Owing to the excellent flame retardant properties, brominated flame retardants (BFRs) have been extensively used in many fields to improve their fire resistance, such as furniture, textiles, carpets, electronics castings, building materials, insulators [1]. Therefore, BFRs are ubiquitous in the environment and have recently been aroused because of their persistence, bioaccumulation and potential adverse effects to environment and human health, such as environmental endocrine-disrupting effects [2-5]. For example, tetrabromobisphenol A (TBBPA) and its derivatives have been widely detected in multiple seafood samples collected from Chinese marginal sea [6] and offshore of the Netherlands [7], including mollusks, fish, crabs, etc. Moreover, TBBPA is considered to be the most widely used BFRs with the highest production, accounting for about 60% of the total market for BFRs [8]. In recent years, TBBPA and its congeners have been identified as one of the common pollutants in marine mediums including sediment, seawater, and seafood [9]. Therefore, it is critical to develop sensitive and efficient detection methods to determine them from complex matrices.
Typically, investigations of TBBPA analogs are mainly based on high performance liquid chromatography-mass spectrometer (HPLC-MS) or liquid chromatography-diode array detector (HPLC-DAD) [10]. However, these methods are often limited by complicated, expensive, and time-consuming sample pretreatment techniques. Up to now, few reports have referred to the efficient extraction and advanced enrichment of TBBPA and its analogs, which would lead to the establishment of incomplete analytical methods to them. Solid-phase microextraction (SPME), developed by Pawliszyn et al., has become a mature and popular sample pretreatment technique because it is fast, miniaturized, highly efficient, and integrates sampling, isolation and enrichment into a single step [11, 12]. Thus, as a novel kind of solid phase microextraction (SPME) technology, capillary microextraction (CME), also called in-tube SPME, has emerged as an effective technique to achieve high extraction efficiency and repeatability due to its series of superiorities, such as good permeability, fast mass transfer, easy preparation and chemical modification, and small column volume [13]. Especially, monolithic capillary column is a more suitable mesoporous or macroporous sorbent for CME. According to the matrix nature, monolithic stationary phases mainly include three types: silica-based, organic polymer-based and organic-silica hybrid monolith [14, 15]. However, the deficiencies of mechanical stability for the organic polymer monolith and tedious post-modification process for the inorganic silica-based monoliths hinder their applications. As an alternative, the organic-inorganic hybrid monolithic column (HMC) could overcome the drawbacks and combine the advantages of two other kind monoliths, such as simple preparation, high mechanical strength, and strong solvent tolerance [16, 17].
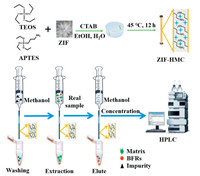
In this work, a novel flower-shaped zeolitic imidazolate framework (ZIF) doped organic-inorganic hybrid monolithic column (ZIF-HMC) was prepared via sol-gel "one-step" method, the effective length of ZIF-HMC for CME is 3 cm. It was utilized for CME to the separation and enrichment of four BFRs in environmental sewage with the combination of HPLC. Scheme 1 showed the schematic diagram for the preparation of ZIF-HMC and the application of CME. The detailed preparation process was presented in Supporting information.
|
Download:
|
| Scheme 1. Schematic diagram for the preparation of ZIF-HMC and the application of CME. | |
The structures and properties of four BFRs (TBBPA, TBBPS, TBBPA-ae, TBBPA-dbpe) were given in Table S1 (Supporting information).
To investigate the extraction performance of ZIF-HMC, before CME of target analytes, the extraction column was washed with 300 μL MeOH to remove out the residuary reactant. Afterward, 1 mL of BFRs standards solution or sample solution was injected into the monolithic column to enrich target analytes, then washed with 800 μL MeOH. The eluted sample solution was collected, followed by concentration with nitrogen at 80 ℃, then diluted to 200 μL using methanol, finally injected into the HPLC-UV for further analysis after a filtration with 0.22 μm membrane filter.
Fig. 1 presented the SEM images of ZIF-HMC. It could be seen that a continuous porous monolithic matrix with a homogeneous inner network (Fig. 1a) and uniform flower-shaped structure (Fig. 1c), abundant large flow channels (through pore in few micrometers) and some mesopores embedded (Fig. 1b). This kind of porous monolithic network was beneficial to the rapid mass transfer and the efficient extraction capacity of the monolith. Moreover, the cross section image of ZIF-HMC in Fig. 1a demonstrated the prepared monolith was well connected to the column inner wall and was uniformly distributed in the capillary column.

|
Download:
|
| Fig. 1. SEM images of ZIF-HMC, magnification: (a) 320×, (b) 4000×, (c) 30, 000×. | |
Original ZIF and ZIF doped organic-inorganic hybrid stationary phase in fused silica capillary were characterized by FT-IR. As shown in Fig. 2a, the absorption peaks at 2924 cm−1 and 2849 cm−1were assigned to C-H stretching vibration, the peaks at 1476 cm−1 and 1642 cm−1 belonged to C-N and N-H stretching vibration respectively. The adsorption peak at 1388 cm−1 were assigned to C=N stretching vibration on the imidazole ring, the peaks at 1146 cm−1 and 1303 cm−1 are assigned to in-plane bending, and the peak at 759 cm−1 corresponds to out-of-plane bending, which proved that ZIF was successfully doped to HMC by one-step sol-gel method.

|
Download:
|
| Fig. 2. (a) FT-IR of ZIF doped stationary phase (black line), original ZIF (red line). (b) N2 adsorption-desorption isotherms and pore size distribution of ZIF-HMC. (c) The energy disperse spectroscopy (EDS). | |
The N2 adsorption-desorption isotherms of hybrid monolith ZIF was type IV isotherms with H1-type hysteresis loop (Fig. 2b), which confirmed the existence of mesoporous structure. The BET surface areas and pore size distributions (PSDs) were 16.42 m2/g and 21.5 nm respectively (Fig. 2b). The energy disperse spectroscopy (EDS) of hybrid monolith ZIF showed the presence of elements C, N, O, Si and Zn, which indicated the incorporation of ZIF, tetraethoxysilane (TEOS) and aminopropyltriethoxy silane (APTES) monomer in the column matrix (Fig. 2c). The XPS (Fig. S1 in Supporting information) analysis was also carried out to explore the element composition of hybrid monolith ZIF, the result was consistent with EDS results.
In order to achieve the best extraction efficiency, the optimized experiment parameters were obtained by orthogonal array design (OAD) as follows, elution volume was 1000 μL, extraction rate was 10 μL/min, salt was 0 g. Table S2 (Supporting information) showed the effect of some parameters on the CME of four BFRs by ZIF-HMC.
Optimized CME conditions were employed to validate the analytical performance of the monolithic column. As shown in Table 1, the proposed method displayed good linearity with (R2) higher than 0.9972, wider linear range 1−500 μg/L, lower detection limits (0.52~3.1 μg/L), and good reproducibility (Relative standard deviation, RSD < 0.14%), which indicated the method possessed excellent adsorption performance of BFRs.
|
|
Table 1 Analytical performance of the proposed method (n = 3). |
Four real water samples were used to demonstrate the practical applicability of the ZIF-HMC. No BFRs were detected in real samples, the recoveries and precisions were shown in Table S3 (Supporting information). The samples were spiked with standard solutions of BFRs at 50 μg/L and 80 μg/L, the recoveries ranged from 92.6% to 110.0% and 88.8% to 116.6%, respectively.
The comparison between the developed method and other reported methods [18-20] was shown in Table S4 (Supporting information). The proposed method in this work exhibited low LODs, satisfactory recoveries, and small sample volume, short extraction time for the targets, which could be ascribed to the multi-modal noncovalent interactions between the analytes and monolithic sorbents, including hydrophilic effect, electrostatic, hydrogen bonding, and π-π interactions.
In this work, a ZIF doped organic-inorganic hybrid monolithic column (ZIF-HMC) was developed through simple sol-gel one-step method. After optimization of the CME conditions, ZIF-HMC achieved the best enrichment efficiency, it provided a potential alternative for the high efficient extraction and rapid determination of organic pollutants in complex samples. It is worth mentioning that the ZIF-HMC possessed great potential for separating small molecules and the strategy used here could be extended to prepare other derivatized HMC functionalized monoliths.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was generously supported by the National Natural Science Foundation of China (Nos. 21467028 and 21777129), the Program for Innovative Research Group of Gansu Province, China (No. 1210RJIA001), Special Fund Project for the Central Government to Guide Local Science and Technology Development (2020), Key Laboratory of Polymer Materials of Gansu Province and the Key Laboratory of Ecological Environment Related Polymer Materials of Ministry of Education.
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.034.
| [1] |
A. Besis, C. Christia, G. Poma, A. Covaci, C. Samara, Environ. Pollut. 230 (2017) 871-881. DOI:10.1016/j.envpol.2017.07.032 |
| [2] |
L. Liu, A. Liu, Q. Zhang, et al., J. Chromatogr. A 1497 (2017) 81-86. DOI:10.1016/j.chroma.2017.03.040 |
| [3] |
A. Covaci, S. Harrad, M.A.E. Abdallah, et al., Environ. Int. 37 (2011) 532-556. DOI:10.1016/j.envint.2010.11.007 |
| [4] |
S. Yue, T. Zhang, Q. Shen, et al., Environ. Int. 140 (2020) 105729. DOI:10.1016/j.envint.2020.105729 |
| [5] |
M.A. Abdallah, G. Pawar, S. Harrad, Environ. Int. 74 (2015) 13-22. DOI:10.1016/j.envint.2014.09.012 |
| [6] |
A. Liu, G. Qu, M. Yu, et al., Environ. Sci. Technol. 50 (2016) 4203-4211. DOI:10.1021/acs.est.5b06378 |
| [7] |
S. Morris, C.R. Allchin, B.N. Zegers, et al., Environ. Sci. Technol. 38 (2004) 5497-5504. DOI:10.1021/es049640i |
| [8] |
X. Wang, J. Liu, Q. Liu, X. Du, G. Jiang, Talanta 116 (2013) 906-911. DOI:10.1016/j.talanta.2013.08.011 |
| [9] |
Y. Qiu, K. Liu, S. Zhou, et al., Bull. Environ. Contam. Toxicol. 103 (2019) 41-47. DOI:10.1007/s00128-019-02637-7 |
| [10] |
A. Liu, Z. Zhao, G. Qu, Trends Analyt. Chem. 111 (2019) 85-99. DOI:10.1016/j.trac.2018.12.003 |
| [11] |
Y. Yang, P. Qin, J. Zhang, et al., J. Chromatogr. A 1570 (2018) 47-55. DOI:10.1016/j.chroma.2018.07.080 |
| [12] |
J. Zhang, W. Li, W. Zhu, et al., Chem. Commun. (Camb.) 55 (2019) 10019-10022. DOI:10.1039/C9CC04348A |
| [13] |
W. Li, X. Zhou, S. Tong, Q. Jia, Talanta 105 (2013) 386-392. DOI:10.1016/j.talanta.2012.10.065 |
| [14] |
Q. Wang, S. Shen, Talanta 194 (2019) 649-657. DOI:10.1016/j.talanta.2018.09.112 |
| [15] |
H. Liu, G. Raffin, G. Trutt, et al., J. Chromatogr. A 1595 (2019) 174-179. DOI:10.1016/j.chroma.2019.02.033 |
| [16] |
L. Zhao, X. Hu, Y. Chen, H. Lian, Environ. Monit. Forewar. 9 (2017) 21-24. |
| [17] |
H. Zheng, Q. Liu, Q. Jia, J. Chromatogr. A 1343 (2014) 47-54. DOI:10.1016/j.chroma.2014.03.067 |
| [18] |
J. Yang, J. Qiao, S. Cui, et al., J. Sep. Sci. 38 (2015) 1969-1976. DOI:10.1002/jssc.201500167 |
| [19] |
K.A. Sarpong, K. Zhang, Y. Luan, Y. Cao, W. Xu, Microchem. J. 154 (2020) 104526. DOI:10.1016/j.microc.2019.104526 |
| [20] |
E. Blanco, M.C. Casais, M.C. Mejuto, R. Cela, J. Chromatogr. A 1071 (2005) 205-211. DOI:10.1016/j.chroma.2004.10.075 |
 2021, Vol. 32
2021, Vol. 32 


