b School of Chemical Sciences, University of Chinese Academy of Sciences, Beijing 100049, China;
c College of Chemistry & Environmental Science, Hebei University, Baoding 071002, China
Enzymes with highly substrate specificity and enantio-selectivity under mild physiological conditions are ideal biological catalysts. Compared with conventional catalytic strategies, reactions and bio-procedures based on enzymes are environmentally friendly, specificity, and sustainable. However, the low reusability and stability, which results in high industrial cost and inefficient recycling, hampers greater application of free enzymes in solution. The immobilization of enzymes onto solid carriers has become one of the most promising approaches to overcome the problems [1, 2]. After immobilization, the resulting enzyme reactors can enhance the overall productivity and enzyme robustness, as well as enabling better control of the catalysis process owing to different environmental conditions provided by the carriers [3-7]. For example, porous polymer membranes containing nano- to micro-scale pores are important materials [8, 9] for efficient enzyme immobilization and can impact the performance of the enzyme immobilized on the polymer skeletons [10-13]. Besides the carrier types, its structure, especially the topography of the carriers, is important, not only regarding its influence on the enzyme loading capacity, but also with respect to enzyme activity, enzymolysis and reusability [1]. Although previous studies have developed different immobilization strategies to exploit the influence of different carriers' properties on the enzyme-catalyzed reactions, the carriers' topography effect on their enzymolysis efficiencies still remains a great challenge.
The topography of the enzyme carriers, especially the porous polymer membrane based enzyme reactors (PMER), play a significant role owing to their large surface area to volume ratio and their low thickness structure. The topography of the porous polymer membrane, including the surface roughness, porosity, permeability, brushes on the surface and so on, would influence the properties of the PMER [3-7]. To better understand the role of PMER topography, the effect of surface roughness on the enzyme immobilization has been studied. Results showed rough surfaces have much larger surface areas than smooth surfaces, which facilitated enzyme immobilized and led to greater enzymolysis efficiency [3, 4]. Additionally, it has proved that the pore size of the porous membrane also affected the enzymolysis efficiency of PMER by the confinement effect [7]. Furthermore, by modifying the polymer chains on the membrane pores, 'hairy'-porous polymer membranes have been developed, which can be used to control its enzymolysis efficiency by tuning the chain length or chain topography [5]. Obviously, the enhancement of enzymolysis efficiency depends not only on the PMER surface topography, but also on changes in polymer chains topography induced by conditions, such as pH or temperature. However, to the best of our knowledge, there are no studies considering the impact of both the surface topography and modified polymer chains changing on PMER enzymolysis efficiency.
To investigate how the topography of porous polymer membrane influences the enzyme efficiency of PMER, different porous polymer membrane morphologies and various types of 'hairy'-porous polymer membranes were considered in designing the carriers. In this regards, phase separation [14-16] is of interest from both fundamental and applied perspectives; this occurs when a polymer blend changes from one-phase to unstable two-phase region during the membrane formation. The application of this strategy for membrane topography control has been mainly in tailoring the macroscopic properties of composites [17, 18]. To alter the topography of porous polymer membrane, incompatible blocks of block copolymers can be used as the structure enabling phase separation and formation of nano-scale topography by self-assembly. The topography and the domain size of porous polymer membrane depend on the polymer molecular mass, relative length of the hydrophilic and hydrophobic segments [19, 20]. The typical size of the domains made by phase-separation is in the range of 10−200 nm and affected by the length of the blocks. Obviously, phase separation of block copolymers must be optimized for topography design of porous polymer membrane. Additionally, regarding 'hairy'-porous polymer membrane, the topography of the polymer chains brush, which affects the PMER's enzymolysis efficiency, can be tuned according to their stimuli-responsive properties. The physiochemical properties and structure of stimuli-responsive "hairy" brush polymers can change with changes to the external environment, such as temperature, pH, light or magnetism [8]. Therefore, it is assumed that the enzymolysis efficiency of stimuli-responsive "hairy"-PMER can be increased by changing the external environment to cause morphological changes to the polymer self-assembly.
Herein, a kind of pH-responsive "hairy"-porous polymer membrane fabricated by phase separation was designed and prepared. It composed of poly(styrene- co-maleic anhydride-acrylic acid) (PS-PMAn-PAA), poly(styrene-ethylene glycol) (PS-PEO) and Fe3O4 magnetic nanoparticles. Subsequently, d-amino acid oxidase (DAAO) was chemically immobilized onto the porous polymer membrane to construct a pH-responsive "hairy"-porous polymer membrane enzyme reactor (DAAO@PMER). Using d-methionine (d-Met) as the substrate, a chiral ligand-exchange capillary electrophoresis (CLE-CE) method was established and optimized to evaluate the enzymolysis efficiency of DAAO@PMER with d-methionine (d-Met) as the substrate. The relative merits of DAAO@PMER were due to the ability to tune the topography of the porous polymer membrane by phase separation, and to tune the "hairy" topography by the buffer pH. The pH-responsive "hairy"-PMER provides a platform for construction of unique enzyme reactors with high catalytic velocity and good reusability, and exhibits a new concept for design of porous PMER.
PS-PMAn-PAA was synthesized by reversible addition-fragmentation chain transfer (RAFT) polymerization (Fig. S1 in Supporting information). The "hairy"-porous polymer membrane was fabricated by heating the mixture of PS-PMAn-PAA, PS-PEO and magnetic Fe3O4 nanoparticles at 80.0 ℃ for 5 min. The PAA moiety of PS-PMAn-PAA provided the 'hairy' brush, while PS-PMAn and PS-PEO constructed the topography of the porous polymer membrane.
The synthesis and characterization of PS-PMAn-PAA and the "hairy" property of PAA in PS-PMAn-PAA were investigated and the results displayed in Tables S1–S3, Figs. S2 and S3 (Supporting information).
The pH-tunable property of the PAA "hairy" brush on the porous polymer membrane was evaluated by the contact angle measurements (Fig. S4 in Supporting information) at pH 4.7 and 8.3, respectively. The contact angles of porous polymer membrane were 85.9° at pH 4.7 and 57.0° at pH 8.3, respectively, which indicated that the membrane became more hydrophilic in basic condition [21, 22]. These results confirmed the pH-tunability of porous polymer membrane, and that this approach could provide a simple way to tune the thickness or topography of the "hairy" brush on the porous polymer membrane.
PS-PMAn-PAA was mixed with PS-PEO at different mass ratios and dissolved in THF to afford yield of membrane (Fig. S5 in Supporting information). During the formation of the polymer membrane, owing to the different solubility of PS-PMAn-PAA and PS-PEO dissolved in THF, the polymer blend changed from one-phase to unstable two-phase region [14-16]. Consequently, the concentration fluctuations of the two block co-polymers became unstable and grew, rather than blend, giving domains rich in one polymeric species. During the late stages, the characteristic porous topography was obtained by the phase separation of PS-PMAn-PAA and PS-PEO. After the porous polymer membrane was constructed, the DAAO enzyme was immobilized onto the membrane (Fig. S5). Characterization of DAAO@PMER was performed using Fourier transform infrared spectroscopy (FT-IR, Fig. S6 in Supporting information), thermo gravimetric analysis (TGA, Fig. S7 in Supporting information), vibrating sample magnetometer (VSM, Fig. S8 in Supporting information) and N2 adsorption-desorption (Fig. S9 in Supporting information).
Following confirmation of the successfully fabrication of the PS-PMAn-PAA and the controllable changes of the PAA moiety for the topography study of porous polymer membrane, the structure of the DAAO@PMER was investigated. The effect of the polymer mass ratio of PS-PMAn-PAA to PS-PEO, the topography of the porous polymer membrane and the DAAO immobilization on the enzymolysis efficiency of DAAO@PMER were all explored.
The topography of the fabricated series of porous polymer membranes was observed by SEM (Fig. 1). As the polymer mass ratio of PS-PMAn-PAA to PS-PEO decreased from 90:10 (Figs. 1A and B) to 50:50 (Figs. 1C and D) and 10:90 (Figs. 1E and F), although the pores difference on the surface of the membrane was not very obviously (Figs. 1A, C and E), there was less pores inside the polymer membrane (Figs. 1D and F). This was due to PS-PEO enhancing the hydrophobicity of the porous polymer membranes, which affected the phase separation processes during the pores formation [5]. Notably, much more nano- and micro-scale pores could be observed in the cross-section of the membrane (Fig. 1B) than that in Figs. 1D and F, which would affect the enzymolysis efficiency of the carrier. The amount of PS-PMAn-PAA also impacted the anchor site in porous polymer membrane and the pH-responsive property of PAA moiety, which could tune the collision probability of the substrate and DAAO and improve the enzymolysis efficiency [7].

|
Download:
|
| Fig. 1. SEM images of the porous polymer membrane (3 layers) with different polymer mass ratio of PS-PMAn-PAA to PS-PEO. (A, B) 90:10; (C, D) 50:50; (E, F) 10:90. Surface images of the porous polymer membrane: (A), (C), (E). Cross-section images of the porous polymer membrane: (B), (D), (F). | |
To investigate the enzymolysis efficiency of DAAO@PMER using D-Met as the substrate, a chiral ligand exchange capillary electrophoresis (CLE-CE) protocol was developed. A quantitative analysis of Dns-d, l-Met was then performed under the optimized CLE-CE conditions. Linear equations with good linearity relationships and low detection limits were obtained (Fig. S10 and Table S4 in Supporting information). Consequently, the effect of polymerization time, the amount of immobilized DAAO, the duration of DAAO immobilization, the polymer mass ratio and the number of membrane layers on the enzymolysis efficiency of DAAO@PMER were investigated by using the developed CLE-CE method.
The enzymolysis efficiency of DAAO@PMER was measured according to the decrease in D-Met decreased after incubation with the enzyme reactor (Fig. S11 in Supporting information), which was calculated by Eq. 1:
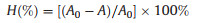
|
(1) |
Where A0 and A are the peak areas of D-Met before and after incubation with DAAO@PMER, respectively, and H% is the enzymolysis efficiency.
The enzymolysis efficiency of DAAO@PMER was optimized. First, the effect of the mass ratio of PS-PMAn to AA (100:300, 300:300 or 300:100) and polymerisation duration (6.0 h, 12.0 h or 24.0 h) on the enzymolysis efficiency of DAAO@PMER was investigated. Figs. S12A and S12B (Supporting information) indicated that a 300:300 mass ratio of PS-PMAn to AA monomer and polymerization duration of 12.0 h afforded the highest enzymolysis efficiency. Then, the effect of different amounts of DAAO (1.0, 2.5 or 4.0 mg/mL) and different immobilization durations (1.0, 3.0 or 5.0 h) on the enzymolysis efficiency of DAAO@PMER was studied. Figs. S12C and S12D (Supporting information) that optimal enzymolysis efficiency could be obtained when the amount of fixed DAAO was 2.5 mg/mL and the immobilization duration was 3.0 h. Next, the influence of PS-PMAn-PAA to PS-PEO mass ratio (90:10, 50:50 or 10:90) and the number of porous polymer membrane layers (1, 3 or 6 layers) on the enzymolysis efficiency was investigated. Fig. S12E (Supporting information) exhibits that the enzymolysis efficiency increased with increasing PS-PMAn-PAA from 10.0–90.0 mg. Fig. S12F (Supporting information) shows that 3 layers of porous polymer membrane resulted the highest the enzymatic hydrolysis efficiency of DAAO@PMER. Importantly, the thicker the PAA was, the higher the hydrolysis efficiency was. Finally, optimized conditions of 10.0 mgPS-PEO, 90.0 mgPS-PMAn-PAA (300:300 of PS-PMAn: AA) 12.0 h polymerization, 2.0 mg Fe3O4 and 2.5 mg/mL DAAO immobilized onto the porous polymer membrane for 3.0 h at 4 ℃ were selected for further study.
Finally, the classical proteins detection method (Coomassie blue binding assay) was used to measure the amount of immobilized DAAO (Fig. S13 in Supporting information). The results indicated that 1.31 mg/mL DAAO was anchored onto the porous polymer membrane.
An enzyme kinetics study of free DAAO and DAAO@PMER was performed at different pH values. The decrease in the amount of D-Met substrate was determined by the CLE-CE assay, and this data was used to calculate the affinity and kinetic reaction rate of the prepared DAAO@PMER via Michaelis-Menten Eq. 2:

|
(2) |
where, V and Vmax are the initial and maximum velocities, [S] is the substrate concentration and Km is Michaelis-Menten constant.
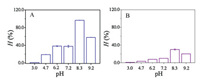
To prove that the 'hairy' brush could be tunable based on the pH-based stimulation of PAA moiety, which would further impact the enzymolysis efficiency, the enzyme kinetics of DAAO@PMER and free DAAO were investigated at different pH values raging (3.0–9.2). Fig. 2 displays that the highest enzymolysis efficiency ( H%) of DAAO@PMER was obtained at pH 8.3, which was 3.2-folds higher than that of free DAAO in solution.
|
Download:
|
| Fig. 2. Effect of buffer pH on the enzymolysis efficiency of (A) DAAO@PMER and (B) free DAAO in solution. | |
The Lineweaver Burk plots of DAAO@PMER and free DAAO were determined using D-Met as the substrate (Fig. S14 in Supporting information). The Km and Vmax were calculated to be 30.40 mmol/L and 2.13 mmol L−1 min−1 for DAAO@PMER, and 2.43 mmol/L and 0.12 mmol L−1 min−1 for free DAAO in solution, respectively. Notably, although the Km of DAAO@PMER was lower than that of free DAAO, the Vmax of DAAO@PMER was 17.7-folds higher than that of free DAAO, indicating the superior performance of DAAO@PMER.
To evaluate the repeatability of the DAAO@PMER fabrication, the batch-to-batch ( n=4) fabrication of the DAAO@PMER was performed. The H% of each batch indicated good fabrication repeatability (RSD≤0.8%). Moreover, the H% of DAAO@PMER remained 70.9% after five continuous runs and kept 77.8% after five days stored at 4 ℃ (Fig. S15A in Supporting information). While, the H% of free DAAO decreased to 39.2% within half a day (Fig. S15B in Supporting information). These results exhibited good reusability and stability of the DAAO@PMER.
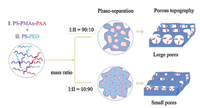
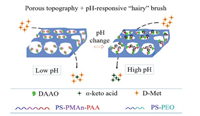
The mechanism of precise enzymolysis rate control by the prepared DAAO@PMER could be attributed to its special topographical structure and pH-responsive "hairy" brush on the porous polymer membranes [9]. First, the different hydrophilic/hydrophobic properties of PS-PMAn-PAA and PS-PEO resulted in phase separation of this two-polymer mixture during the formation of the porous polymer membranes (Fig. 3): The hydrophobic properties of the PS moiety meant that it formed the backbone in the porous polymer membranes, whereas the hydrophilic PMAn and PEO moieties formed other part of the porous polymer membranes; this led to phase separation and formation of the porous topography [5]. Second, the PAA moieties anchored onto the porous polymer membranes to form a pH-responsive "hairy" brush [9]; this structure controlled the topography of the pores (Fig. 4) [7], producing a significant change in the enzymolysis efficiency of DAAO@PMER at different pH values (Fig. 2A). The improved enzymolysis efficiency was mainly dependant on the porous topography of DAAO@PMER, which could enhance the possibility of collisions between the substrate (d-Met) and the enzyme reactor. Furthermore, at lower pH (pH 4.7), the pH-responsive PAA moiety exhibited hydrophobicity and curled onto the enzyme, which would further block the substrate to contact the enzyme. While, at higher pH (pH 8.3), the PAA moiety exhibited hydrophilicity and stretched into the aqueous solution [21, 22], which made the substrate to rapidly pass through the polymer brush, and contact the enzyme. Consequently, there was a marked enhancement in the enzymolysis efficiency of DAAO@PMER due to the pH-responsive "hairy" brush [9]. These results demonstrated that high enzmolysis efficiency can be achieved via topography and pH-responsive "hairy" brush structures arising from polymer phase separation. This is an interesting and promising exploration research area, warranting further exploration.
|
Download:
|
| Fig. 3. Process of porous polymer membrane topography formation by phase separation. | |
|
Download:
|
| Fig. 4. Schematic illustration of the tunable enzymolysis efficiency based on the "hairy" brush pH-responsive property of DAAO@PMER. | |
In summary, a concept for PMER fabrication composed of PS-PMAn-PAA and PS-PEO was developed based on the special topography achieved by phase separation and a pH-responsible "hairy" brush. Combining the merits of the topography and the pH-tunable property, the enzymolysis efficiency of the as-prepared DAAO@PMER could be tuned simply by adjusting the polymer mass ratio and buffer pH. Furthermore, its enzymolysis efficiency was evaluated by the proposed CLE-CE technique with d-Met as the substrate. Notably, the DAAO@PMER performed well and the Vmax represented 17.7-folds higher than that of free DAAO in solution at high pH. This approach has paved a new research path for the construction of special PMER topographies based on phase separation and stimuli-responsible "hairy" brushes, providing great potential in the enzymatic kinetics study and screening of enzyme inhibitors.
Declaration of competing interestThere are no conflicts of interest.
AcknowledgmentsThe authors are grateful for the financial support from the National Natural Science Foundation of China (Nos. 21874138, 22074148, 21727809, 21635008). We also thank Yuying Song for her kind help in surface area and pore-size characterizations.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.021.
| [1] |
G.R. Cheng, J.P. Xing, Z.F. Pi, et al., Chin. Chem. Lett. 30 (2019) 656-659. DOI:10.1016/j.cclet.2018.12.003 |
| [2] |
Y. Rui, X.M. Wu, B.D. Ma, Y. Xu, Chin. Chem. Lett. 29 (2018) 1387-1390. DOI:10.1016/j.cclet.2017.10.033 |
| [3] |
F. Wu, L. Su, P. Yu, et al., J. Am. Chem. Soc. 139 (2017) 1565-1574. DOI:10.1021/jacs.6b11469 |
| [4] |
F. Wu, P. Yu, X.T. Yang, et al., J. Am. Chem. Soc. 140 (2018) 12700-12704. DOI:10.1021/jacs.8b08020 |
| [5] |
S.A. Currivan, W.Q. Chen, R. Wilson, et al., Analyst 143 (2018) 4944-4953. DOI:10.1039/c8an01330f |
| [6] |
J. Qiao, J.Y. Kim, Y.Y. Wang, et al., Anal. Chim. Acta 906 (2016) 156-164. DOI:10.1016/j.aca.2015.11.042 |
| [7] |
J.L. Dong, W.J. Ning, W.J. Liu, et al., Analyst 142 (2017) 2578-2586. DOI:10.1039/C7AN00778G |
| [8] |
J.S. Yang, J. Qiao, J.Y. Kim, et al., Anal. Chem. 90 (2018) 3124-3131. DOI:10.1021/acs.analchem.7b04273 |
| [9] |
Y. Eygeris, E.V. White, Q.Y. Wang, et al., ACS Appl. Mater. Interfaces 11 (2019) 3407-3416. DOI:10.1021/acsami.8b17483 |
| [10] |
J.F. Jiang, J. Qiao, Y.M. Xiao, et al., Talanta 165 (2017) 251-257. DOI:10.1016/j.talanta.2016.12.055 |
| [11] |
M.M. Zhang, J. Qiao, L. Qi, Anal. Chim. Acta 1035 (2018) 70-76. DOI:10.1016/j.aca.2018.07.019 |
| [12] |
H.J. Yang, L. Wang, S.L. Chen, et al., Polym. Int. 64 (2015) 915-923. DOI:10.1002/pi.4865 |
| [13] |
N. Tsujioka, N. Ishizuka, N. Tanaka, et al., Polym. Sci. Part A: Polym. Chem. 46 (2008) 3272-3281. DOI:10.1002/pola.22665 |
| [14] |
J.H. Li, Z.J. Du, H.Q. Li, et al., Polymer 50 (2009) 1526-1532. DOI:10.1016/j.polymer.2009.01.049 |
| [15] |
I.C. Henderson, N. Clarke, Macromolecules 37 (2004) 1952-1959. DOI:10.1021/ma034718l |
| [16] |
A.C. Balazs, V.V. Ginzburg, F. Qui, et al., Phys. Chem. B 104 (2000) 3411-3422. DOI:10.1021/jp993356+ |
| [17] |
G. Peng, F. Qiu, V.V. Ginzburg, et al., Science 288 (2000) 1802-1804. DOI:10.1126/science.288.5472.1802 |
| [18] |
N.R. Cameron, Polymer 46 (2005) 1439-1449. DOI:10.1016/j.polymer.2004.11.097 |
| [19] |
M.J. Fasolka, A.M. Mayes, Annu. Rev. Mater. Res. 31 (2001) 323-355. DOI:10.1146/annurev.matsci.31.1.323 |
| [20] |
B. Szilagyi, Z. Skok, A. Racz, et al., Bioorg. Med. Chem. Lett. 28 (2018) 1693-1698. DOI:10.1016/j.bmcl.2018.04.048 |
| [21] |
H. Lim, Y. Lee, S. Han, et al., J. Polym. Sci. Part B: Polym. Phys. 41 (2003) 1791-1797. DOI:10.1002/polb.10536 |
| [22] |
T. Luo, S. Lin, R. Xie, et al., J. Membr. Sci. 450 (2014) 162-173. DOI:10.1016/j.memsci.2013.09.002 |
 2021, Vol. 32
2021, Vol. 32 

