b State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, Integrative Microbiology Research Centre, Guangdong Province Key Laboratory of Microbial Signals and Disease Control, South China Agricultural University, Guangzhou 510642, China;
c Guangdong Laboratory for Lingnan Modern Agriculture, Guangzhou 510642, China
Isoquinoline constitutes an important structural motif in a range of natural alkaloids and bioactive molecules [1, 2], displaying a broad range of biological activities, such as anti-HIV, antimalarial, antitumor, antimicrobial, antileukemic antibacterial and insect growth retarding properties [3, 4]. Natural isoquinolinium salts such as magnocurarine [5], Quinocitrinines [6], chelerythrine [7], berberine [8], sanguinarine [9], and latifolians [10], have been reported to show a wide range of biological activities (Fig. 1A). In the majority of these natural isoquinolinium salts, the paired anions are often halogen ions. Very few examples of mesoionic isoquinolinium salts, namely the paired cation and anion present in one molecule, have been reported [11]. To the best of our knowledge, no reports for the synthesis of mesoionic thiazoloisoquinolinium thiolate have been divulged (Fig. 1C, compound I). Problems for the synthesis of such compounds include: (1) The design of a [2 + 2 + 1] cyclization system for the formation of a thiazole ring via C-H sulfurization of the isoquinoline with elemental sulphur [12]. Up until now, elemental sulfur has not been used for the reaction with isoquinoline to form carbon-sulfur bonds. (2) Trapping the elemental sulfur for the formation of the paired sulfur anion along with the [2 + 2 + 1] cyclization, and (3) maintaining the stability of the zwitterinic thiolates which are used as transitional active intermediates for cyclization [13, 14]. These challenges prompted us to further explore the synthesis of mesoionic isoquinolinium thiolates. In 1975, Bradsher reported a cyclization of isoquinolinium derivatives to form an unstable 2, 3-dihydrothiazolo[2, 3-a]isoquinolin-4-ium (Fig. 1B) [15]. A severe limitation of this chemistry was the tendency of the cyclized product to undergo ring opening with the resonance energy of the restored aromatic system. To overcome this problem, we designed a stable model for the mesoionic thiazoloisoquinoliniums (Fig. 1C, compound I) that stabilized by the aromaticity and the 1, 3-dipolarization of the ion pairs.

|
Download:
|
| Fig. 1. (A) Examples of isoquinolinium alkaloids. (B) Bradsher's work on the cyclization of quaternary ammonium salts. (C) Our design for the synthesis of thiazoloisoquinolinium and S-bridged fused tetracyclic compounds. | |
Herein, we report a metal- and additive-free synthesis of 1, 3-dipolar isoquinolinium thiolates via the [2 + 2 + 1] cycloaddition of isoquinoline, ethyl propiolate and elemental sulfur (Fig. 1C). This strategy provides the desired specimen for the synthesis of unique structures stabilized by conjugation and the 1, 3-dipolarization effect. In addition, the corresponding thiazoloisoquinolinium thiolates are versatile intermediates for the synthesis of thioether-containing isoquinolinium salts and S-bridged fused tetracyclic compounds. Although polycyclic isoquinolines are well reported in the literature [16-19]. We reveal for the first time, a new and unique type of S-bridged fused tetracyclic structure consisting of thiothiamide ring and two quarternary carbon centres.
Our study began with the synthetic analysis of mesoionic thiazoloisoquinolinium thiolates (Scheme 1). In order to obtain mesoionic compounds, a terminal alkyne bearing an electron-withdrawing group is preferable for the addition with isoquinoline. Such an addition may produce an unstable mesoionic species. We reasoned that the anion in the mesoionic species can be trapped by the electrophilic elemental sulfur to form an aromatic conjugated 1, 4-zwitterionic intermediate A. At this stage, similar to Bradsher's work [15], the loss of the stable non-aromatic intermediate B may occur. The enol-type intermediate A may be transformed to a ketone-type intermediate C, which reacts with sulfur to form a 1, 3-zwitterionic intermediate D. We speculated that further oxidative cyclization could produce intermediate E, the elemental sulfur may play the role as an oxidant. And the aromatization is the predominant driving force leading to the formation of the stable 1, 3-zwitterionic isoquinolinium thiolate F from intermediate E.

|
Download:
|
| Scheme 1. A plausible mechanism for the synthesis of 1, 3-zwitterionic thiazoloisoquinolinium thiolates. | |
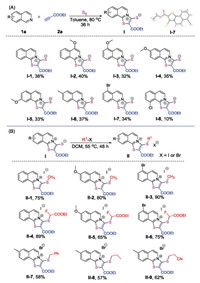
Based on the above analysis, ethyl propionate was selected to activate the isoquinoline for the [2 + 2 + 1] cyclization. In the cyclization process, the in situ formed paired anion can be trapped by elemental sulfur to form a mercapto anion. Thus, aprotic solvents are preferable for such a transformation. In the reaction of isoquinoline with ethyl propionate and elemental sulfur at room temperature in different aprotic solvents (e.g., DMF, CH3CN, DCE, dioxane and toluene), the target product was detected by LCMS in all cases. Toluene was found to be the most effective solvent for the transformation. After extensive screening of the reaction conditions, including temperature, addition of additives, type of sulfur agents, and loading quantities (Table S1 in supporting information), we finally found that the reaction proceeded well in toluene at 80 ℃ for 36 h using 3 equivalents of ethyl propionate. The target product was obtained in 38% yield. This result is acceptable for the synthesis of these unique 1, 3-zwitterionic thiazoloisoquinolinium thiolates.
Further evaluation of the reaction scope shows that substrates possessing electron-donating substituents are more effective than substrates bearing electron-withdrawing groups (Scheme 2A). Electron-donating groups, such as a methoxy group at the 4-, 5-, 6- and 7-positions, as well as a methyl group at the 6-position, gave satisfactory results affording the corresponding products in yields of 32%–40% (compounds I-2–I-5). When the reaction was performed with substrates bearing electron-withdrawing groups, such as chloro and bromo substituents, only 5-bromo isoquinoline was able to give the corresponding product I-7 in 34% yield, while 8-chloro isoquinoline furnished its corresponding product I-8 in a very low yield of 10% yield. Isoquinoline with a cyano group was not suitable for such transformation, only trace product was observed on ESI-MS. It is worth noting that the structure of the product was fully confirmed by X-ray single crystal analysis of compound I-7.
|
Download:
|
| Scheme 2. Synthesis of thiazoloisoquinolinium thiolate and thiazoloisoquinolinium halides. | |
The mercapto anion in compounds I is a useful nucleophilic species for the construction of thioether moieties. Compounds I can react with methyl iodide, ethyl 2-fluoro-2-iodoacetate, and alkyl bromides respectively, affording the corresponding thiazoloisoquinolinium iodides or bromides. Nine examples are shown in Scheme 2B. These nucleophilic substitution reactions were successful in DCM at 55 ℃ for 48 h, affording the corresponding products from II-1 to II-9 in moderate to good yields (Scheme 2B).
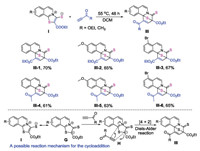
1, 3-Zwitterionic thiazoloisoquinolinium thiolates are potential cycloaddition partners for the construction of fused polycyclic compounds. We reasoned that the [4 + 2] cycloaddition of compound I with ethyl propionate may occur at the nucleophilic mercapto anion and at the 2nd electrophilic position of isoquinolinium [20]. Unexpectedly, the [4 + 2] cycloaddition selectively occurs at the α-C of the ketone possessing larger hindrance, rather than with the mercapto anion, thus a class of unique S-bridged pyrido[2, 1-α]isoquinoline-4-thiones bearing two quarternary carbon centres was obtained (Scheme 3). These reactions proceeded well in DCM at 55 ℃ over 48 h without the use of any metal catalyst or additives, affording the products III-1–III-6 in moderate to good yields (Scheme 3). Ethyl propionate and but-3-yn-2-one were effective reaction partners for the cycloaddition. The structures of these compounds were confirmed by NMR and HRMS analysis; a characteristic peak in the 13C NMR spectrum corresponding to the carbon atom of the thione is present (see NMR spectra in Supporting information). A possible reaction mechanism for the cycloaddition is also outlined in Scheme 3. Compound I process a resonance 1, 3-dipole G, which is more favorable than structure I for the [4 + 2] cycloaddition with a terminal alkyne. Because the location of S anion and the carbon-nitrogen double bond in the structure I is trans, the energy barrier of the cycloaddition is high. Thus the reaction should undergo a Diels-Alder transition state H to afford products III.
|
Download:
|
| Scheme 3. Synthesis of S-bridged tetracyclic-fused rings and a possible reaction mechanism. | |
In order to confirm the hypothesis of the synthetic pathway for the synthesis of isoquinolinium thiolates (Scheme 1), we employed ESI-MS real time analysis to explore the generation of potential intermediates. MS peaks of 260 were found in the samples after reaction over 15 min and 1 h, respectively. The MS peak of 260 was designated to be intermediate I as shown in Scheme S1 (Supporting information). However, the trace amounts of the intermediate cannot be isolated so their structures cannot be determined more accurately. Interestingly, in the reaction of 3-methoxy pyridine 1h with ethyl propionate and sulfur under the standard conditions, no cyclization product V was observed, but a non-cyclized product IV was obtained in 25% yield (Scheme S1) [21]. This suggests that compound IV cannot be transformed to compound V. Thus, the reaction of isoquinoline with ethyl propionate and elemental sulfur probably involves an intermediate I, suggesting that the proposed synthetic pathway shown in Scheme 1 is plausible.
The herbicidal activity of compound I-1 was tested on L. minor. The duckweed was badly withered when treated with 100 μg/mL and 50 μg/mL concentration of compound I-1 for 7 days, respectively. The inhibition rates of chlorophyll were positively correlated with the increase of the concentration, and the EC50 value was 51.5 μg/mL (see the detailed experiments and results in Supporting information). These results indicate that such zwitterionic compounds I might be a promising scaffold for the development of a new herbicide.
In summary, unique 1, 3-zwitterionic thiazoloisoquinolinium thiolates have been prepared via the reaction of isoquinolines, ethyl propiolate and elemental sulfur. It is noteworthy that the reaction does not involve the use of any metal catalyst or additive. Although the obtained yields of the products are not particularly high, this approach provides a simple multicomponent process for the preparation of unique thiozoisoquinolinium thiolates, which can be further transformed to thioether-containing thiazoloisoquinolinium halides and unique S-bridged fused tetracyclic compounds bearing a thiothiamide ring and two quarternary carbon centres. Importantly, preliminary bioassay shows that compound I-1 possesses bioactivity against chlorophyll of L. minor with an inhibition rate of EC50 51.5 μg/mL. This study opens up a new pathway to the synthesis of 1, 3-zwitterionic polycyclic compounds and their effective transformation to S-bridged tetracyclic compounds. Further studies concerning the selective transformation of thiazoloisoquinolinium thiolates are ongoing in our laboratories and the evaluation of the biological properties for these synthesized compounds will be reported in due course.
Declaration of competing interestThe authors declared that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
AcknowledgmentsThis work was financially supported by Natural Science Foundation of Guangdong Province for Distinguished Young Scholars (Nos. 2019B151502052, 2021B1515020107), the programs of National Natural Science Foundation of China (No. 32072450), the International Science and Technology Cooperation Program in Guangdong (No. 2020A0505100048), the Program of Science and Technology of Guangzhou (No. 202002030295), and the open Project of the State Key Laboratory of Crop Stress Biology for Arid Areas (No. CSBAAKF2021009).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.065.
| [1] |
P. Joseph, M. Quinoline, Nat. Prod. Rep. 14 (1997) 605-618. DOI:10.1039/np9971400605 |
| [2] |
M.D. Menachery, G.L. Lavanier, M.L. Wetherly, et al., J. Nat.Prod. 49 (1986) 745-778. DOI:10.1021/np50047a001 |
| [3] |
K.W. Bentley, Nat. Prod. Rep. 9 (1992) 365-391. DOI:10.1039/NP9920900365 |
| [4] |
H.J. Ni, Y. Martínez, G.-P. Guan, et al., BioMed. Res. Int. 2016 (2016) 1352146. |
| [5] |
M. Bala, S. Kumar, K. Pratap, et al., Nat. Prod. Res. 33 (2019) 622-627. DOI:10.1080/14786419.2017.1402319 |
| [6] |
A.G. Kozlovsky, V.P. Zhelifonova, T.V. Antipova, et al., J. Antibiot. 56 (2003) 488-491. DOI:10.7164/antibiotics.56.488 |
| [7] |
F. Miao, X.J. Yang, L. Zhou, et al., Nat. Prod. Res. 25 (2011) 863-875. DOI:10.1080/14786419.2010.482055 |
| [8] |
M. Tillhon, L.M.G. Ortiz, P. Lombardi, et al., Biochem. Pharmacol. 84 (2012) 1260-1267. DOI:10.1016/j.bcp.2012.07.018 |
| [9] |
N. Ahmad, S. Gupta, M.M. Husain, et al., Clin. Cancer Res. 6 (2000) 1524-1528. |
| [10] |
S.J. Rochfort, L. Towerzey, A. Carroll, et al., J. Nat. Prod. 68 (2005) 1080-1082. DOI:10.1021/np049616i |
| [11] |
(a) H. Hou, Y. Zhanga, C.G. Yan, Chem. Commun. 48 (2012) 4492-4494; (b) A. Shaabani, H.A. Rezayan, A. Sarvary, et al., Tetrahedron 65 (2009) 6063-6068; (c) K.T. Potts, D.R. Choudhury, J. Org. Chem. 43 (1978) 2700-2702. |
| [12] |
(a) T.B. Nguyen, Adv. Synth. Catal. 359 (2017) 1066-1130; (b) J.R. Zhang, Y.Y. Liao, J.C. Deng, et al., Chem. Commun. 53 (2017) 7784-7787; (c) K.W. Wang, Z.F. Jia, Y. Bai, et al., J. Am. Chem. Soc. 142 (2020) 11131-11138; (d) M.Z. Wu, Y. Jiang, Z.Y. An, et al., Adv. Synth. Catal. 360 (2018) 4236-4240; (e) X.Z. Che, J.J. Jiang, F.H. Xiao, et al., Org. Lett. 19 (2017) 4576-4579. |
| [13] |
B. Cheng, Y.T. Li, T.M. Wang, et al., Chem. Commun. 55 (2019) 14606-14608. DOI:10.1039/C9CC08326J |
| [14] |
B. Cheng, Y.T. Li, T.M. Wang, et al., J. Org. Chem. 85 (2020) 6794-6802. DOI:10.1021/acs.joc.0c00374 |
| [15] |
T.L. Wimmer, F.H. Day, C.K. Bradsher, J. Org. Chem. 40 (1975) 1198-1201. DOI:10.1021/jo00897a003 |
| [16] |
B.F. Jiang, Y. Zhou, Q.Y. Kong, et al., Molecules 18 (2013) 814-831. DOI:10.3390/molecules18010814 |
| [17] |
M.V.R. Reddy, M.R. Rao, D. Rhodes, et al., J. Med. Chem. 42 (1999) 1901-1907. DOI:10.1021/jm9806650 |
| [18] |
R.R. Jha, R.K. Saunthwal, A.K. Verma, Org. Biomol. Chem. 12 (2014) 552-556. DOI:10.1039/C3OB42035C |
| [19] |
Z.Y. Chen, J. Wu, Org. Lett. 12 (2010) 4856-4859. DOI:10.1021/ol101988q |
| [20] |
V. Jaiswal, B. Mondal, J. Saha, Asian J. Org. Chem. 9 (2020) 1466-1477. DOI:10.1002/ajoc.202000238 |
| [21] |
(a) J.C. Deng, Y.C. Gao, Z. Zhu, et al., Org. Lett. 21 (2019) 545-548; (b) J.C. Deng, J.H. Chen, J.R. Zhang, et al., Adv. Synth. Catal. 360 (2018) 4795-4806. |
 2021, Vol. 32
2021, Vol. 32 

