b Department of Physics, Dongguk University, Seoul 04620, Republic of Korea
In recent years, the electrocatalytic ozonation process has received widespread attention because of its highly efficient removal of pharmaceutical micropollutants [1-5]. In 2016, a new electrocatalytic ozonation process was developed by our research group, namely: Electrochemical heterogeneous catalytic ozonation (E-catazone); this as achieved by using a self-made titanium dioxide nanoflower coated porous Ti gas diffuser (TiO2-NF@PTGD) as the anode [6], and carbon-coated electrode as the cathode. Owing to the integration of an ozone aerator, ozone catalyst, and anode into TiO2-NF@PTGD, O3 was transferred to the surface of porous titanium under forced convection and subsequently converted into reactive oxygen species (ROS) under the action of electrochemistry and the TiO2-NF catalytic layer. Thus, the E-catazone process resulted in a hydroxyl radical production that was two orders of magnitude greater than that obtained using electrochemical oxidation and ozonation alone. Furthermore, this process exhibits excellent mass transfer and oxidation characteristics, while demonstrating great potential for the fast and effective degradation of ozone-resistant micropollutants [7, 8]. Our previous study has confirmed that the TiO2-NF catalyst layer on the porous Ti anode plays an important role in the augmented pollutant removal and enhances the generation of ROS in E-catazone [7]. Notably, the TiO2-NF not only promotes the interface reaction with the H2O surface under an electrochemical action (Eq. 1), but also improves the interface adsorption of O3 and its subsequent conversion into ROS (Eq. 2):
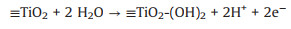
|
(1) |
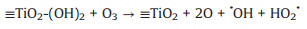
|
(2) |
The morphology, hierarchical structure, and crystal form of the catalyst play a vital role in its catalytic ability [9-11]. TiO2-NF exhibits a three-dimensional [12, 13], hierarchical, and porous structure [14] that provides sufficient interface area for gas/solid/bulk solution reactions to occur [13, 15]. The TiO2-NF coating is prepared in situ and grown on the porous titanium substrate using the alkaline hydrothermal method; subsequently it is crystallized by sintering at a temperature of 450 ℃ [6]. It is well known that the sintering temperature can significantly affect the crystal form of TiO2 [16], which in turn affects its catalytic activities. At a sintering temperature of lower than 500 ℃, TiO2 predominantly takes the form of anatase, and an increase in temperature gradually transforms the anatase into rutile [17]. In our previous studies, we have determined that TiO2-NF@PTGD prepared at a sintering temperature of 450 ℃ exists in the form of anatase and produces excellent catalytic effects in the E-catazone system [6-8]. Moreover, Song et al. [18] prepared different crystal types of nano-TiO2 by altering the sintering temperature and found that the anatase phase of TiO2 prepared at 450 ℃ displayed better catalytic activities than those of rutile TiO2 prepared at a temperature of 750 ℃, while also exhibiting a higher specific surface area. Such results can be explained by the fact that anatase TiO2 has a higher surface hydroxyl density than that of rutile TiO2, and that surface hydroxyl groups are major reaction sites for the interfacial catalysis of O3 and TiO2. In previous E-catazone studies, there has been no systematic evaluation of the preparation process parameters of TiO2 and it is not clear whether the sintering temperature of TiO2 affected the catalytic performance of TiO2-O3 and the degradation efficiency of refractory organic compounds. Furthermore, it has been noted that the effective removal of ozone-resistant drugs is essential for the control of pharmaceutical micropollutants. Therefore, in the present study, an ozone-resistant drug with a second-order reaction kinetic constant of kp-CBA, O3 < 1 L mol−1 s−1 was chosen as the target pollutant, namely, para-chlorobenzoic acid (p-CBA) [19-21]. The effects of preparing TiO2-NF@PTGDs at different sintering temperatures on the degradation of p-CBA by E-catazone were investigated by analyzing the physical characteristics of TiO2-NF and the degradation characteristics of p-CBA. The influence mechanism was also revealed by analyzing interfacial reaction kinetics, measuring the exposure of hydroxyl radicals, and simulating the O3-TiO2 interface reaction characteristics via kinetic modeling methods. The details of the experiment have been listed in the supporting information, including the preparation and characterization of TiO2-NF@PTGDs, E-catazone treatment of p-CBA, hydroxyl radical exposure, and kinetic modeling.
The TiO2-NF coatings were all uniformly grown on the porous titanium substrate. The morphologies of TiO2-NF@PTGDs prepared without sintering (TiO2-NF@PTGD-ws), and at sintering temperatures of 350 ℃ (TiO2-NF@PTGD-350), 450 ℃ (TiO2-NF@PTGD-450), and 550 ℃ (TiO2-NF@PTGD-550) were similar, with TiO2-NF comprising multiple TiO2 nanosheets arranged in an array (Figs. 1a–d and f–i). Notably, the morphology of the TiO2-NF@PTGD prepared at 750 ℃ (TiO2-NF@PTGD-750) was slightly different. That is, the TiO2 nanosheets took the form of nanorod arrays (Figs. 1e and j) because the nanosheets were broken at higher temperatures and subsequently exhibited rod shapes. Furthermore, the XRD data of TiO2-NF@PTGDs at different sintering temperatures indicated that the TiO2-NF@PTGD surface layers exhibited a crystal structure (Fig. S1 in Supporting information). The crystallite phase composition of TiO2-NF@PTGDs was analyzed using the JADE.6 software. Results highlighted that variations in the sintering temperature significantly affected the surface crystal composition of TiO2-NF@PTGDs (Table 1). For TiO2-NF@PTGD-ws, the surface was mainly composed of Ti8O15 (Table 1). In contrast, the surfaces of TiO2-NF@PTGD-350, TiO2-NF@PTGD-450, and TiO2-NF@PTGD-550 were mainly composed of anatase TiO2 and titanium matrix, and the sintering temperature affected the proportion of anatase TiO2 (Table 1). When the sintering temperature increased from 350 ℃ to 450 ℃, the mass proportion of anatase TiO2 increased from 69.1% to 81.2%; however, as temperature increased to 550 ℃, the value gradually decreased to 79.3%. Finally, as the temperature further increased to 750 ℃, the proportion of anatase TiO2 decreased to 7.2%, and the proportion of rutile TiO2 reached 70.6%; this indicates that anatase TiO2 transforms to rutile TiO2 at high temperatures. Notably, this trend is similar to that reported in other studies [22, 23].

|
Download:
|
| Fig. 1. Morphologies of nanoflower-shaped titanium oxide-coated porous Ti gas diffusers prepared at different sintering temperatures. The diffusers were prepared without sintering (a, f), and at sintering temperatures of 350 ℃ (b, g), 450 ℃ (c, h), 550 ℃ (d, i) and 750 ℃ (e, j), respectively. | |
|
|
Table 1 Physicochemical parameters and observed pseudo-first-order kinetic constant (kobs) for p-CBA degradation by E-catazone with different nanoflower-shaped titanium oxide-coated porous Ti gas diffuser (TiO2-NF@PTGD) anodes. |
The XPS spectra of the O 1 s bands in the 529–535 eV binding energy region for all the prepared TiO2-NF@PTGD samples are displayed in Fig. 2 and Fig. S2 in Supporting information. Except for that of TiO2-NF@PTGD-750, all spectra could be fitted into two O 1 s peaks, 529.4–530.3 eV [24] and 531.7–532.3 eV [25], which corresponded to the metallic oxides (i.e., lattice oxygen [OL] of the TiO2-NF) [26] and adsorbed surface oxygen groups (Oads) such as ≡OH or other hydrated species [26], respectively. These are major reaction sites for heterogeneous catalytic reactions of O3. The peak area data for both OL and Oads are listed in Table 1. Interestingly, the Oads values indicated that our self-made TiO2-NF@PTGD at the sintering temperature of 450 ℃ may comprise more surface oxygen sites during the interfacial catalysis of O3; therefore, it can react with O3 and convert it to ROS more effectively, as compared to other TiO2-NF@PTGDs.

|
Download:
|
| Fig. 2. XPS spectra of O 1 s on the surface of nanoflower-shaped titanium oxide-coated porous Ti gas diffusers (TiO2-NF@PTGDs) without sintering (TiO2-NF@PTGD-ws) (a); at 350 ℃ (TiO2-NF@PTGD-350) (b); 450 ℃ (TiO2-NF@PTGD-450) (c); 550 ℃ (TiO2-NF@PTGD-550) (d); 750 ℃ (TiO2-NF@PTGD-750) (e). | |
The sintering temperature of TiO2-NF@PTGD can remarkably influence p-CBA degradation (Figs. 3a and b). When the sintering temperature increased from room temperature to 450 ℃, p-CBA removal increased from 69.6% to 98.5% within 5 min. Notably, the p-CBA degradation curves were fitted with the pseudo-first-order kinetic characteristics and p-CBA removal rates. Further, the pseudo-first-order removal rate constant k-CBA increased by a magnitude of 2.91, from 2.82 × 10−1min−1 to 8.20 × 10−1min−1 (Table 1). However, when the sintering temperature was further increased to 750 ℃, the p-CBA removal efficiency after 5 min and k-CBA were decreased to 15.9% and 3.8 × 10-2min−1, respectively. The same trend was also observed in the hydroxyl radical exposure (∫[·OH]dt) obtained with different TiO2-NF@PTGDs. As the sintering temperature increased to 450 ℃, the ∫[·OH] dt obtained for TiO2-NF@PTGD-450 increased from 2.38 × 10-10 mol L−1 s to 8.41 × 10-10 mol L−1 s; however, ∫[·OH] dt rapidly decreased to 3.45 × 10-11 mol L−1 s for TiO2-NF@PTGD-750 when the sintering temperature further rose to 750 ℃ (Figs. 3c and d). The positive correlation between the ∫[·OH] dt and the degradation of p-CBA indicates that the sintering temperature of TiO2-NF@PTGDs influences the degradation of p-CBA by affecting the ∫[·OH]dt of E-catazone.p-CBA is a typical ozone-inert drug, therefore, it can hardly be oxidized by O3 alone but can be effectively degraded by ·OH. Therefore, the rapid degradation of p-CBA in E-catazone is closely related to the excellent interface properties of the TiO2-NF@PTGD-450 electrode and its ability to produce ·OH. Finally, the highest content of Oads for TiO2-NF@PTGD was obtained at the sintering temperature of 450 ℃, which indicates that the TiO2-NF@PTGD-450 contained the highest amount of surface oxygen groups; therefore, it functioned as a reaction site for the catalysis of molecular O3 into ROS [27, 28].

|
Download:
|
| Fig. 3. Removal of p-CBA (a), pseudo-first-order kinetic constant (kobs) of p-CBA degradation (b), evolution of hydroxyl radical exposure (∫[OH] dt) with reaction time (c), and hydroxyl radical exposure (∫[OH] dt) at 5 min (d) in E-catazone system using different nanoflower-shaped titanium oxide-coated porous Ti gas diffusers (TiO2-NF@PTGDs). Experimental conditions: p-CBA concentration of 8 mg/L; current of 150 mA; gas flow rate of 0.2 L min−1; O3 concentration of 10 mg/L; and an initial pH of 7.2. | |
As indicated in our previous study [7], the O3 interface catalytic process of TiO2-NF@PTGD in E-catazone mainly involves the reaction of TiO2-NF with water molecules under an electrochemical action to produce surface oxygen groups (Eq. 1), as well as the interface conversion between hydroxylated TiO2-NF and O3 to produce ROS (Eq. 2).
These two reactions are the important rate-limiting steps for the catalytic conversion of ozone to produce ROS and promote the rapid degradation of organic compounds. To further analyze the effects of the TiO2 sintering temperature on the reaction rate of the limiting reactions, the kinetic model of the E-catazone reaction system was established using the Kintecus software (V6.80). By fitting the degradation curves of p-CBA (Fig. S3 in Supporting information), the kinetic rate constants of Eqs. 1 (kEq.1) and 2 (kEq.2) were obtained. The sintering temperature of TiO2-NF@PTGD significantly influenced the reaction rates, kEq.1 and kEq.2 (Fig. 4). At the sintering temperature of 450 ℃, TiO2-NF@PTGD-450 exhibited kEq.1 and kEq.2 values of 5.00 × 10−1 s−1 and 4.00 × 10-3 L mol−1 s−1, respectively, which were much higher than those of TiO2-NF@PTGD-ws (2.00 × 10−1 s−1 and 2.00 × 10-3 L mol−1 s−1) and TiO2-NF@PTGD-750 (5.00 × 10-2 s−1 and 5.00 × 10-4 L mol−1 s−1) (Table S1 in Supporting information).

|
Download:
|
| Fig. 4. Kinetic rate constants of two key TiO2-O interface reactions with different nanoflower-shaped titanium oxide-coated porous Ti gas diffusers (TiO2-NF@PTGDs). | |
Interestingly, we found that the effect of the sintering temperature on the interfacial reaction rate was the same as that of Oads and hydroxyl radical exposure. The sintering temperature affects the crystal type and content of TiO2-NF and controls the content of Oads on the surface of TiO2-NF, thereby causing TiO2-NF to exhibit different surface energies [29]. In the E-catazone system, the electrochemical action can further promote the reaction of the TiO2-NF interface with water molecules to form surface-active groups such as ≡TiO2-(OH)2 [7, 30, 31]. This active group is rich in electrons and can be enhanced and induced by electrostatic forces to ensure that ozone molecules are adsorbed on the interface of TiO2. In the present study, the adsorbed ozone was further catalyzed and formed surface atomic oxygen (O) or HO2·. These ROS were then converted to ·OH through a series of homogeneous elementary reactions (Table S1c). Thus, TiO2-NF@PTGD sintered at 450 ℃ demonstrates the highest interfacial activity to react with water molecules and catalyze O3, converting it into ROS for the rapid degradation of pollutants. In addition, the above results indicate that E-catazone exhibits an excellent degradation efficiency for ozone inert drugs and can achieve rapid removal. This observation provides new technical means and ideas for the efficient removal of micropollutants of refractory drugs and for the effective management and control of pharmaceutical and personal care products (PPCPs).
In summary, this study demonstrated that the sintering temperature of TiO2-NF is the primary factor affecting the oxidation ability of the E-catazone system. The mechanism governing the influence of sintering temperature on the oxidation activity of E-catazone was revealed, and the optimum sintering temperature was defined. The results indicated that TiO2 prepared at the sintering temperature of 450 ℃ was the most efficient against the ozone-inert drug p-CBA. The underlying explanation for this result is that the TiO2-NF electrode generated the highest content of both anatase and Oads and had higher surface energy, thereby laying the foundation for the subsequent O3-TiO2 interface catalysis.
Therefore, under the electrochemical action of the TiO2 electrode, the TiO2 interface binds rapidly and produces active groups, which further promote the adsorption and catalytic transformation of the O3 interface, resulting in a large amount of ROS to achieve the rapid degradation of refractory organic compounds.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis research was supported by the Beijing Outstanding Young Scientist Project (No. C19H100010), Beijing Outstanding Talent Training Foundation, China (No. 2018000020124G056) with title 'Efficient removal and toxicity study of typical antibiotics from waste water of high-speed railway trains in Beijing', the National Natural Science Foundation of China (No. 52042201).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.072.
| [1] |
K.L. Li, Y. Zhang, L.L. Xu, et al., Appl. Catal. B 264 (2000) 118512. |
| [2] |
K.L. Li, L.L. Xu, Y. Zhang, et al., Appl. Catal. B 249 (2019) 316-321. DOI:10.1016/j.apcatb.2019.03.015 |
| [3] |
J. Li, Y. Li, Z. Xiong, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 2139-2146. DOI:10.1016/j.cclet.2019.04.057 |
| [4] |
Y.J. Wang, G. Yu, S.B. Deng, J. Huang, B. Wang, Chemosphere 208 (2018) 640-654. DOI:10.1016/j.chemosphere.2018.05.095 |
| [5] |
T. Wang, Y.Q. Song, H.J. Ding, et al., Chem. Eng. J. 394 (2020) 124852. DOI:10.1016/j.cej.2020.124852 |
| [6] |
X. Li, G. Liu, M. Shi, et al., Sep. Purif. Technol. 165 (2016) 154-159. DOI:10.1016/j.seppur.2016.03.048 |
| [7] |
X. Li, F. Ma, Y. Li, et al., Chem. Eng. J. 389 (2020) 124411. DOI:10.1016/j.cej.2020.124411 |
| [8] |
X. Li, S. Sun, X. Zhang, et al., Sep. Purif. Technol. 178 (2017) 189-192. DOI:10.1016/j.seppur.2016.12.052 |
| [9] |
J.P. Zou, Y. Chen, S.S. Liu, et al., Water Res. 150 (2019) 330-339. DOI:10.1016/j.watres.2018.11.077 |
| [10] |
F. Yu, L. Wang, Q. Xing, et al., Chin. Chem. Lett. 31 (2020) 1648-1653. DOI:10.1016/j.cclet.2019.08.020 |
| [11] |
M. Zhu, L. Zhang, S. Liu, et al., Chin. Chem. Lett. 31 (2020) 1961-1965. DOI:10.1016/j.cclet.2020.01.017 |
| [12] |
Y. Wang, M.M. Zhang, S.H. Lv, et al., ACS Omega 5 (2020) 13994-14005. DOI:10.1021/acsomega.0c01390 |
| [13] |
H.X. Li, C. Yang, X.Y. Wang, et al., J. Am. Ceram. Soc. 103 (2020) 1187-1196. DOI:10.1111/jace.16807 |
| [14] |
X. Li, G. Liu, M. Shi, et al., Electrochim. Acta 218 (2016) 318-324. DOI:10.1016/j.electacta.2016.08.098 |
| [15] |
M.S. Akhtar, A. Umar, S. Sood, et al., Materials 12 (2019) 566. DOI:10.3390/ma12040566 |
| [16] |
H. Sutrisno, E.D. Siswani, K.S. Budiasih, Sci. Sinter. 50 (2018) 291-298. DOI:10.2298/SOS1803291S |
| [17] |
A.L. Li, Z.L. Wang, H. Yin, et al., Chem. Sci. 7 (2016) 6076-6082. DOI:10.1039/C6SC01611A |
| [18] |
S. Song, Z.W. Liu, Z.Q. He, A.L. Zhang, J.M. Chen, Sci. Technol. 44 (2010) 3913-3918. DOI:10.1021/es100456n |
| [19] |
R.H. Huang, J. Liu, L.S. Li, et al., Chin. Chem. Lett. 22 (2011) 683-686. DOI:10.1016/j.cclet.2010.11.037 |
| [20] |
J.X. Yang, J. Li, W.Y. Dong, et al., Chem. Eng. J. 295 (2016) 443-450. DOI:10.1016/j.cej.2016.03.014 |
| [21] |
B.Y. Lan, R.H. Huang, L.S. Li, et al., Chem. Eng. J. 219 (2013) 346-354. DOI:10.1016/j.cej.2012.12.083 |
| [22] |
D.A.H. Hanaor, C.C. Sorrell, J. Mater, Sci. Mater. Electron. 46 (2011) 855-874. DOI:10.1007/s10853-010-5113-0 |
| [23] |
G. Chen, J. Chen, Z.K. Song, C. Srinivasakannan, J.H. Peng, J. Alloys. Compd. 585 (2014) 75-77. DOI:10.1016/j.jallcom.2013.09.056 |
| [24] |
A.R. Babar, S.S. Shinde, A.V. Moholkar, et al., J. Alloys. Compd. 509 (2011) 3108-3115. DOI:10.1016/j.jallcom.2010.12.012 |
| [25] |
K. Srinivas, M. Vithal, B. Sreedhar, M.M. Raja, P.V. Reddy, J. Phys. Chem. C 113 (2009) 3543-3552. |
| [26] |
T. Wu, G.H. Zhao, Y.Z. Lei, P.Q. Li, J. Phys. Chem. 115 (2011) 3888-3898. |
| [27] |
C. Mansas, J. Mendret, S. Brosillon, A. Ayral, Sep. Purif. Technol. 236 (2020) 116221. DOI:10.1016/j.seppur.2019.116221 |
| [28] |
J. Wang, H. Chen, Sci. Total Environ. 704 (2020) 135249. DOI:10.1016/j.scitotenv.2019.135249 |
| [29] |
X.F. Lang, Y.H. Liang, J. Zhang, et al., Phys. Chem. Chem. Phys. 22 (2020) 1371-1380. DOI:10.1039/C9CP05409J |
| [30] |
F. Rodríguez-Hernández, D.C. Tranca, B.M. Szyja, et al., J. Phys. Chem. 120 (2016) 437-449. |
| [31] |
X. Yin, H. Wang, E.H. Han, Surf. Sci. 691 (2020) 121504. DOI:10.1016/j.susc.2019.121504 |
 2021, Vol. 32
2021, Vol. 32 


