, leading to the anisotropic growth of MOFs [61]. Due to the interaction of the hydrophobic chains, the intercalated bulk MOFs assemble with the surfactant, which could weaken the interactions of interlayers and disassemble to form new ultrathin MONs with the thickness of 3-4 nm, the lateral size of 0.5–2.0 μm, and a high yield of 67%. Moreover, by varying the alkyl chain length of the surfactants, the thickness of the MONs could be facilely tuned from 3 nm to 60 nm. In addition, when BDC containing different electron-withdrawing and electron-donating groups was used as ligands, Zr-MONs could only be formed by using dicarboxylate ligands with electron-donating groups, such as 2, 5-dihydroxyterephthalic acid, 2-aminoterephthalic acid and 2-bromotere-phthalic acid.
Cetyltrimethylammonium bromide (CTAB) was reported as a surfactant in the synthesis of Cu-BDC MONs [62]. The cationic surfactant CTAB can interact with the organic linkers or metal ions, resulting in crystal growth in a preferred direction. Seoane and Jorge et al. prepared the nonlayered NH2-MIL-53(Al) lamellae with the thickness of 35-45 nm and the lateral dimension of 140-400 nm assisted by CTAB [63]. The mixed solution containing Al3+ and CTAB was heated to obtain a preorganized vesicular system before the surfactant was replaced by 2-amino-terephthalic ligands in alkaline aqueous solution. Furthermore, the Al-CTAB preorganized vesicular system is also feasible for synthesis of CAU-10(Al) and NH2-CAU-10(Al) MONs. In addition, using this method, other trivalent metals could be extended to synthesize MONs once the metal preassembled in the presence of CTAB.
Altogether, the surfactant molecules can provide the more stable environment for producing MONs, which could block the growth of MONs in the third direction through covalent action or interaction with the nodes and linkers of the MONs. Moreover, the surfactants could contribute to avoiding the aggregation and stacking between layers and improving the stability of MONs by reducing the high surface energy of MONs.
2.2.3. Modulated methodSmall modulators, such as acetic acid, trifluoroacetic acid, and benzoic acid, have been successfully applied for synthesis of MONs. These molecules usually coordinate with the metal nodes of MOFs to regulate the growth kinetics.
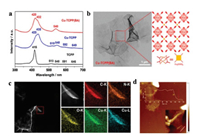
Gu et al. reported using formic acid as a saturated modulator to prepare Zr-BTB (benzene-1, 3, 5-tribenzoate) MONs [64]. It is noted that formic acid could overcome the high surface energy of nanosheets and suppress the growth along the vertical direction, resulting in twisted stacking MONs with thickness of 8 nm. Furthermore, they found that soaking in ethanol was an effective way to untwist the tilted nanosheet stacking at 80 ℃ for 6 h under vacuum. Kitagawa and co-workers reported the modulated synthesis of [Cu2(BDC)2(BPY)]n MONs (denoted as 1-meso) [65]. The 2D square grid of [Cu(BDC)(S)]n (S=solvent) was formed by reacting copper ions with H2BDC via using acetic acid as a modulator. With increasing the concentration of acetic acid, the average crystal size of 1-meso was increased from ~50×50×20 nm3 to ~300×300×30 nm3. Pei et al. reported a simple and feasible method for the preparation of micron-size ultrathin MONs with benzoic acid (BA) as an adjuvant [66]. The obtained Cu-TCPP MONs displayed a relatively thick lamellar morphology and a particle size of 100-400 nm, while the obtained Cu-TCPP(BA) displayed the average thickness of 1-2 nm, and a well-defined ultrathin structure with the particle size of 3-8 μm (Figs. 5a-d). Benzoic acid plays a key role in the formation of the Cu-TCPP(BA) MONs. With the increase in the concentration of benzoic acid, the resultant Cu-TCPP(BA) MONs gradually transformed into a micron-scale graphene-like sheet structure, and the thickness was also reduced.
|
Download:
|
| Fig. 5. (a) UV–vis absorption of the TCPP, Cu-TCPP and Cu-TCPP(BA). (b) TEM, (c) elemental mapping and (d) AFM height images of the prepared Cu-TCPP(BA) MONs. Reproduced with permission [66]. Copyright 2020, American Chemical Society. | |
In addition, pyridine could be used as a modulator for synthesis of MONs, because it could combine with metal nodes and limit the growth of MOF in the third direction. Ma and co-workers reported the synthesis of a specific (3, 4)-connected Ni2(BDC)2(DABCO) (DABCO=1, 4-diazabicyclo [2.2.2]-octane) MONs by using pyridine via a substitution-suppression process [67]. During the synthesis process, pyridine could act as ligand to coordinate with Ni2+ ions over DABCO. The morphology of final product can be modified from hexagonal nanorods to nanosheets with controllable thickness tuned by the dosage of pyridine. Typically, the obtained MONs are characteristic with the average thickness of 20 nm and a lateral size ranging from 600 nm to 800 nm.
In brief, it is of great importance to select the appropriate modulators for interacting with organic linkers or metal centers to limit the growth of MOFs, or avoid the restacking of the obtained MONs.
2.2.4. Sonication methodUltrasonic treatment is not only effective in the top-down exfoliation process, but also a practical way to synthesize MONs from the reagents via the bottom-up method. During sonication process, cavitation could lead to the rapid release of energy with heating and cooling rates of > 1010 K/s, temperatures up to 5000 K, and pressures up to 1000 bar [68], which will be a fast, energy-efficient and environmentally friendly method for synthesis of MONs.
For example, Tang et al. reported the synthesis of ultrathin MONs using sonication method [69]. In detail, Ni2+, Co2+ ions and H2BDC were first dissolved in a mixture of DMF, water and ethanol, and then triethanolamine was quickly injected with stirring for 5 min to obtain a uniform colloidal suspension. Afterward, the colloidal solution was continuously ultrasonicated for 8 h (40 kHz) under airtight condition to obtain ultrathin NiCo-MONs. As shownFig. 6a, Co and Ni atoms are connected in the (200) plane to form 2D bimetal layers, which are separated by BDC ligands. TEM and AFM images clearly show the ultrathin morphology of the as-synthesized NiCo-MONs with a thickness of about 3.1 nm (Figs. 6b and c). The high-angle annular dark-field scanning TEM (HAADF-STEM) imaging shows the alignment of metal atoms on the (200) planes in a hexagonal array pattern, in good agreement with the theoretical model (Fig. 6d). Similarity, Wang et al. developed a simple ultrasonic oscillation method for synthesizing MONs [70]. The solution was ultrasonically shocked (25 kHz) for 8 h after the metal precursor was added into the solution and formed an emulsion in the presence of triethylamine acid. The thickness of the NiFe-MONs is around 10 nm and the interplanar spacing of lattice plane (200) is about 1.01 nm. Similarly, sonication synthesis was also used for the synthesis of MONs with different metal nodes and organic ligands, such as [Zn(BDC)(H2O)] MONs [71] and Co-TDA MONs (TDA=2, 5-thiophenedicarboxylate) [72].

|
Download:
|
| Fig. 6. (a) Crystal structure of NiCo-MONs. (b) TEM image of NiCo-MONs. The inset shows the Tyndall light scattering of NiCo-MONs in an aqueous solution. (c) HAADF-STEM image of the (200) plane for NiCo-MONs showing the hexagonal arrangement of the metal atoms. The pink color represents metal atoms, blue is for light elements (carbon and oxygen), and green is for the background. (d) AFM image of as-prepared NiCo-MONs, showing measured dimensions of individual flakes. Reproduced with permission [69]. Copyright 2016, Nature Publishing Group. | |
Other bottom-up methods such as inverse microemulsion and drop-casting synthesis were also developed to synthesize MONs. For example, chiral MONs were fabricated using an inverse microemulsion method [73]. Through dispersing the aqueous precursors into isooctane with the assistance of NaAOT (dioctyl sulfosuccinate sodium), the limitation of microreactors and association of AOT- surfactant contribute to the anisotropic growth of chiral MONs with thickness of 3.7 nm (6 layers). In addition, Cu-Fe-NH2 MONs were synthesized by using "drop-casting" method [74]. In detail, the solvothermally synthesized reaction mixtures containing the bulk Cu-Fe-NH2 MOFs, reaction residues and solvents is subjected to ultrasonically agitating in ice-cold water bath for obtaining the inks. And then, the MOF inks were dropped onto the tips of clean nickel foam substrates, and the drops instantly spread through the porous nickel foam network due to capillary action. The resulted Cu-Fe-NH2 MOF/nickel foam film had a 2D sheet-like structure with an average size of ~390 nm.
Combining bottom-up methods with top-down methods is also an effective strategy to produce the MONs. Lotsch et al. reported the synthesis of ultrathin MONs via the surfactant-assisted bottom-up synthesis and the subsequent exfoliation method [75]. With the help of CTAB, the [Zn(BeIM)OAc] (BeIM=benzimidazole) MONs were self-assembled with about 8 nm lattice period at the interface of the reversed microemulsion. After that, the nanosheets were obtained with thickness of ~10 nm after 72-h vibration stripping in the mixed solvents of THF and chloroform. Tan and co-workers reported the synthesis of (HNEt3)2[Zn3BDC4] MONs by the rapid supramolecular self-assembly method and subsequent sonication exfoliation [76]. The DMF solution of Zn(NO3)2 was mixed with H2BDC and NEt3 solution, and quickly self-assembled to form a gel-like supramolecular material. After washing with a variety of polar solvents and ultrasonic treatment, the supramolecular fibers were decomposed to obtain (HNEt3)2[Zn3BDC4] MONs.
3. MONs based compositesTo extend the application of MONs, it is of great importance to combine with other active components to form the composites, which could not only integrate the functions of each component, but also generate the novel synergy property for achieving high performances. To date, many kinds of MONs based composites have been reported, like polymer/MONs [77-79, 80], carbon cloth/MONs [81], carbon nanotube/MONs [82, 83], graphene oxide/MONs [84]. Herein, we will focus on the synthesis of various kinds of MONs based composites for catalysis, mainly including the single metal sites, metal NPs and metal oxides and metal sulfides.
3.1. Single metal site supported by MONsSingle-atom catalysts have been arousing great interest because the well-defined active center with high activity, selectivity and stability [85, 86]. Considering the adjustable organic ligand groups on MONs that could provide anchoring sites for metal ions, various MONs supported single-site catalysts have been designed for heterogeneous catalysis.
One of the typical ways is to directly graft metal sites onto the organic ligands of MONs for constructing single-site catalysts. For example, Lin et al. synthesized Hf12-Ru nMON by directly reacting Ru(H2DBB)(bpy)22+ (bpy=2, 2′-bipyridine, DBB=4, 4′-di(4-benzoato)-2, 2′-bipyridine) ligands with HfCl4 [87]. During the synthesis process, trifluoroacetic acid (TFA) and water act as modulators, and the obtained Hf12-Ru nMON is characteristic with a thickness of ~1.7 nm and a lateral dimension of ~150 nm. Similarly, they synthesized Hf12-Ir-F nMON by reacting the Ir(DBB)[dF(CF3)ppy]2+ (DBB=4, 4′-di(4-benzoato)-2, 2′-bipyridine; dF(CF3)ppy=2-(2, 4-difluor-ophenyl)-5-(trifluoromethyl)pyridine) ligands with [Hf6O4(OH)4(HCO2)6] secondary building units (SBUs) [88]. During this synthesis process, Ni(MBA)Cl2[MBA=2-(4′-methyl-[2, 2′-bipyridin]-4-yl)acetate] was used as capping agents, and the molar ratio of Ni to Ir is 1 in the final product. It is noted that the obtained Hf12-Ir-Ni retained the monolayer structure of Hf12-Ir-F, with a thickness of ~2.9 nm and a lateral dimension of ~300 nm. In this case, two kinds of single metal sites were gained with adjacent distance ~0.85 nm, where Ir centers were used for photosensitive agents and Ni centers are the active sites for catalysis. In addition, post synthetic strategy was developed to graft single metal sites onto organic ligands of MONs. For example, Lin and Sun et al. prepared single Pd sites on the MONs [89], which is synthesized by reacting H3TPYTC with HfCl4 in a solvent mixture of DMF, formic acid, and water. The as-prepared MONs were metalated with Pd(OAc)2 in acetonitrile (MeCN) via coordinating with pyridines in TPY, and then the acetate groups on the Pd precursors were removed by treatment with HBF4·OEt2. Subsequently, the single-site Pd in Pd-MONs was coordinated with 2-chloro-110-phenanthroline (phenCl) to form the [PdII(TPY)(phenCl)]2+ center. Then, the Pd-MOL-phenCl was reacted with 2 equiv. of Selectfluor to obtain F-Pd-MOL-phenCl. It is noted that F-Pd-MOL-phenCl was measured to be 1.8 nm in thickness, slightly thicker than the unfunctionalized MONs (1.3 nm).
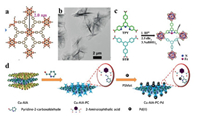
Another way is to functionalize the organic ligands in MONs with more functional groups for chelating with single metal sites. For example, Li et al. prepared the Cu-MONs with thickness of about 76 nm, and then coordinated amino groups in the ligand 2-aminoiophthalic acid (AIA) with the carbonyl group in the 2-pyridine formaldehyde to form Cu-AIA-PC [90]. After that, Pd(II) ions could chelate with pyridyl and C=N groups on the Cu-AIA-PC with the content of 8.3 wt% (Fig. 7d), and they are mainly in the form of divalent state in the target Cu-AIA-PC-Pd. Energy dispersive X-ray spectroscopy (EDX) analysis illustrates that the elements of C, O, Cu and Pd are homogeneously dispersed throughout the nanosheets.
|
Download:
|
| Fig. 7. (a) Formation of the 2D kgd lattice from 6-connected SBUs and 3-connected BTB ligand. (b) TEM image of Hf6O4(OH)4(HCO2)6(BTB)3 MONs. (c) Preparation of the Fe-TPY-MONs. Reproduced with permission [91]. Copyright 2016, Wiley-VCH. (d) Schematic illustration for the preparation of Cu-AIA-PC-Pd catalyst. Reproduced with permission [90]. Copyright 2020, Wiley-VCH. | |
Besides, mixed organic ligands are introduced into the MONs for grafting single metal sites. For example, Lin et al. grafted single iron site onto MONs with thickness of 1.2±0.2 nm [91], which were composed by [Hf6O4(OH)4(HCO2)6] SBUs, and mixed ligands of benzene-1, 3, 5-tribenzoate (BTB) and 4′-(4-benzoate)-(2, 2′, 2′′-terpyridine)-5, 5′′-dicarboxylate (TPY) (Figs. 7a and b). To provide the coordination environment for single iron site, 30% BTB in MONs is replaced by TPY, which could be metalated with FeBr2 (1.05 eq. relative to TPY) via coordinating with three adjacent pyridines in one TPY ligand, and then activated through reducing agent NaBHEt3 to get the target products (Fig. 7c). Single iron centers in the catalyst were confirmed to be 1:1 with respect to TPY. Powder X-ray diffraction (PXRD) and TEM studies confirmed that the Fe-TPY-MONs catalyst adopts the same structure as the undoped MONs.
In a word, MONs supported single-site metal catalysts could be successfully constructed by taking full advantages of ligands in the MONs. On the one hand, metal ions could be grafted on the functional groups of ligands before or after the synthesis of MONs. On the other hand, post-synthetic modification of organic ligands in MONs provides the appropriate coordination environment for single metal sites.
3.2. Metal NPs supported by MONsGenerally, synthesis of composites of metal NPs and MONs can be divided into two methods. One method is to directly introduce metal NPs or their precursors in/on the as-prepared MONs. The other method is to synthesize the MONs around as-prepared metal NPs.
3.2.1. Metal NPs grown on MONsMetal NPs are often used as active components for catalysis, and now, various MONs supported metal NPs have been developed, such as Pt [92-94], Pd [95], Au [96-98], Ag [99], Ru [100], Ir [101] NPs and their alloys [99]. For examples, Sun et al. successfully achieved Pt NPs of ~3 nm in diameter deposited on Ni-BDC MONs via in situ reduction of chloroplatinic acid (H2PtCl6) in ethanol without adding any capping agent (Fig. 8a) [92]. Abundant surface oxygen atoms with unpaired electrons in Ni-BDC MONs could provide uniformly distributed anchoring sites for Pt NPs, and moreover, the zero-valent Pt is mainly present in the composite, while there is also less amount of Pt(II) ions. HRTEM image showed the exposed interplanar spacing of 0.23 nm (Fig. 8b), which can be ascribed to the face-centered cubic (fcc) (111) facets of Pt. In addition, Shea et al. prepared Pd NPs on the surface of Zr-MONs, in which the 110-ferrocene dicarboxylic acid (FcDA) acts as both the organic ligand and a reductant for synthesizing Pd NPs [95]. The Zr-Fc MONs was synthesized by reacting FcDA ligand with Zr6 SBUs, and they are characteristic with a thickness of 11.6±1.2 nm and an average lateral size of 500 nm. Owing to the presence of ferrocene moieties in the ligand FcDA, Pd2+ ions were reduced into Pd NPs under mild condition (Fig. 8c). Pd NPs of 3-5 nm in diameter were clearly observed in the composites, which showed a lattice spacing of about 0.23 nm, assignable to the Pd (111) planes. Zhao and co-workers prepared Au/Cu-TCPP composites by in situ growth of Au NPs on Cu-TCPP MONs [96]. In detail, Cu-MONs with average thickness of 3.4 nm were obtained through sonication exfoliation of Cu-TCPP membranes. The Au NPs with an average size of 9.0±1.6 nm were synthesized by reducing HAuCl4 with sodium citrate on as-prepared Cu-TCPP MONs, and the lattice spacing of 0.23 nm of Au NPs corresponded to the (111) plane.

|
Download:
|
| Fig. 8. (a) TEM image of MON@Pt hybrid nanosheet. (b) HRTEM image of MON@Pt hybrid nanosheets. Reproduced with permission [92]. Copyright 2019, American Chemical Society. (c) Schematic of the preparation of Pd/Zr-Fc MONs. Reproduced with permission [95]. Copyright 2019, Royal Society of Chemistry. | |
Different from above, the MONs could grow around other nanomaterials to form the composites. For example, Yaghi, Yang and co-workers grew Al2(OH)2TCPP MONs on the surface of Ag nanocrystals (NCs) [102]. Octahedral Ag NCs in ethanol solution were drop-casted on a silicon substrate, and the deposition of Al2O3 films on the Ag NCs could be used as the Al source for synthesis of MONs. Typically, the Al2O3 films are deposited at 60 ℃ using trimethylaluminum as precursor with the deposition rate of 0.1 nm/cycle, and the total thickness is controlled to be less than 3 nm. The alumina coated on Ag NCs is converted into MONs by reacting with the TCPP linker at 140 ℃. The average thickness of the MON enclosures is ~50, 25 and 10 nm in accordance with the alumina layer thicknesses of 3 (30 cycle), 0.5 (5 cycle) and 0.1 nm (1 cycle), respectively. The orientation of Al2(OH)2TCPP MONs at the interface confirmed that the TCPP ligands of the MONs are perpendicular to the Ag surface. In addition, Zhang et al. synthesized MONs based composites by growing Ni-BDC MONs directly with the well-dispersed noble metal nanocrystals (NMNCs) [101]. A series of NMNCs including Ir, Ru and Pt were prepared with ethylene glycol (EG) as reductant and PVP as stabilizer. Then, the obtained NMNCs were quickly mixed with the precursors nickel acetate and terephthalic acid in DMF solvent. After vigorous stirring for only 1 h, the NMNCs/Ni-BDC MONs were obtained with the thickness of ~10 nm, in which Ir, Ru and Pt nanocrystals with size of 1.74, 1.84 and 2.46 nm are observed, respectively. Moreover, abundant M-O-Ni bridging bonds were spontaneously formed on the surface of Ni-BDC MONs. The NMNCs/MON hybrids were generated by the attachment of (111) plane of the noble metals onto the (100) plane of the nanosheets.
Briefly, different metal NPs could be composited with MONs to form the target products via as-prepared MONs or in situ synthesis of MONs.
3.3. Metal sulfides or metal oxides supported by MONsMetal sulfides are introduced to combine with MONs for preparing the composites [103-106]. For example, Zhang and co-workers synthesized the CuS/Cu-TCPP composites [104]. In detail, thioacetamide (TAA) as the sulfide source was mixed with Cu-TCPP MONs in the ethanol solution, and then was heated at 75 ℃ for 3 h to get the target composites. During the synthesis process, the average size of CuS NPs is increased from 7.3±3.2 nm to 13.9±5.2 nm when the sulfidation reaction time increased from 1 h to 6 h. The interplanar distance of the lattice fringes of CuS is ~0.19 nm, corresponding to the (110) plane. Furthermore, this synthesis method can be applied for synthesis of other MONs based metal sulfide, such as CdS/Cd-TCPP and CoSX/Co-TCPP. In addition, Miao et al. prepared a 2D Co-TCPP and MoS2 composites [106]. The Co-TCPP MONs and MoS2 nanosheets was synthesized previously by solvothermal method, and then mixed in the ethanol solution. After ultrasonic treatment and stirring for 12 h, the MoS2/Co-TCPP composite was obtained through the weak interactions between MoS2 and Co-TCPP MONs.
In addition, metal oxides are used to combine with MONs for preparing the composites [107-109]. For example, Quan et al. synthesized the composites of Cu(BTC)-1 MONs, Fe3O4 and Au NPs [108]. In detail, Au NPs were first anchored onto the Cu(HBTC)-1 MONs by in situ reduction of HAuCl4 with NaBH4. Then, the coprecipitation method was applied to heat the mixture of Fe2+ and Fe3+ with NH3·H2O under a nitrogen atmosphere to obtain Fe3O4 NPs. The composite nanosheets showed a thickness of monolayer around 1.00–3.00 nm and a lateral dimension around 1.00–4.00 μm with anchored Au and Fe3O4 NPs with a mean size of 5.99±2.58 nm. XPS data further proved the presence of zero-valent Au and Fe3O4. In addition, Meenakshi et al. synthesized TCS@MOF (TCS means TiO2 and chitosan) composites via the one-pot solvothermal process [109], which were made by growing granular TiO2 on the surface of MIL-88(Fe) nanosheet. Chitosan, which has abundant dynamic functional groups such as hydroxyl (-OH) and amine (‒NH2), played an important role in tethering TiO2 NPs with MIL-88(Fe) nanosheet. The nanosheets had a size of 1 μm and single-layered length of ~0.5 μm with well-spread TiO2, in which the TiO2 NPs overlay the MIL-88(Fe) nanosheet with a d-spacing of 6.2 nm.
4. Catalytic applications of MON based nanocatalystsTo date, MOFs are widely used in heterogeneous catalysis due to their unique properties including diversity, tailorability, high porosity as well as 2D structure [110]. Compared with bulk MOFs, MONs possess a large number of highly accessible active sites and could diminish the diffusion barriers between substrates and active sites, thus facilitating the reaction process. By combination with other active components, some novel catalytic properties and more catalytic functions of MONs based composites could be achieved. As below, we will introduce the catalytic applications of MONs based nanocatalysts according to the properties of metal nodes, organic linkers, or both in the MONs, even the synergistic properties of other active components with MONs in the composites.
4.1. Metal nodes or organic ligands as active sites in MONsMetal nodes in the MONs can be used as Lewis acid sites for catalysis. Furthermore, the ultrathin nanosheets possess sufficient accessible active sites, and their nanoscale thicknesses are favorable for mass diffusion and transfer of substrates and products, thus leading to the excellent catalytic performances.
Unsaturated coordinated Cu sites in Cu-IMA MONs could serve as catalytic centers for the N-arylation of imidazole under mild condition [111]. Each Cu2+ center has a square-planar geometry of two carboxylate groups and two imidazole nitrogen atoms from 1H-imidazole-1-acetic acid (IMA) ligands, but is chemically accessible in the axial position. When used as catalyst, Cu-IMA MONs (5 mol%) showed 86% yield for the N-arylation reaction between imidazole and phenylboronic acid in methanol at room temperature for 5 h with respect to only 27% yield for CuBr under the same condition. Furthermore, when arylboronic acids with electron-donor groups were used as substrates, the higher yield over 90% was achieved.
Ultrathin Ni-BDC MONs were used as Lewis acid catalysts for the Knoevenagel condensation reaction of propane dinitrile with different aldehydes [58]. Ni-BDC MONs showed a conversion rate of 98.2% at room temperature (25 ℃) for 2 h, but as for bulk MOFs, it was only 73.8%. The excellent catalytic performance of Ni-BDC MONs should originate from the high exposure of active Ni sites and the facile mass transfer and diffusion of substrates and products. On the other hand, the catalytic activity of ultrathin Ni-BDC MONs remains 97.7% after five successive catalytic cycles, without structural and morphological destruction (Figs. 9a and b), which confirmed the stability and reusability of the catalyst. In addition, Kurup and Tom et al. synthesized [Cd(DDIH)2·H2O]n (H2DDIH = (1, 2-diphenylethane-1, 2-dione bisisonicotinylhydrazone) MONs with a hexagonal shape and an average particle size of 15 μm [112]. When used as catalyst for Knoevenagel condensation of benzaldehyde and malononitrile with 2 mol% catalyst in methanol, 96% conversion was achieved within 15 min. at room temperature. Furthermore, the catalyst could still exhibit over 90% conversion at the fifth run, suggestive of the good stability and reusability. The excellent catalytic performance was attributed to the abundant labile water coordinated Cd(II) ions as the catalytic centers. Zhang et al. reported in situ growth of Al2(OH)2(TCPP) MONs in the channels (pore size of 198±14 nm) of anodized aluminum oxide (AAO) membranes as catalytic reactors [113] (Fig. 9c). When the catalytic reactor was used for the Knoevenagel condensation reaction between benzaldehyde and malononitrile, the mixture solution flowed into the channels of the composite membrane and initiated reaction as long as the reactants contacted the active sites. Finally, about 50% yield was achieved for the continuously reaction for one hour and repeated 3 times. The excellent catalytic performance and good stability come from the intrinsic structure, which can remove the products from the reaction zone quickly, prevent the catalyst aggregation, and minimize the loss of the catalyst during the catalytic reaction and recycling process.

|
Download:
|
| Fig. 9. (a) Powder XRD patterns of fresh and used Ni-BDC MONs. (b) SEM and TEM images of ultrathin Ni-BDC MONs after catalytic reactions. Reproduced with permission [58]. Copyright 2018, Tsinghua University Press and Springer-Verlag GmbH Germany, part of Springer Nature. (c) Scheme (upper) and the corresponding SEM image (lower) of the AAO/Al-MOF composite membrane reactor (right). Reproduced with permission [113]. Copyright 2017, Wiley-VCH. | |
[Sc(L)(C2O4)0.5(H2O)2]·H2O (H2L=pyrazine-2, 5-dicarboxylic acid) MONs were synthesized by microwave heating method. They exhibited a brick-like 2D network with a size of 200 nm×600 nm, and were investigated as heterogeneous Lewis acid catalysts for the cyanosilylation of p-nitrobenzaldehyde [114]. After activating for 4 h at 330 ℃, 99% conversion was achieved for reaction between p-nitrobenzaldehyde and trimethylsilyl cyanide (1:2 molar ratio) at room temperature for 2 h. As contrast, other kinds of catalysts could achieve the complete conversion for at least 7.5 h. Besides, the Sc-MONs could be easily recovered by filtration and reused at least 4 cycles without obvious loss in activity. The excellent activity and high stability could be attributed to the increased density of Sc sites, where the small rigid ligands link the metal sites intensively with less modes of coordination.
Zhao et al. reported Zr/Hf MONs named NUS-8 composed of Zr6O4(OH)4 or Hf6O4(OH)4 clusters and 1, 3, 5-benzenetribenzoate (BTB3-) with a thickness of 10-20 nm and a lateral size up to 500-1000 nm as catalyst for thioanisole oxidation [115]. NUS-8 showed the highest PhSO2CH3 selectivity (~100%) and conversion (> 99%) in the presence of 30% H2O2 at room temperature. More importantly, the C/Zr ratio in NUS-8 nanosheets remained almost intact [0.87 to 0.83 for NUS-8(Zr)] after three catalytic runs. It is noted that coordinatively unsaturated Zr/Hf sites might serve as Lewis acid sites for the oxidation reaction of thioethers into sulfoxides and sulfones. Compared with bulk MOFs, the faster reaction kinetics can be attributed to the 2D nature of NUS-8 that facilitates the diffusion of substrates and products during the catalytic process. Sun et al. stabilized double S2- templated Ag27 clusters by 5, 10, 15, 20-tetra(4-pyridyl)porphyrin (TPyP-H2) ligands to afford a robust Ag27-MONs [116]. When used as catalyst for carbonylative cyclization of benzylprop-2-ynylamine with CO2, the 97% yield was achieved at room temperature within 6 h reaction. Moreover, when various propargylamine with different sizes were used as substrates, more than 94% yield was also obtained. This can be ascribed to the saddle-shaped cluster node and the exposed catalytic sites of Ag27-MONs. Furthermore, DFT calculation shows the easy binding of substrate to the exposed Ag atoms through π-interactions of C≡C bonds.
In addition, organic ligands with Lewis base sites in the MONs could be used for catalysis. For example, Shi et al. reported the TATMA (TATMA=4, 4, 4′′-s-triazine-1, 3, 5-triyltri-m-aminobenzoate) organic ligands with abound Lewis basic sites (1, 3, 5-triazine groups) and various coordination nodes (carboxylate groups) in the MONs for the Knoevenagel condensation of benzaldehyde and malononitrile [117]. The synthesized Nd-TATMA MONs could serve as good base catalysts, and a yield of 95% for 2-benzylidenemalononitrile was obtained at 80 ℃ for 3 h. Moreover, when benzaldehyde with withdrawing group (4-fluorobenzaldehyde and 4-nitrobenzaldehyde) was used as substrate, a higher conversion rate > 99% was achieved.
Altogether, using metal nodes or organic ligands in MONs as catalytic active sites is a simple way to obtain the excellent catalytic properties. The MONs could facilitate the diffusion of the substrates, allow them to the active sites faster and then make the products easily away from the active sits.
4.2. Cooperative catalysis of metal nodes and organic ligands in MONsThe organic ligands in the catalyst can change the spatial and electronic states of adjacent metal active centers, the synergistic catalysis of metal sites and organic ligands in the MONs can further enhance the catalytic activity for some specific reactions.
The metal site as Lewis acid and the functional group on the ligand as Lewis base can provide another schematic design for bimolecular synergistic reactions. Yin et al. reported a hybrid catalyst called PCN-222(Co)@MTTB by combining 2D MTTB MONs and 3D PCN-222(Co) MOFs via a one-step solvothermal method [118]. The PCN-222(Co) MOFs were synthesized by Co-TCPP ligands and Zr6 SBUs, while MTTB was consisted of TTB (TTB=4, 4′, 4″-s-triazine-2, 4, 6-triyl-tribenzoate) ligands and Zr6 SBUs. Due to the combination of the exposed acidic sites (Co2+, Zr4+) and basic sites (-N- groups) within the structure, the composites showed the high performance for the cycloaddition of CO2 with different epoxides, and the yields are 100% for propylene epoxide, 98% for epichlorohydrin, and 95% for allyl glycidyl ether. It is noted that the epoxide is attached to the metal site (Co2+), while the CO2 is attached to the base site (-N- groups). Both of them could cyclize into the corresponding epoxy carbonates.
Cd-PBA(5-(4-pyridin-3-yl-benzoylamino)-isophthalic) MONs, which are characteristic with rich open metal sites and Lewis basic sites [119], could be used as a catalyst for Knoevenagel condensation and cyanosilylation with trimethylsilyl cyanide. For example, aromatic benzaldehyde and malononitrile compounds with a molar ratio of 1:2 could be catalyzed in ethanol to generate the product with a yield of 91% at room temperature for 2 h. But once the Cd-PBA catalyst was not activated, which means the -NH groups of amides were blocked by solvent molecules, the yield of 54% was obtained under the similar condition. These results indicate that -NH groups of amides could promote the catalytic reaction together with metal nodes.
In a word, the synergistic effect between metal sites and organic ligands could achieve the enhanced catalytic performance for specific reactions.
4.3. Metal ions anchoring onto MONs for catalysisMonatomic metal sites can be grafted onto organic ligands of MONs for catalysis. For example, Lin et al. anchored iron centers in MONs to afford highly active and reusable single-site solid catalysts for the hydrosilylation of terminal olefins (Fig. 10a) [100]. Fe-TPY-MONs (Fe loading of 0.02%) could catalyze the hydrolyzation of styrene to afford the pure anti-Markovnikov product with complete conversion over 48 h and a turnover number (TON) of > 5000 (Figs. 10b and c), which is much better than only 30% for bulk MOFs, declaring the advantage of MONs over traditional MOFs as heterogeneous molecular catalysts. Also, the amino group of MONs could act as a modifiable site for supporting single Pd site, which showed the excellent performance for the Suzuki reaction of iodobenzene and phenylboronic acid [91]. The aryl iodides containing electron-withdrawing groups such as 4-CHO, 4-CN, 4-NO2, 4-CH2-OH proceed with the satisfactorily isolated yields (> 90%). Furthermore, as for the typical macromolecule substrate, 9-Iodophenanthrene, the excellent yield of 92.1% for the target product 9-phenylphenanthrene was also achieved by single Pd sites supported by MONs.

|
Download:
|
| Fig. 10. (a) Preparation of the Fe-TPY-MON. HRTEM and FFT images of Fe-TPY-MON (b) before and (c) after catalysis. Reproduced with permission [91]. Copyright 2016, Wiley-VCH. | |
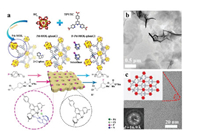
Wang and Lin et al. constructed Pd-MOL-phenCl catalyst for the fluoridation of aromatic hydrocarbons [89]. A terpyridine (TPY)-based ligand on the MONs, together with a 2-chloro-110-phenanthroline (phenCl) as a co-ligand, chelates with Pd(II) to form the active center (Fig. 11a). The Pd-MON-phenCl was subsequently reacted with 2 equiv. of Selectfluor to obtain F-Pd-MON-phenCl (Figs. 11b and c), which can be used as a solid reagent for C–H fluorination of arenes. Aromatic hydrocarbons with various functional groups including nitrile, hydroxyl, heterocycles, ketones, chlorides and ethers, were converted into the corresponding fluorination products, and the yields were 81% for 4-phenylbenzonitrile, 100% for phenol, 81% for 2-phenylpyridine, 81% for 4-phenylcyclohexanone, 7% for 4, 6-dichloro-2, 5-diphenylpyrimidine and 52% for an aromatic ether. The F-loaded solid reagent retained some activity after storage for even one month (20% yield). The presence of free radical intermediate states of the phenCl ligand is observed during the catalytic fluorination process, and can further react with Selectfluor (1 equiv.) to form (N-(FO)-phenCl·) groups, which could achieve the synergistic catalysis with Pd active center. Pd-MON-phenCl can also be used as catalyst for the fluorination of various arenes by using Selectfluor directly, and the yields are 90% for 4-phenylbenzonitrile, 76% for phenol, 82% for 2-phenylpyridine and 62% for 4-phenylcyclohexanone.
|
Download:
|
| Fig. 11. (a) Schematic showing the fluorination of a ciprofibrate using F-Pd-MON-phenCl as a solid reagent. (b) TEM image of F-Pd-MON-phenCl. (c) HRTEM and FFT images of F-Pd-MON-phenCl after catalytic reaction. Reproduced with permission [89]. Copyright 2020, Tsinghua University Press and Springer-Verlag GmbH Germany, part of Springer Nature. | |
Briefly, monatomic metal sites combining with organic ligands in MONs exhibited the satisfactory catalytic performances. It is highly desired for clearly designing the coordination environment of metal ions and further improving the long-term stability of single atomic sites.
4.4. Metal NPs supported by MONs for catalysisUsing metal NPs as the active centers is one of the most common ways to take advantage of the accessible surface of MONs. For example, Zhao and co-workers prepared Au/Cu-MONs by in situ hydrothermal growth of Au NPs for the reduction of 4-nitrophenol to 4-aminophenol [96]. The composite showed a rate constant of 0.200 min-1, which threefold higher than that of pure Au NPs (0.082 min-1). High reaction rate was achieved by shortening the diffusion process due to the decorated Au NPs on the surface of nanosheets. Kenneth et al. prepared the Pd NPs on Zr-based (Zr-Fc) MONs for hydrogenation of styrene [95]. The Pd/Zr-Fc MONs showed the excellent activity with the TOF value (7968 h-1), higher than other Pd-based heterogeneous catalysts like 271 h-1 for Pd@UiO-66. Moreover, the Pd NPs loaded onto the 4.4 nm thick Zr-Fc MOFs showed the higher TOF value than those with a thickness of 11.6 nm and 15.9 nm, further proving the importance of ample accessible active sites and low diffusion barrier for reactants.
Sub-2 nm Ru NPs distributed on the Zr-BDC MONs were reported as catalyst for the hydrogenation of levulinic acid (LA) to γ-valerolactone (GVL) [100]. The Ru/MONs showed over 99% conversion and yield of GVL with a TOF value of 349 h-1 at 90 ℃ for 1 h. As comparison, Ru/bulk Zr-BDC catalyst exhibited only 28.0% yield of GVL and a TOF value of 38.1 h-1 under the same condition. The exposed abundant active sites and avoidable geometric constraints contribute to the enhanced catalytic activity. Moreover, no obvious change in the catalytic performance as well as the structure and morphology were observed after successive six runs, which reflects the excellent stability of the catalyst.
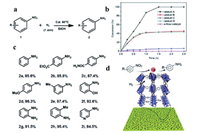
Besides, Wang et al. reported the Pd clusters with a diameter of 2.1 nm supported by hierarchical Zn/Ni-MOF-2 nanosheet-assembled hollow nanocubes (NAHNs) [120], which act as catalyst for alkoxycarbonylation reaction of aryl iodides. Using H2BDC as organic linkers, zinc and nickel ions as metal precursors, the obtained NAHNs had a side length of 400-600 nm, which were constituted by flexible nanosheets with an average thickness of sub-10 nm. The catalyst exhibited the excellent catalytic properties towards CO-based multicomponent alkoxy carbonylation reactions. Iodobenzene with both electron-donating and withdrawing groups reacted smoothly to give the corresponding products and the yields are 95.6% for R=H, 85.6% for 4-Me, 97.5% for 2-OMe, 82.8% for 4-F and 75.4% for 4-NO2. They suggested that the unique structure of Zn/Ni-MOF-2 NAHNs and gas adsorption property for CO might accelerate the reaction process catalyzed by Pd clusters. Similarly, Pd-H-MOF-5 nanosheets with a thickness of approximately 19 nm and Pd NPs with a size of sub-10 nm were applied for the reduction of nitro compounds (Fig. 12a) [121]. Compared with other catalysts including Pd-H-MOF-5 nanosheets (catalyst A), Pd-H-MOF-5 post-treated with H2O (catalyst B), Pd-H-MOF-5 nanosheets with surfactants (catalyst C) and Pd@bulk MOF-5 (catalyst D) (Fig. 12b), Pd-H-MOF-5 nanosheets showed the highest catalytic activity and reaction conversion (nearly 100%) with respect to ~40% for others catalysts under the same reaction condition. The reduction of nitrobenzene derivatives with both electron-withdrawing and -donating groups showed satisfied reaction conversion (over 85%), and besides, the reduction of 3-nitropyridine had a conversion of 84.5% (Fig. 12c). The unique structures and gas adsorption capacity of H-MOF combined with the surfactant-free immobilized Pd NPs contribute to the excellent catalytic performance (Fig. 12d).
|
Download:
|
| Fig. 12. (a) Reduction of nitroarene. Reaction conditions: 1 (0.5 mmol), Pd-H-MOF-5 MONs (0.01 equiv.), H2 (balloon) in EtOH at 60 ℃. (b) Conversion (%) as a function of time in the reduction reaction. (c) Yields of isolated products from the reduction of nitroarene catalyzed by Pd-H-MOF-5 MONs for 4 h. (d) Schematic representation of the reduction of nitroarene over Pd-H-MOF-5 MONs. Reproduced with permission [121]. Copyright 2019, Royal Society of Chemistry. | |
In a word, metal NPs supported by MONs also showed the excellent catalytic performance and stability due to the accessible catalytic center and stable skeleton structure. However, controlling the uniform size of metal NPs and avoiding particle aggregation remains a challenge under harsh reaction condition.
4.5. Molecular sieving effect of MONs for catalysisThe MONs could make the substrate more accessible to the catalytic center, but at the same time, the shape selectivity of the MOF channels for the diffusion of reactants or products was weakened. Based on MONs structure, building multi-level structures could realize shape-selective catalysis by taking full advantage of the cavities and channels in the MONs.
Based on 2D layered zeolitic imidazolate framework layers (ZIF-L), Chen et al. made a unique crosshair-star shape Pd@ZIF-L hybrid catalyst with a size of about 20 μm, in which Pd NPs with an average particle size of about 3 nm were loaded in ZIF-L unique cushion-shaped cavities between layers with a size of 6.64Å [122]. Thus, the alkenes with a kinetic diameter smaller than 6.64Å can pass through the cavities of ZIF-L, reach the Pd active sites and undergo hydrogenation reaction. As for 1-hexene, cyclohexene and cyclooctene with the kinetic diameters of 1.7, 4.2 and 5.5Å as substrates, respectively, the Pd@ZIF-L catalyst showed the conversion rates of 75.7%, 32.6% and 7.4%, which is consistent with the kinetic diameters. Moreover, when a larger molecular size tetraphenylethylene (6.7Å) was used as substrate, no activity is found over Pd@ZIF-L.
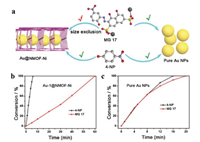
Au@Ni-MONs was reported as a new mixed-ligand MONs for the reduction of nitroaromatic compounds [97]. The nanosheets were characteristic with thickness of ~20 nm and a narrow distribution of diameter of 2-3 μm by facile surfactant-assisted method. After that, Au NPs were reduced within the pores of MONs, which could grow a little large from 0.94 nm (Au-1@Ni-MONs) to 1.58 nm with the increasing amount of gold precursors (Fig. 13a). Au-1@Ni-MONs possesses the uniform micropores with an average pore size of 1.16 nm. Compared with 4-NP (2.4×6.1 Å2), the reduction reaction of Mordant Green 17 (MG 17) with a larger molecular size (7.2×14.0 Å2) in the presence of NaBH4 aqueous solution need tenfold time (Figs. 13a and b), but slightly different when using pure Au NPs as catalyst (Fig. 13c), presumably owing to the retarded molecular diffusion through small pore apertures of Au-1@Ni-MONs.
|
Download:
|
| Fig. 13. (a) Schematic illustration of size selection effect of Au-1@Ni-MONs for 4-NP and MG 17. (b) Catalytic conversion of MG 17 and 4-NP over Au-1@Ni-MONs. (c) Catalytic conversion of MG 17 and 4-NP over pure Au NPs. Reproduced with permission [97]. Copyright 2015, Wiley-VCH. | |
Pd@Ni-MONs (0.15 mol%), in which highly dispersed ultra-fine Pd NPs (~1.0 nm) were observed within ultrathin Ni-MONs nanosheets (~8 nm), showed almost 100% conversion of styrene hydrogenation under the atmosphere pressure of H2 with a TOF value of 1230 h-1 [123]. Moreover, as the size of substrates increased, the conversion decreased significantly. For example, when β-methylstyrene (5.3×9.1 Å2) was used as substrate, the conversion dropped to 77.7%, while as for trans-stilbene (6.5×12.5 Å2), no conversion was found within 50 min. These results further confirmed that the well-defined pore structure of Pd@Ni-MONs can act as a "molecular sieve" for selective catalysis.
In a word, using the unique pore size feature of MONs based catalysts bring the shape selection character in the catalytic process.
4.6. Tandem catalysis over MONs based catalystsThrough the design of multiple reaction sites in MONs, tandem reactions can be further realized. Based on the rich modularity of the ligands and metal nodes of MONs, we could introduce active sites with different functions and even load metal NPs on MONs to achieve the continuous transformation of the substrates, thus greatly increasing the overall efficiency of the multistep reactions.
Recently, Jonathan and co-workers demonstrated the synthesis of ultrathin Cu-MONs based tandem catalyst through the covalent post-synthetic functionalization [124]. The post-synthetic Cu-MONs were obtained after treated in a suspension of 1, 3-propanesultone, in which sulfonate groups as a larger negative surface charge facilitate the ultrasonic exfoliation process to obtain Cu-MONs with 1.4 nm in thickness. When used as catalyst, the obtained MONs showed totally 82% conversion rate for acid hydrolysis of benzaldehyde dimethyl acetal to benzaldehyde, followed by a Knoevenagel condensation with malononitrile to form benzylidene malononitrile (Fig. 14a). However, bulk MOFs displayed only 3% conversion under the same reaction condition. To explain the possible reason, they pointed out that sulfonic acid groups provided the basic amine site for the first hydrolysis reaction and the remaining basic amine sites for catalyzing the subsequent Knoevenagel condensation reaction.

|
Download:
|
| Fig. 14. (a) Scheme for the acid hydrolysis of benzaldehyde dimethyl acetal (1) to benzaldehyde (2), followed by base-catalysed Knoevenagel condensation of benzaldehyde with malononitrile (3) to form nitrostyrene. Reproduced with permission [124]. Copyright 2019, Royal Society of Chemistry. (b) The direct C-H hydroxyalkynylation tandem reaction catalyzed by Cu-MOF membrane 1a in H2O. Reproduced with permission [125]. Copyright 2019, Royal Society of Chemistry. | |
Cu-MONs, which were synthesized from H2BBDC and CuCN with PVA (poly(vinyl alcohol)) by solvothermal method, were supported by Cu foam substrate for catalyzing three-component tandem hydroxyalkynylation reaction [125]. The Cu-MONs were well anchored on the Cu foam substrate and distributed homogeneously with a thickness of about 600 nm. Typically, 10 mol% of Cu-MONs as catalysts reacted in H2O/acetone/CH3CN solution with (NH4)2S2O8 at 110 ℃ for 4 h, and generated the desired hydroxyalkynylated naphthoquinone (R = H) with 91% yield (Fig. 14b). When used as catalyst, it displayed the excellent activity and regioselectivity for the tandem three-component C–H hydroxyalkynylation reactions, generating hydroxyalkynylated naphthoquinone as the single products with yields ranging from 86% to 91%. The high catalytic activity was ascribed to the well-defined active centers in the Cu-MONs. Also, the open rectangle channel with size of 12.94×10.34 Å2 could facilitate the transport of substrates and products.
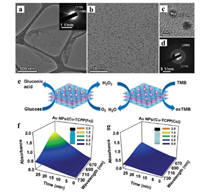
Zhang and co-workers prepared Au NPs on metalloporphyrin MONs as biomimetic catalysts for cascade reactions (Fig. 15a) [98], in which the Cu-TCPP (M=Fe, Co) acts as the peroxidase mimics for the decomposition of hydrogen peroxide, and Au NPs serve as artificial glucose oxidase. Cu-TCPP(Fe) MONs are characteristic with a thickness of 4.1±1.3 nm and Au NPs with a size of 2.1±0.5 nm (Figs. 15b-d). Au NPs/Cu-TCPP(Fe) hybrid nanosheets can catalyze glucose to produce gluconic acid and H2O2 in the presence of O2. The in situ generated H2O2 can be used to oxidize TMB (3, 3′, 5, 5′-tetramethylbenzidine) to produce the oxTMB (Fig. 15e), which showed the maximum absorption peak at 652 nm. Time-dependent absorption spectra of the reaction solution showed the increase of absorption at 652 nm as the time goes, which indicated the production of oxTMB (Figs. 15f and g). As a result, the absorption intensity displayed a good linear relationship with the glucose concentration from 10×10-6 mol/L to 300×10-6 mol/L, giving a detection limit of 8.5×10-6 mol/L for glucose when using Au NPs/Cu-TCPP hybrid nanosheets as a detector.
|
Download:
|
| Fig. 15. (a, b) TEM images of Au NPs/Cu-TCPP(Fe) hybrid nanosheet. Inset in (a): The corresponding SAED pattern of Cu-TCPP(Fe) MON. (c) HRTEM image and (d) SAED pattern of Au NPs. (e) Schematic illustration of the enzyme-mimetic cascade reaction catalyzed by Au NPs/Cu-TCPP(M) hybrid nanosheets (M=Fe, Co). (f, g) Time-dependent absorption spectra of the solution obtained after the reaction of TMB in the presence of glucose and Au NPs/Cu-TCPP(M) hybrid nanosheets incubated for different time. Inset: The corresponding photographs of solutions after reactions for 30 min. Reproduced with permission [98]. Copyright 2017, Wiley-VCH. | |
Altogether, MONs itself could be used as efficient catalyst and they can further combine with other active sites for catalysis, such as single metal site or inorganic nanoparticles. Besides, the active metal center together with functionalized ligand groups and pore structure of MONs could achieve the synergistic catalysis, shape-selective catalysis and tandem reactions, etc.
5. Conclusion and perspectivesWe have summarized the recent progresses on synthesis methods of diverse MONs as well as a variety of MONs based composites for heterogeneous thermal catalysis. It is noted that MONs as the emerging materials are still in its fancy, and there are enough spaces to develop new kinds of MONs based catalysts.
To date, two main strategies including top-down and bottom-up synthesis have been introduced for synthesis of various MONs. As for top-down strategy, some typical methods including physical exfoliation, sonication exfoliation, solvent-assisted exfoliation, freeze-thaw exfoliation and intercalation exfoliation have been summarized. Using the exfoliation method is more suitable for synthesis of MONs such as M-bim and M-BDC MOFs. Moreover, the obtained MONs are characteristic with smaller size and thinner thickness [24]. However, it is difficult to gain uniform peeling thickness, and the yield of MONs is generally very low. As for bottom-up strategy, various methods including interfacial synthesis, surfactant-assisted synthesis, modulated synthesis, and sonication synthesis have been successfully developed to synthesize MONs. Bottom-up strategy is very convenient to synthesize MONs with narrow distribution, and they are generally used to synthesize M-THT, M-BDC, M-TCPP and M-BTB MONs. However, it is still challengeable to control the MONs with specific layers and avoid aggregation between nanosheets during synthesis. To effectively address above problems, one possible way is to integrate the advantages of suitable methods together for precise control of the thickness and size of MONs. For example, by using the intercalation and chemical exfoliation approach could realize a yield of MONs about 57%, in which 4, 4′-dipyridyl disulfides were intercalated into MOFs layers, and then rapidly cleavage of intercalated disulfide was performed to obtain layered MOF crystals [39]. The other way is to develop the novel and effective synthesis methods for synthesis of MONs with specific thickness and large size. Further, during the synthesis process, selecting the suitable solvents and surfactants could not only contribute to tuning the size and thickness of MONs, but also help to reduce the high surface energy of MONs and improve the stability to some extent. Post-processing for the synthesis of MONs also make sense, such as the ligands exchange and surface modification on the surface of MONs [126].
Diverse metal nodes and easy-tailorable organic linkers could provide more opportunities for combining MONs with other active ingredients such as single metal sites, metal NPs, metal sulfides and metal oxides to construct composites. However, it still needs to introduce new components onto MONs and extend the kinds of composites for heterogeneous catalysis. Moreover, active sites on MONs are often adsorbed weakly, and lack of specific interfacial interaction between them, thus leading to the low stability. It is of great importance to introduce the diverse anchoring sites for different kinds of components, and further design novel hierarchical composite structures for specific catalytic applications.
To date, owing to diverse metal sites and organic ligands of MONs, the MONs could serve as active centers for different catalytic reactions such as Lewis acid, Bronsted acid and Lewis base catalysis. Moreover, they possess the advantage for large reaction substrate and internal molecular reactions due to the exposed active sites and short pathway of mass diffusion and transfer with respect to bulk MOFs. As for the composites, they could integrate the functions of both MONs and other active sites for specific catalytic reactions, even for synergistic catalysis or tandem catalysis. However, some improvements on MONs based catalysts still need to be done in the future: (a) To further test their catalytic capability, some important but challengeable catalytic reactions need to be extended in the future, while different MONs based catalysts are required to be designed carefully. (b) To avoid deactivation, such as leaching of metal sites and active site aggregation, it is of great importance to control the coordination of atoms in monatomic catalysts and design the anchoring sites for metal particles on MONs, which might better take advantage of the organic ligands in MONs rather than simple macerating method. Furthermore, some robust MONs based catalysts need to be constructed in different reaction medium. (c) The relationships among the atomic coordination environment, electrical properties of catalytic centers, microstructure and surface morphology are still unclear. To address above, it is highly desirable for developing some in-situ/operando characterization techniques to monitor the reaction behaviors of MONs based catalysts and the product distribution during the reaction process in real time, and moreover, theoretical calculation could provide some fundamental understanding on the catalytic process.
In addition to thermal catalysis, a variety of MONs based nanocatalysts have already been applied in the fields of electrocatalysis and photocatalysis [22, 32, 127-129]. However, there still remain some challenges for further applications. As for electrocatalysis, the low conductivity of organic ligands and low stability of MONs in the hydrolysis reaction often limited the electrocatalytic performances of MONs. The design of π-conjugated MONs with redox-active organic ligands could provide an effective strategy to overcome this challenge to some extent. Moreover, making compounds with conductive materials or growing directly on conductive substrates could significantly improve the conductivity of MONs for electrocatalysis application. As for photocatalysis [12, 16, 87, 88, 94, 128-130], the ultrathin structure of MONs results in the great dispersibility in solvents and less light scattering. Meanwhile, the proximity of photosensitizers and catalytic centers in MONs provide the accessible transfer progress as the photocatalysis excited state has a limited lifetime [128]. However, the electron/hole injection may be accompanied with side reactions, and the unwanted e--h+ recombination would waste the absorbed energy [130]. To address above, adding co-catalyst such as metallic NPs, metal oxides, and sensitizers such as dyes or complexes may improve the charge flow and prevent the unwanted e--h+ recombination.
In conclusion, the precise synthesis of MONs based catalysts is still in its fancy, and it is highly required to construct the catalysts with well-defined structures for achieving superior performances and revealing the relationships among components, structure and catalytic performance in the future. We hope that this review will further stimulate this exciting area of MONs and look forward to contributing to the rapid development of heterogeneous catalysis.
Declaration of competing interestThe authors declare no competing financial interests or associative interest in connection with the work submitted.
AcknowledgmentsThe authors acknowledge financial support from the Strategic Priority Research Program of Chinese Academy of Sciences (No. XDB36000000), National Key Basic Research Program of China (No. 2016YFA0200700, Z. Tang), National Natural Science Foundation of China (Nos. 92056204, 21890381 and 21721002, Z. Tang; Nos. 21722102 and 51672053, G. Li), Beijing Natural Science Foundation (No. 2182087, G. Li), Frontier Science Key Project of Chinese Academy of Sciences (No. QYZDJ-SSW-SLH038, Z. Tang), K. C. Wong Education Foundation (Z. Tang) and Youth Innovation Promotion Association CAS (No. 2016036, G. Li).
| [1] |
K.S. Novoselov, A.K. Geim, S.V. Morozov, et al., Science 306 (2004) 666-669. DOI:10.1126/science.1102896 |
| [2] |
C. Tan, X. Cao, X.J. Wu, et al., Chem. Rev. 117 (2017) 6225-6331. DOI:10.1021/acs.chemrev.6b00558 |
| [3] |
Y. Yang, M. Wu, X. Zhu, et al., Chin. Chem. Lett. 30 (2019) 2065-2088. DOI:10.1016/j.cclet.2019.11.001 |
| [4] |
M. Chhowalla, H.S. Shin, G. Eda, et al., Nat. Chem. 5 (2013) 263-275. DOI:10.1038/nchem.1589 |
| [5] |
Q. He, Z. Wang, L. Meng, et al., Chem. Res. Chin. Univ. 2 (2021) 523-538. |
| [6] |
H. Liu, A.T. Neal, Z. Zhu, et al., ACS Nano 8 (2014) 4033-4041. DOI:10.1021/nn501226z |
| [7] |
A. Molle, J. Goldberger, M. Houssa, et al., Nat. Mater. 16 (2017) 163-169. DOI:10.1038/nmat4802 |
| [8] |
P. Vogt, P. De Padova, C. Quaresima, et al., Phys. Rev. Lett. 108 (2012) 155501. DOI:10.1103/PhysRevLett.108.155501 |
| [9] |
A.J. Mannix, X.F. Zhou, B. Kiraly, et al., Science 350 (2015) 1513-1516. DOI:10.1126/science.aad1080 |
| [10] |
W.J. Ong, L.L. Tan, Y.H. Ng, et al., Chem. Rev. 116 (2016) 7159-7329. DOI:10.1021/acs.chemrev.6b00075 |
| [11] |
T. Li, X.J. Hao, S. Bai, Y.F. Zhao, Y.F. Song, Acta Phys. Chim. Sin. 36 (2020) 1912005. |
| [12] |
H. Yin, Z. Tang, Chem. Soc. Rev. 45 (2016) 4873-4891. DOI:10.1039/C6CS00343E |
| [13] |
A. Pakdel, Y. Bando, D. Golberg, Chem. Soc. Rev. 43 (2014) 934-959. DOI:10.1039/C3CS60260E |
| [14] |
W. Shi, L. Cao, H. Zhang, et al., Angew. Chem. Int. Ed. 56 (2017) 9704-9709. DOI:10.1002/anie.201703675 |
| [15] |
D. Sheberla, L. Sun, M.A. Blood-Forsythe, et al., J. Am. Chem. Soc. 136 (2014) 8859-8862. DOI:10.1021/ja502765n |
| [16] |
T. Wen, D.X. Zhang, J. Zhang, Inorg. Chem. 52 (2013) 12-14. DOI:10.1021/ic302273h |
| [17] |
R. Sakamoto, K. Takada, X. Sun, et al., Coord. Chem. Rev. 320 (2016) 118-128. |
| [18] |
J. Zhou, Q. Sun, J. Am. Chem. Soc. 133 (2011) 15113-15119. DOI:10.1021/ja204990j |
| [19] |
S. Yang, W. Niu, A.L. Wang, et al., Angew. Chem. Int. Ed. 56 (2017) 4252-4255. DOI:10.1002/anie.201701134 |
| [20] |
M. Zhao, Y. Huang, Y. Peng, et al., Chem. Soc. Rev. 47 (2018) 6267-6295. DOI:10.1039/C8CS00268A |
| [21] |
Q. Wang, D. Astruc, Chem. Rev. 120 (2020) 1438-1511. DOI:10.1021/acs.chemrev.9b00223 |
| [22] |
J. Lei, M. Zeng, L. Fu, Chem. Res. Chin. Univ. 36 (2020) 504-510. DOI:10.1007/s40242-020-0190-3 |
| [23] |
G. Chakraborty, I.H. Park, R. Medishetty, et al., Chem. Rev. 121 (2021) 3751-3891. DOI:10.1021/acs.chemrev.0c01049 |
| [24] |
D.J. Ashworth, J.A. Foster, J. Mater. Chem. A 6 (2018) 16292-16307. DOI:10.1039/C8TA03159B |
| [25] |
J. Liu, H. Yu, L. Wang, et al., Inorg. Chim. Acta 483 (2018) 550-564. DOI:10.1016/j.ica.2018.09.011 |
| [26] |
M. Ko, L. Mendecki, K.A. Mirica, Chem. Commun. 54 (2018) 7873-7891. DOI:10.1039/C8CC02871K |
| [27] |
D. Zhu, M. Qiao, J. Liu, et al., J. Mater. Chem. A 8 (2020) 8143-8170. DOI:10.1039/D0TA03138K |
| [28] |
W.M. Liao, J.H. Zhang, S.Y. Yin, et al., Nat. Commun. 9 (2018) 1179. DOI:10.1038/s41467-018-03205-z |
| [29] |
Y. Peng, Y.S. Li, Y.J. Ban, et al., Science 346 (2014) 1356-1359. DOI:10.1126/science.1254227 |
| [30] |
T. Araki, A. Kondo, K. Maeda, Chem. Commun. 49 (2013) 552-554. DOI:10.1039/C2CC36703C |
| [31] |
P. Amo-Ochoa, L. Welte, R. Gonzalez-Prieto, et al., Chem. Commun. 46 (2010) 3262-3264. DOI:10.1039/b919647a |
| [32] |
J. Wang, N. Li, Y. Xu, et al., Chem. Eur. J. 26 (2020) 6402-6422. DOI:10.1002/chem.202000294 |
| [33] |
A. Abherve, S. Manas-Valero, M. Clemente-Leon, et al., Chem. Sci. 6 (2015) 4665-4673. DOI:10.1039/C5SC00957J |
| [34] |
J.C. Tan, P.J. Saines, E.G. Bithell, et al., ACS Nano 6 (2012) 615-621. DOI:10.1021/nn204054k |
| [35] |
M. Jian, R. Qiu, Y. Xia, et al., Sci. Adv. 6 (2020) eaay3998. DOI:10.1126/sciadv.aay3998 |
| [36] |
J.A. Foster, S. Henke, A. Schneemann, et al., Chem. Commun. 52 (2016) 10474-10477. DOI:10.1039/C6CC05154E |
| [37] |
X. Wang, C. Chi, K. Zhang, et al., Nat. Commun. 8 (2017) 14460. DOI:10.1038/ncomms14460 |
| [38] |
H.S. Wang, J. Li, J.Y. Li, et al., NPG Asia Mater. 9 (2017) e354. DOI:10.1038/am.2017.7 |
| [39] |
Y. Ding, Y.P. Chen, X. Zhang, et al., J. Am. Chem. Soc. 139 (2017) 9136-9139. DOI:10.1021/jacs.7b04829 |
| [40] |
X. Huang, P. Sheng, Z. Tu, et al., Nat. Commun. 6 (2015) 7408. DOI:10.1038/ncomms8408 |
| [41] |
F.L. Li, P. Wang, X. Huang, et al., Angew. Chem. Int. Ed. 131 (2019) 7125-7130. DOI:10.1002/ange.201902588 |
| [42] |
A.J. Clough, J.M. Skelton, C.A. Downes, et al., J. Am. Chem. Soc. 139 (2017) 10863-10867. DOI:10.1021/jacs.7b05742 |
| [43] |
A.J. Clough, J.W. Yoo, M.H. Mecklenburg, et al., J. Am. Chem. Soc. 137 (2015) 118-121. DOI:10.1021/ja5116937 |
| [44] |
R. Dong, P. Han, H. Arora, et al., Nat. Mater. 17 (2018) 1027-1032. DOI:10.1038/s41563-018-0189-z |
| [45] |
K. Hoshiko, T. Kambe, R. Sakamoto, et al., Chem. Lett. 43 (2014) 252-253. DOI:10.1246/cl.130882 |
| [46] |
T. Zhang, B. Zheng, L. Li, et al., Appl. Surf. Sci. 539 (2021) 148255. DOI:10.1016/j.apsusc.2020.148255 |
| [47] |
R. Dong, M. Pfeffermann, H. Liang, et al., Angew. Chem. Int. Ed. 54 (2015) 12058-12063. DOI:10.1002/anie.201506048 |
| [48] |
S. Sakaida, K. Otsubo, O. Sakata, et al., Nat. Chem. 8 (2016) 377-383. DOI:10.1038/nchem.2469 |
| [49] |
L. Huang, G. Gao, H. Zhang, et al., Nano Energy 68 (2020) 104296. DOI:10.1016/j.nanoen.2019.104296 |
| [50] |
H. Jiang, P. Zhang, X. Wang, Y. Gong, Nano Res. 14 (2021) 1789-1801. DOI:10.1007/s12274-020-3020-5 |
| [51] |
L.Y. Song, B. Yang, F.F. Liu, et al., J. Phys. Chem. C 124 (2020) 12390-12396. DOI:10.1021/acs.jpcc.0c00931 |
| [52] |
Z. Gao, C.H. Hsu, J. Liu, et al., Nanoscale 11 (2019) 878-881. DOI:10.1039/C8NR08477G |
| [53] |
S. Stepanow, N. Lin, D. Payer, et al., Angew. Chem. Int. Ed. 46 (2007) 710-713. DOI:10.1002/anie.200603644 |
| [54] |
H. Spillmann, A. Dmitriev, N. Lin, et al., J. Am. Chem. Soc. 125 (2003) 10725-10728. DOI:10.1021/ja0362353 |
| [55] |
Q. Zuo, T. Liu, C. Chen, et al., Angew. Chem. Int. Ed. 58 (2019) 10198-10203. DOI:10.1002/anie.201904058 |
| [56] |
Y. Wang, M. Zhao, J. Ping, et al., Adv. Mater. 28 (2016) 4149-4155. DOI:10.1002/adma.201600108 |
| [57] |
L. Hang, T. Zhang, H. Wen, et al., Nano Res. 14 (2021) 660-666. DOI:10.1007/s12274-020-3093-1 |
| [58] |
X.F. Zhang, L. Chang, Z.J. Yang, et al., Nano Res. 12 (2019) 437-440. DOI:10.1007/s12274-018-2235-1 |
| [59] |
Z.Y. Wang, G. Wang, H.Y. Qi, et al., Chem. Sci. 11 (2020) 7665-7671. DOI:10.1039/D0SC01408G |
| [60] |
H. Fan, H. Yu, X. Wu, et al., ACS Appl. Mater. Interfaces 8 (2016) 25261-25267. DOI:10.1021/acsami.6b07300 |
| [61] |
X. Zhang, P. Zhang, C. Chen, et al., Green Chem. 21 (2019) 54-58. DOI:10.1039/C8GC02835D |
| [62] |
H.N. Abdelhamid, Nanotechnology 30 (2019) 435601. DOI:10.1088/1361-6528/ab30f6 |
| [63] |
A. Pustovarenko, M.G. Goesten, S. Sachdeva, et al., Adv. Mater. 30 (2018) 1707234. DOI:10.1002/adma.201707234 |
| [64] |
Z.R. Tao, J.X. Wu, Y.J. Zhao, et al., Nat. Commun. 10 (2019) 2911. DOI:10.1038/s41467-019-10971-x |
| [65] |
Y. Sakata, S. Furukawa, M. Kondo, et al., Science 339 (2013) 193-196. DOI:10.1126/science.1231451 |
| [66] |
Y. Zhao, J. Wang, R. Pei, J. Am. Chem. Soc. 142 (2020) 10331-10336. DOI:10.1021/jacs.0c04442 |
| [67] |
Y. Lin, H. Wan, D. Wu, et al., J. Am. Chem. Soc. 142 (2020) 7317-7321. DOI:10.1021/jacs.0c01916 |
| [68] |
N. Stock, S. Biswas, Chem. Rev. 112 (2012) 933-969. DOI:10.1021/cr200304e |
| [69] |
S. Zhao, Y. Wang, J. Dong, et al., Nat. Energy 1 (2016) 1-10. DOI:10.1038/NENERGY.2016.184 |
| [70] |
G.T. Hai, X.L. Jia, K.Y. Zhang, et al., Nano Energy 44 (2018) 345-352. DOI:10.1016/j.nanoen.2017.11.071 |
| [71] |
Z.Q. Li, L.G. Qiu, W. Wang, et al., Inorg. Chem. Commun. 11 (2008) 1375-1377. DOI:10.1016/j.inoche.2008.09.010 |
| [72] |
Y. Ning, X. Lou, C. Li, et al., Chem. Eur. J. 23 (2017) 15984-15990. DOI:10.1002/chem.201703077 |
| [73] |
J. Guo, Y. Zhang, Y. Zhu, et al., Angew. Chem. Int. Ed. 57 (2018) 6873-6877. DOI:10.1002/anie.201803125 |
| [74] |
N.K. Shrestha, S.A. Patil, S. Cho, et al., J. Mater. Chem. A 8 (2020) 24408-24418. DOI:10.1039/D0TA07716J |
| [75] |
S.C. Junggeburth, L. Diehl, S. Werner, et al., J. Am. Chem. Soc. 135 (2013) 6157-6164. DOI:10.1021/ja312567v |
| [76] |
A.K. Chaudhari, H.J. Kim, I. Han, et al., Adv. Mater. 29 (2017) 1701463. DOI:10.1002/adma.201701463 |
| [77] |
Y. Peng, R. Yao, W. Yang, Chem. Commun. 55 (2019) 3935-3938. DOI:10.1039/C9CC00349E |
| [78] |
Y. Peng, Y. Li, Y. Ban, et al., Angew. Chem. Int. Ed. 56 (2017) 9757-9761. DOI:10.1002/anie.201703959 |
| [79] |
F. Xiang, E.J. Popczun, D.P. Hopkinson, Nanotechnology 30 (2019) 345602. DOI:10.1088/1361-6528/ab19f4 |
| [80] |
W. Zhu, Y. Yang, Q. Jin, et al., Nano Res. 12 (2019) 1307-1312. DOI:10.1007/s12274-018-2242-2 |
| [81] |
Y. Fu, H. Zhou, S. Yin, et al., Compos. Part A Appl. Sci. Manuf. 134 (2020) 105910. DOI:10.1016/j.compositesa.2020.105910 |
| [82] |
F. Ran, X. Xu, D. Pan, et al., Nanomicro Lett. 12 (2020) 46. |
| [83] |
Q. Xia, H. Liu, M. Jin, et al., Nanoscale 12 (2020) 8969-8974. DOI:10.1039/D0NR00992J |
| [84] |
J. Cheng, J. Liang, L. Dong, et al., RSC Adv. 8 (2018) 40813-40822. DOI:10.1039/C8RA08410F |
| [85] |
A. Wang, J. Li, T. Zhang, Nat. Rev. Chem. 2 (2018) 65-81. DOI:10.1038/s41570-018-0010-1 |
| [86] |
Z. Yan, B. Ma, S. Li, et al., Sci. Bull. 64 (2019) 976-985. DOI:10.1016/j.scib.2019.05.014 |
| [87] |
G. Lan, K. Ni, E. You, et al., J. Am. Chem. Soc. 141 (2019) 18964-18969. DOI:10.1021/jacs.9b11024 |
| [88] |
G. Lan, Y. Quan, M. Wang, et al., J. Am. Chem. Soc. 141 (2019) 15767-15772. DOI:10.1021/jacs.9b08956 |
| [89] |
W. Shi, L. Zeng, L. Cao, et al., Nano Res. 14 (2021) 473-478. DOI:10.1007/s12274-020-2698-8 |
| [90] |
J. Tang, M. Cai, G. Xie, et al., Chem. Eur. J. 26 (2020) 4333-4340. DOI:10.1002/chem.201905249 |
| [91] |
L. Cao, Z. Lin, F. Peng, et al., Angew. Chem. Int. Ed. 55 (2016) 4962-4966. DOI:10.1002/anie.201512054 |
| [92] |
K. Rui, G. Zhao, M. Lao, et al., Nano Lett. 19 (2019) 8447-8453. DOI:10.1021/acs.nanolett.9b02729 |
| [93] |
Z. Xia, J. Fang, X. Zhang, et al., Appl. Catal. B 245 (2019) 389-398. DOI:10.1016/j.apcatb.2018.12.073 |
| [94] |
Z. Gao, Y. Li, Y. Zhang, et al., ACS Appl. Mater. Interfaces 12 (2020) 1963-1972. DOI:10.1021/acsami.9b14958 |
| [95] |
Z. Deng, H. Yu, L. Wang, et al., J. Mater. Chem. A 7 (2019) 15975-15980. DOI:10.1039/C9TA03403J |
| [96] |
S. Wu, L. Qin, K. Zhang, et al., RSC Adv. 9 (2019) 9386-9391. DOI:10.1039/C9RA00662A |
| [97] |
R. Yan, Y. Zhao, H. Yang, et al., Adv. Funct. Mater. 28 (2018) 1802021. DOI:10.1002/adfm.201802021 |
| [98] |
Y. Huang, M. Zhao, S. Han, et al., Adv. Mater. 29 (2017) 1700102. DOI:10.1002/adma.201700102 |
| [99] |
G. Zhan, H.C. Zeng, Adv. Funct. Mater. 26 (2016) 3268-3281. DOI:10.1002/adfm.201505380 |
| [100] |
Y. Lin, M. Zhang, L. Zhao, et al., Appl. Surf. Sci. 536 (2021) 147952. DOI:10.1016/j.apsusc.2020.147952 |
| [101] |
Q. Jiang, J. Xu, Z. Li, et al., Adv. Mater. Interfaces 8 (2021) 2002034. DOI:10.1002/admi.202002034 |
| [102] |
Y. Zhao, N. Kornienko, Z. Liu, et al., J. Am. Chem. Soc. 137 (2015) 2199-2202. DOI:10.1021/ja512951e |
| [103] |
J.D. Yi, T.T. Liu, Y.B. Huang, R. Cao, Sci. China Mater. 62 (2019) 965-972. DOI:10.1007/s40843-018-9393-x |
| [104] |
Q. Lu, M. Zhao, J. Chen, et al., Small 12 (2016) 4669-4674. DOI:10.1002/smll.201600976 |
| [105] |
S. Jin, H.J. Son, O.K. Farha, et al., J. Am. Chem. Soc. 135 (2013) 955-958. DOI:10.1021/ja3097114 |
| [106] |
M. Zhu, Q. Ma, S.Y. Ding, et al., Mater. Lett. 239 (2019) 155-158. DOI:10.1016/j.matlet.2018.12.108 |
| [107] |
J. Yi, R. Xie, Z. Xie, et al., Angew. Chem. Int. Ed. 59 (2020) 23641-23648. DOI:10.1002/anie.202010601 |
| [108] |
B. Tan, H. Zhao, W. Wu, et al., Nanoscale 9 (2017) 18699-18710. DOI:10.1039/C7NR05541B |
| [109] |
S. Vigneshwaran, P. Sirajudheen, P. Karthikeyan, et al., J. Hazard. Mater. 406 (2021) 124728. DOI:10.1016/j.jhazmat.2020.124728 |
| [110] |
L. Jiao, Y. Wang, H.L. Jiang, Q. Xu, Adv. Mater. 30 (2018) 23. |
| [111] |
Z.H. Li, L.P. Xue, L. Wang, et al., Inorg. Chem. Commun. 27 (2013) 119-121. DOI:10.1016/j.inoche.2012.10.035 |
| [112] |
L. Tom, M.R.P. Kurup, J. Solid State Chem. 294 (2021) 121846. DOI:10.1016/j.jssc.2020.121846 |
| [113] |
Y. Yu, X.J. Wu, M. Zhao, et al., Angew. Chem. Int. Ed. 56 (2017) 578-581. DOI:10.1002/anie.201610291 |
| [114] |
Z. Zhu, Y. Tao, Y. Jiang, et al., CrystEngComm 21 (2019) 5261-5268. DOI:10.1039/C9CE00969H |
| [115] |
Z. Hu, E.M. Mahdi, Y. Peng, et al., J. Mater. Chem. A 5 (2017) 8954-8963. DOI:10.1039/C7TA00413C |
| [116] |
M. Zhao, S. Huang, Q. Fu, et al., Angew. Chem. Int. Ed. 59 (2020) 20031-20036. DOI:10.1002/anie.202007122 |
| [117] |
F. Guo, B. Yuan, W. Shi, Inorg. Chem. Commun. 86 (2017) 285-289. DOI:10.1016/j.inoche.2017.11.007 |
| [118] |
E. Liu, J. Zhu, W. Yang, et al., ACS Appl. Nano Mater. 3 (2020) 3578-3584. DOI:10.1021/acsanm.0c00305 |
| [119] |
L. Hu, G.X. Hao, D. H.-Luo, et al., Cryst. Growth Des. 18 (2018) 2883-2889. DOI:10.1021/acs.cgd.7b01728 |
| [120] |
S. Xue, H. Jiang, Z. Zhong, et al., Micropor. Mesopor. Mater. 221 (2016) 220-227. DOI:10.1016/j.micromeso.2015.09.053 |
| [121] |
S. He, Y. Chen, Z. Zhang, et al., Chem. Sci. 7 (2016) 7101-7105. DOI:10.1039/C6SC02272C |
| [122] |
Z. Zhang, Y. Chen, S. He, et al., Angew. Chem. Int. Ed. 53 (2014) 12517-12521. DOI:10.1002/ange.201406484 |
| [123] |
T. Guo, K. Mo, N. Zhang, et al., Dalton Trans. 50 (2021) 1774-1779. DOI:10.1039/D0DT03877F |
| [124] |
J. Nicks, J. Zhang, J.A. Foster, Chem. Commun. 55 (2019) 8788-8791. DOI:10.1039/C9CC02061F |
| [125] |
C. Huang, K. Zhu, G. Lu, et al., CrystEngComm 22 (2020) 802-810. DOI:10.1039/C9CE01719D |
| [126] |
P. Chen, Y. Liu, X. Hu, et al., Nano Res. 13 (2020) 3151-3156. DOI:10.1007/s12274-020-2986-3 |
| [127] |
L.P. Tang, S. Yang, D. Liu, et al., J. Mater. Chem. A 8 (2020) 14356-14383. DOI:10.1039/D0TA03356A |
| [128] |
L. Cao, C. Wang, ACS Cent. Sci. 6 (2020) 2149-2158. DOI:10.1021/acscentsci.0c01150 |
| [129] |
Y. Xue, G. Zhao, R. Yang, et al., Nanoscale 13 (2021) 3911-3936. DOI:10.1039/D0NR09064F |
| [130] |
G.E. Gomez, F. Roncaroli, Inorg. Chim. Acta 513 (2020) 119926. DOI:10.1016/j.ica.2020.119926 |
 2021, Vol. 32
2021, Vol. 32 

