b Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China
Providing safe drinking water is a critical issue for public health [1, 2]. Waterborne pathogens in developing and rural areas causing serious health issues and fatal diseases have attracted global attention. As of 2015, over 800 million people lacked access to safe drinking water, which resulted in the death of over 480, 000 people because of waterborne illnesses [3]. Consequently, effective water disinfection materials and technologies are of great significance to ensure healthy life of people in underdeveloped areas [4-6]. To date, several strategies, such as chlorination, membrane filtration, and electroporation disinfection, have been implemented to obtain clean and safe drinking water [7-10]. However, these water disinfection strategies still possess many challenges. For example, although chlorination could inactivate more than 99.99% bacteria in water, which could produce toxic chlorinated by-products and reduce the disinfection efficacy due to the increase of turbidity content and existence of natural organic matter (NOM) in water. Traditional membrane filtration of water could separate the microbes from water, but unable to inactivate waterborne pathogens existing in water [11-14]. The electroporation method could efficiently kill the pathogens in water due to the high electric field generated by the nanostructures such as silver nanowires (Ag NWs), carbon nanotubes (CNTs) or copper nanowires [15-18]. However, the complex procedures of materials preparation and high energy consumption restricted the practical application of electroporation disinfection strategies. Therefore, the development of innovative point-of-use (POU) material and method for efficient, high flux, economical and energy-effective disinfection of drinking water are urgently needed. Wood as a biomass material is naturally abundant and renewable [19, 20]. The microstructure of wood consists of numerous open, low tortuous and aligned channels, which naturally exist to transport ions, water, and other ingredients from root to tree branches [21, 22]. The cellulose (major substance in the wood) aggregated into nanofibrils, interaction with hemicellulose and lignin which forms the wood structures, leads to an anisotropic properties of wood [23, 24]. Due to these unique advantages, wood-based materials have attracted great attention for researchers. Functional devices for highly efficient water extraction and highly anisotropic conductors are developed based on the natural wood structures [25, 26]. Wood derived products such as cellulose nanocrystals and nanofibers have also been utilized for transparent papers, biodegradable electronics, etc. [27-29].
Taking advantage of natural wood, we propose wood carbon membrane as low cost, efficient matrix for water disinfection. The wood carbon membrane is able to retain the microstructure of three dimensional (3D) porous, providing low tortuous and aligned channels of natural wood. Furthermore, carbonized nanofibrils inside wood carbon channels offer unique nanoscaled tips structure, which have excellent biological and electronic properties in electric field disinfection process. Ag nanoparticles are widely accepted with excellent antibacterial activity and biocompatibility, which can strongly interacts with thiol groups of vital enzymes and inactivates them [30, 31]. Still, the high cost, separation difficulty and reusability issues largely limit the practical application of Ag nanoparticles for water disinfection. Immobilizing Ag nanoparticles into the aligned microchannels of wood carbon can offer a new way to avoid agglomeration and achieve the reusable Ag NPs/WC composite. In addition, a much stronger electric field can be built up near the tips of nanofibrils in wood. If the tip is small enough, the electric field can be several orders of magnitude stronger to cause irreversible electroporation at a low voltage [15]. Based on that, the conductive Ag NPs/WC composite could be a fantastic candidate for effective electric field water disinfection, due to the synergistic effect of the uniformly distributed the tips structure of carbon nanofibrils, Ag nanoparticles, as well as the unique microchannel structure in wood carbon.
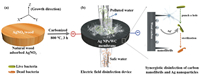
Herein, for the first time, we designed Ag NPs/WC membrane for rapid and highly efficient electric field water disinfection. In this work, balsa wood was chosen as the starting material, which is cost-effective and obtains hollow, large diameter aligned channels [22, 32]. The preparation process of Ag NPs/WC membrane and the mechanism of disinfection are illustrated in Fig. 1. The Ag nanoparticles are in situ formed and well-dispersed in the conductive wood carbon matrix by a simple strategy (Fig. 1a): (i) A piece of balsa wood block is soaked in the aqueous solution of AgNO3 for 2 h to ensure an effective penetration of precursor inside of the wood blocks, (ii) AgNO3/wood composite is dried and carbonized at 800 ℃ for 3 h under nitrogen (N2) atmosphere. The silver ions (Ag+) are reduced to Ag° during wood carbonization process (by carbon), resulting in Ag nanoparticles uniformly forming in the wood carbon channels (Fig. 1b). Under electrical field (radial direction of wood), the electric charge of the microchannel surface of wood carbon are able to introduce the microbial cells in water to approach the channel surface, where the Ag nanoparticles and carbon nanofibrils are well dispersed and anchored. This leads to the occurrence of electric field disinfection process (Fig. 1b). The synergistic effect of the uniformly distributed Ag nanoparticles and the tips structure of carbon nanofibrils in wood carbon result in the highly electric field disinfection performance of our membrane. On the other hand, the open, low tourist, aligned and hollow microchannels enable the aqueous solution flowing through the membrane with high flux. Consequently, the Ag NPs/WC membrane-based electric field disinfection device shows an excellent inactivation performance of more than 5 log for E. coli, E. faecalis, S. Typhimirium, and B. subtilis under an input voltage of 4 V, a high flux of 3.8 × 103 L m−2 h−1 and low energy consumption of 2 J L−1 m−2. The Ag NPs/WC membrane offers a promising point-of-use (POU) strategy for economical, energy-effective, renewable, highly efficient and high flux for drinking water disinfection.
|
Download:
|
| Fig. 1. Schematic of the 3D Ag NPs/WC membrane synthesis and the mechanism of electric field disinfection. (a) A piece of balsa wood adsorbs the precursor of AgNO3. (b) The electric field disinfection device using Ag NPs/WC membrane. | |
Ag nanoparticles in situ formed in the microchannels of wood carbon membrane. The prepared Ag NPs/WC membrane can be used as an electric field disinfection device to inactivate the microbes in water under electric filed (radial direction). The zoomed-in image shows that Ag nanoparticles and carbon nanofibrils distributed in the hollow and aligned microchannels of the wood carbon. Under electric filed, the synergetic effect of tips structure of carbon nanofibrils and Ag nanoparticles in wood carbon matrix results in highly efficient water disinfection performance of the prepared membrane.
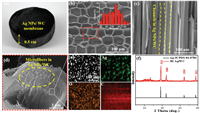
To prepare the Ag NPs/WC membrane, a piece of balsa wood was cut along the growth direction (thickness: 5.0 mm, diameter: 20.0 mm), which was immersed in the AgNO3 aqueous solution (5.0 g/L) for 2 h, followed by dried at 45 ℃ for 24 h and subsequently carbonized at 800 ℃ for 3 h under N2 atmosphere. The photo image of the obtained Ag NPs/WC membrane is shown in Fig. 2a. Scanning electron microscopy (SEM) analysis of the prepared Ag NPs/WC membrane exhibits highly porous microstructure (Fig. 2b). The average diameter of these microchannels is about 35 ± 10 μm, which is large enough to avoid being clogged by microbial cells (several micrometers) as water flowing through [33]. Therefore, the water can be maintained at a high flux without blocking by the microbes in water. Cross-sectional SEM image of Ag NPs/WC membrane (Fig. 2c) reveals several long, low tourist and aligned microchannels (highlighted in yellow dash line). These microchannels combined with the ray cells of wood form a continuous 3D porous matrix that can be utilized for water filtration [21, 34]. The magnified SEM image shows that the carbon nanofibrils well distribute in the microchannels of wood and exhibit tips structure (Fig. 2d). According to the previous report, near the tips structure an enhanced electric field can be built up, even with a relatively low input voltage [15]. The electrical conductivity of the Ag NPs/WC membrane under the wet state reaches 4.5 S/cm (Fig. S1 in Supporting information) which make it as a good candidate for electric field disinfection. Fig. 2e and Figs. S2a–c (Supporting information) illustrate the transmission electron microscope (TEM) images of Ag nanoparticles in situ formed in wood carbon microchannels, which are homogeneously dispersed with an average size of 20 ± 5 nm. The high-resolution TEM (HRTEM) image exhibits that the measured interplanar distance of the (220) lattice plane of Ag nanoparticles is about 0.14 nm, which proves the identity of the nanoparticles (Fig. S2d in Supporting information) [35]. The energy dispersive spectroscopies (EDX) mapping further confirms the above result (Fig. 2e). The content of Ag in Ag NPs/WC membrane is about 11.7 wt% according to inductively coupled plasma mass spectrometry (ICP-MS) analysis. The structure of 3D Ag NPs/WC membrane is further investigated by X-ray diffraction (XRD). As shown in Fig. 2f, the diffraction peaks are in good agreement with metallic Ag (JCPDS No. 04-0783) [36]. Note that the peak at around 26° is indexed to graphitic carbon (002) [37].
|
Download:
|
| Fig. 2. Characterization of the 3D Ag NPs/WC membrane. (a) Photo image of the 3D Ag NPs/WC membrane. (b) SEM image showing the top surface of the designed membrane, inset shows the diameter distribution of the wood channels. (c) SEM image showing the cross-section of the Ag NPs/WC membrane. (d) Magnified SEM image of carbon nanofibrils in 3D Ag NPs/WC membrane. (e) EDX mapping characterization of Ag nanoparticles in the microchannels of wood carbon. (f) XRD pattern of the 3D Ag NPs/WC membrane. | |
To demonstrate the electric field bacterial inactivation performance of 3D Ag NPs/WC membrane, the water sample consisting of E. coli (~107 CFU/mL) was selected as an electric field disinfection model. As well known, E. coli is a kind of Gram-negative bacteria which was most commonly used as an indicator species for water pollution [38, 39]. The schematic illustration of the electric field disinfection device is shown in Fig. 3a. A piece of 3D Ag NPs/WC membrane was used as a filter which was attached to two electrodes in the radial direction of the wood for voltage input. During filtration, water can transport through the long and low tortuous microchannels of wood carbon, where microbial can be fully contacted with carbon nanofibrils and Ag nanoparticles anchoring on the surface of the wood carbon channels to realize the highly efficient electric field disinfection performance of the designed membrane (Fig. 3b). The concentration of bacteria in the influent (Cin), and effluent (Ceff) is analyzed by the colony counting method, and the log efficiency is calculated by the formula (Eq. 1) [16]:
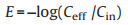
|
(1) |

|
Download:
|
| Fig. 3. Schematic of electric field disinfection device and bacterial inactivation performance of 3D Ag NPs/WC membrane. (a) Schematic of the electric field disinfection device. (b) The high-resolution SEM image of carbon-wood membrane, which shows long and arrayed channels in the radial section of the wood. The direction of water flowing is highlighted with yellow arrows. (c) Disinfection performance of the 3D Ag NPs/WC membrane and pure wood carbon under different input voltages. (d) Electric field disinfection property with different flux. Inset shows the effect of contact time. (e) The disinfection stability of the Ag NPs/WC membrane. (f) The concentrations of Ag within the effluent after filtrated under different voltages. | |
The electric field disinfection properties of 3D Ag NPs/WC membrane and pure wood carbon by using different voltages (0–5 V) at the flux of 1.5 × 103 L m−2 h−1 are shown in Fig. 3c. The bacterial inactivation efficiencies of both pure wood carbon and 3D Ag NPs/WC membrane have enhanced with voltages increasing. For pure wood carbon, the inactivation efficiency reaches to 2.5 log for E. coli at a voltage of 5 V, which could be generated by the electroporation disinfection of carbon nanofibrils tips structure affection in wood carbon microchannels [40]. On the other hand, the inactivation efficiency of 3D Ag NPs/WC membrane is much higher than that of pure wood carbon under the same input voltage. Note that the disinfection efficiency of the designed membrane is reached to more than 5 log at 4 V. The disinfection efficiency of Ag NPs/WC membrane is further investigated at different flux. A syringe pump was utilized for water flux control. The treatment flux is calculated with Eq. 2:
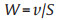
|
(2) |
where W is the flux of the treatment (L m−2 h−1), v is the water flow rate of the syringe pump (L/h) and S is the effective area of the Ag NPs/WC membrane (m2). As shown in Fig. 3d, a high E. coli inactivation efficiency of more than 5 log is maintained even at a high flux of 3.8 × 103 L m−2 h-1 using 4 V voltage. Considering water disinfection efficiency and energy consumption, the input voltage of 4 V is applied in the next water disinfection test. The hydraulic retention time (HRT) is calculated according to the following Eq. 3:
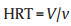
|
(3) |
V is the effective volume of the Ag NPs/WC membrane, v is the water flux controlled by the syringe pump. The effective diameter and height of Ag NPs/WC membrane is about 13.0 mm and 5.0 mm, respectively, and v is the flux rate from 0 to 500 L/h. Inset Fig. shows that the inactivation efficiency reached to more than 5 log as the HRT is reached 5 s. The rapid and highly efficient electric field disinfection performance of the 3D Ag NPs/WC membrane could be ascribed to the following reasons: (1) 3D porous structure and hydrophilic performance (Fig. S3 in Supporting information) of Ag NPs/WC membrane allow fast water flow without agglomeration; (2) the synergistic effect of electroporation and Ag NPs disinfection causing by the carbon nanofibrils and Ag nanoparticles distributed on the surface of wood carbon channels (The detail electric field disinfection cell mechanism of Ag NPs/WC membrane was analyzed in the next part); (3) the long and low tortuous channels of wood increase the chance of microbes contacting with carbon nanofibrils and Ag nanoparticles. Fig. S4 (Supporting information) shows the typical photo images of E. coli colonies after treated with 3D Ag NPs/WC membrane under different voltages. The result indicates that there is no bacteria colony on agar medium after filtrated under the voltage of 4 V, which demonstrating that Ag NPs/WC membrane possessed significant electric field antimicrobial activity against membrane for E. coli is maintained at 5.2 log under the voltage of 4 V after 7 days continuous operation (Fig. 3e). The stability of Ag NPs/WC membrane is further investigated by SEM and XRD analysis. As shown in Fig. S5 (Supporting information), the microstructure of the Ag NPs/WC membrane is well kept even after long term (12 h) utilization. The XRD pattern of the used membrane well agrees with that of before, which demonstrates its fantastic stability (Fig. S6 in Supporting information). The concentration of Ag in the effluent water after filtrated under different input voltage is E. coli. In addition, sustainability is an important property for water disinfection material. The inactivation efficiency of Ag NPs/WC measured with ICP-MS. The result shows that although the content of A increased with the applied voltage enhancing from 0 to 5 V, the content of Ag is still lower than 50 ppb (Fig. 3f), which meets the drinking water standard (100 ppb) setting by world health organization (WHO) [41]. Furthermore, the aqueous solution of Ag+ ions with the concentration of 50 ppb acted as a control sample (Fig. S7 in Supporting information). We compared the inactivation performance of Ag+ ions with that of 3D Ag NPs/WC membrane. The microbe growth result shows that there is no obvious inactivation of Ag+ ions, which further confirms that the excellent disinfection performance of 3D Ag NPs/WC membrane can be caused by the synergetic effect of electroporation and Ag nanoparticles in wood carbon channels.
In order to prove the electric field inactivation mechanism, we designed a series experiments. SEM image shows that there are abundant of carbon nanofibrils in wood carbon channels (Fig. 4a), which can produce higher electric filed due to the tips structure affection (Fig. 4b) [15]. The SEM images of E.coli before and after filtrated with 3D Ag NPs/WC membrane under the applied voltage from 0 to 4 V are shown in Fig. 4c and Fig. S8 (Supporting information). The electroporated pore structures on bacteria membranes can be found obviously, which indicates that tips structure of carbon nanofibrlis in wood could enhance electrical filed which lead to electroporation disinfection occurred. In addition, immediately after filtration under 4 V voltage, E. coli was plated on agar. The percentage of dead bacteria is about 50.4% ± 3.1%, which indicates that some E. coli still could be proliferated and formed colonies on the agar plates (Fig. S9 in Supporting information). The phenomena strongly support the electroporation mechanism [16]. On the other hand, if E. coli with electroporation pores were kept in the filtrate for 2 h, then plating without any nutrients, they were unable to survive. Because of that during the rest period the different osmotic pressure or imbalance of chemical between the intercellular and extracellular environment may occur, which lead to the final death of the microbes (Fig. S9) [42]. Time tracking of E. coil viability after filtrated with Ag NPs/WC membrane under 4 V voltage was investigated. The result further confirmed that E. coli with electroporation pores were not able to reproduce after kept in water for 2 h (Fig. S10 in Supporting information). Propidium iodide (PI), a nuclear DNA staining reagent, can enter cells with incomplete and destroyed membranes, which presents red color under epi-fluorescent microscope. As shown in Fig. 4d, the number of PI-stained bacteria is remarkably increased after filtrated under 4 V voltage comparing with 0 V. The result proves that electroporation has occurred with the help of tips structure of carbon nanofibrils in wood carbon under electric filed.
|
Download:
|
| Fig. 4. Mechanism analysis of electric field disinfection of 3D Ag NPs/WC membrane. (a) SEM image of carbon nanofibrils in wood carbon membrane. (b) Schematic of electroporation disinfection caused by tips structure of carbon nanofibrils in wood carbon cannels. (c) SEM image showing pores formed on E. coli surfaces after filtration under 4 V. (d) Both bright field and fluorescent microscope images of PI-stained E. coli samples after filtration under voltages of 0 and 4 V with. (e) Schematic representation of the electrostatic interaction between bacteria and Ag NPs under electric filed. (f) Fluorescent microscope images of gene constructed E. coli in wood carbon microchannels under the voltage of 0 and 4 V during operation. | |
Due to the electrostatic interaction, the bacteria, which carry negative or positive charges [38], will deflect and attach to the surface of the wood carbon channels under electric field, where the carbon nanofibrils and Ag nanoparticles are uniformly distributed. Excepting porous and low tortuous microchannels of wood carbon, the electrostatic interaction between the bacteria and the surface of the wood carbon channel will further increase the contacting property between bacteria, Ag nanoparticles as well as carbon nanofibrils in wood carbon channels, which facilitate the excellent inactivation performance of 3D Ag NPs/WC membrane (Fig. 4e). The green fluorescent protein (GFP) gene constructed E. coli was used to prove electrostatic attraction between the bacteria and the 3D Ag NPs/WC membrane. As shown in Fig. 4f, strongly green fluorescent can be found on the surface of wood carbon channels in the presence of the voltage of 4 V, comparing with 0 V. The result proved that E. coli can adhere to the surface under the electric field. Ag nanoparticles release Ag+ ions which could easily enter the bacteria with destroyed cell membrane and react with inner cellular components to realize efficient disinfection.
A home-made electric field disinfection setup was designed. As shown in Fig. 5a, a piece of Ag NPs/WC membrane is fixed in a Teflon filter. The voltage is added along the radical direction of wood (red: positive electrode; black: negative electrode), which is adjusted by a direct current (DC) power source from 0 to 5 V. The flux is controlled at 3.8 × 103 L m2 h−1 by a syringe pump. The electric field disinfection occurs as water flowing through Ag NPs/WC membrane. The detail information of the setup is shown in Figs. S11 and S12 (Supporting information). Besides E. coli, we also investigated the electric field disinfection performance of the prepared membrane for other bacteria, such as E. faecalis, B. subtilis and S. Typhimurium (~107 CFU/mL). E. faecalis and B. subtilis are gram-positive bacteria which equip with tougher cell walls and stronger mechanical resistance to cells than gram-negative bacteria [38]. The inactivation efficiencies of 3D Ag NPs/WC membrane for the two kinds of gram-positive bacteria are shown in Fig. 5b. The results exhibit that the disinfection performances of our membrane for the two kinds of bacteria are increased with voltage enhancing and reach 5 log at the voltage of 4 V. Similarly, the disinfection efficiency toward S. Typhimurium (Gram-negative) has also reached 5 log under the 4 V input voltage. The result demonstrates that Ag NPs/WC membrane shows highly efficient inactivation performance for different kinds of bacteria, which can kill more than 99.999% bacteria in water. Furthermore, the energy consumption of the electric field disinfection is calculated to ~2 J for treating 1 L of water with a voltage of 4 V and flux of 3.8 × 103 L m2 h−1 (The detail information was shown in the part of experiment). The disinfection energy consumption of our membrane is much lower than carbon nanotubes (CNTs) filter (~300 J/L), UV irradiation (~1000 J/L), as well as other assisted disinfection technologies reported, previously (Fig. 5c) [8, 16, 42-45].
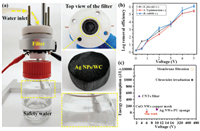
|
Download:
|
| Fig. 5. Home-made water disinfection devices and the inactivation performance for different model of bacteria. (a) Photo image of experimental setup for the electric field disinfection. (b) Inactivation efficiency of 3D Ag NPs/WC membrane for different kinds of bacteria in water. (c) Comparison of the energy consumption of the 3D Ag NPs/WC membrane with recently reported cell inactivation methods. | |
In summary, we have recommended a simple, scalable, stable, and low-energy consumption 3D Ag NPs/WC membrane for rapid and highly efficient water disinfection treatment. An electric field disinfection filter was designed which could achieve excellent cell disinfection performance (over 5 log removal indication no live microbe detection) with low applied voltage (4 V) and the high flux of 3.8 × 103 L m−2 h−1. The designed membrane also exhibits outstanding properties of low energy consumption (~2 J/L) and stability. The 3D Ag NPs/WC membrane can be easily controlled by batteries or solar cells and shows broad application in the field of drinking water disinfection.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe work was supported by the National Natural Science Foundation of China (No. 21876072); the Talent Innovation and Entrepreneurship Project of Lanzhou (No. 2018-RC-04); Special Fund Project for the Central Government to Guide Local Science and Technology Development (2020) and the 111 Project (No. B20027). We would like to thank Dr. Jiayu Wan for the English improvement, Dr. Mingwei Zhu for the SEM test, Dr. Zonglei Zhang for the ICP-MS measurement. We thank the Electron Microscopy Centre of Lanzhou University for the microscopy and microanalysis of our samples.
Appendix A. Supplementary dataSupplementarymaterial related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.02.044.
| [1] |
N. Thapar, I.R. Sanderson, Lancet 363 (2004) 641-653. DOI:10.1016/S0140-6736(04)15599-2 |
| [2] |
X.Q. Li, X.Z. Min, J.L. Li, et al., Joule 2 (2018) 2477-2484. DOI:10.1016/j.joule.2018.08.008 |
| [3] |
WHO Progress on Drinking Water, Sanitation and Hygiene Geneva, Switzerland, 2017.
|
| [4] |
M. Santosham, A. Chandran, et al., Lancet 376 (2010) 63-67. DOI:10.1016/S0140-6736(10)60356-X |
| [5] |
X. Michalet, F.F. Pinaud, L.A. Bentolila, et al., Science 307 (2005) 538-544. DOI:10.1126/science.1104274 |
| [6] |
X.F. Li, W.A. Mitch, Environ. Sci. Technol. 52 (2018) 1681-1689. DOI:10.1021/acs.est.7b05440 |
| [7] |
S.W. Krasner, H.S. Weinberg, et al., Environ. Sci. Technol. 40 (2006) 7175-7185. DOI:10.1021/es060353j |
| [8] |
A.O. Dotson, C.E. Rodriguez, K.G. Linden, J. Am. Water Works Ass. 104 (2012) 318-324. DOI:10.5942/jawwa.2012.104.0075 |
| [9] |
B.E. Logan, M. Elimelech, Nature 488 (2012) 313-319. DOI:10.1038/nature11477 |
| [10] |
V. Heinz, I. Alvarez, A. Angersbach, D. Knorr, Trends Food Sci. Technol. 12 (2001) 103-111. DOI:10.1016/S0924-2244(01)00064-4 |
| [11] |
T.Y. Li, Y.M. Chen, et al., J. Am. Chem. Soc. 132 (2010) 2500-2501. DOI:10.1021/ja908821d |
| [12] |
X.T. Lv, X. Zhang, et al., Water Res. 125 (2017) 162-169. DOI:10.1016/j.watres.2017.08.043 |
| [13] |
S.P. Singh, Y.L. Li, et al., ACS Appl. Mater. Interfaces 9 (2017) 18238-18247. DOI:10.1021/acsami.7b04863 |
| [14] |
K.G. McGuigan, R.M. Conroy, et al., J. Hazard. Mater. 235 (2012) 29-46. |
| [15] |
J.J. Wen, X.J. Tan, et al., Environ. Sci. Technol. 51 (2017) 6395-6403. DOI:10.1021/acs.est.6b06290 |
| [16] |
Z. Huo, Y. Luo, et al., Environ. Sci. Nano 4 (2017) 2010-2017. DOI:10.1039/C7EN00558J |
| [17] |
Z.Y. Huo, X. Xie, et al., Environ. Sci. Technol. 50 (2016) 7641-7676. DOI:10.1021/acs.est.6b01050 |
| [18] |
P.Y. Furlan, A.J. Fisher, et al., J. Chem. Educ. 94 (2017) 488-493. DOI:10.1021/acs.jchemed.6b00692 |
| [19] |
F. Jiang, T. Li, et al., Adv. Mater. 30 (2018) 1703453. DOI:10.1002/adma.201703453 |
| [20] |
R.A. Parham, R.L. Gray, Adv. Chem. Ser. 207 (1984) 1-56. |
| [21] |
M.W. Zhu, Y.J. Li, et al., Adv. Mater. 29 (2017) 1704107. DOI:10.1002/adma.201704107 |
| [22] |
J.W. Song, C.J. Chen, et al., ACS Appl. Mater. Interfaces 9 (2017) 23520-23527. DOI:10.1021/acsami.7b06529 |
| [23] |
Z.-Y. Wu, P. Yin, et al., Research 2019 (2019) 1-11. |
| [24] |
A. Reiterer, H. Lichtenegger, S. Tschegg, P. Fratzl, Philos. Mag. A 79 (1999) 2173-2184. DOI:10.1080/01418619908210415 |
| [25] |
J.Y. Wan, J.W. Song, et al., Adv. Mater. 29 (2017) 1703331. DOI:10.1002/adma.201703331 |
| [26] |
N. Xu, X.Z. Hu, et al., Adv. Mater. 29 (2017) 1606762. DOI:10.1002/adma.201606762 |
| [27] |
M.W. Zhu, J.W. Song, et al., Adv. Mater. 28 (2016) 5181-5187. DOI:10.1002/adma.201600427 |
| [28] |
H.L. Zhu, W. Luo, et al., Chem. Rev. 116 (2016) 9305-9374. DOI:10.1021/acs.chemrev.6b00225 |
| [29] |
S.C. Li, B.C. Hu, et al., Angew. Chem. Int. Ed. 57 (2018) 7085-7090. DOI:10.1002/anie.201802753 |
| [30] |
J.R. Morones, J.L. Elechiguerra, et al., Nanotechnology 16 (2005) 2346-2353. DOI:10.1088/0957-4484/16/10/059 |
| [31] |
C.Y. Zhu, J.F. Xue, J.H. He, J. Nano Sci. Nanotechnol. 9 (2009) 3067-3074. DOI:10.1166/jnn.2009.212 |
| [32] |
J.R. Barnett, V.A. Bonham, Biol. Rev. 79 (2004) 461-472. |
| [33] |
B.P.V. Nellore, R. Kanchanapally, et al., ACS Appl. Mater. Interfaces 7 (2015) 19210-19218. DOI:10.1021/acsami.5b05012 |
| [34] |
F.J. Chen, A.S. Gong, et al., ACS Nano 11 (2017) 4275-4282. DOI:10.1021/acsnano.7b01350 |
| [35] |
H. Zhang, X.B. Xu, et al., Nanoscale 9 (2017) 13334-13340. DOI:10.1039/C7NR03906A |
| [36] |
A.M. Fayaz, K. Balaji, et al., Nanomed. Nanotechnol. 6 (2010) 103-109. DOI:10.1016/j.nano.2009.04.006 |
| [37] |
Y. Yang, Y. Yang, et al., Nat. Commun. 8 (2017) 1559. DOI:10.1038/s41467-017-00850-8 |
| [38] |
H. Nikaido, T. Nakae, Adv. Microbial Physiol. 20 (1979) 163-250. |
| [39] |
B.D. Shepard, M.S. Giltnore, Methods Mol. Biol. 47 (1995) 217-226. |
| [40] |
W.Y. Zhao, B. Ni, Langmuir 33 (2017) 8070-8075. DOI:10.1021/acs.langmuir.7b01274 |
| [41] |
WHO, Safe Drinking-Water from Desalination, 2011, https://www.who.int/water_sanitation_health/publications/gdwq4-1st-addendum/en/.
|
| [42] |
A. Joss, H. Siegrist, et al., Water Sci. Technol. 57 (2008) 251-255. DOI:10.2166/wst.2008.825 |
| [43] |
C. Liu, X. Xie, et al., Nano Lett. 13 (2013) 4288-4293. DOI:10.1021/nl402053z |
| [44] |
C. Liu, X. Xie, et al., Nano Lett. 14 (2014) 5603-5608. DOI:10.1021/nl5020958 |
| [45] |
M.R. Liu, A. Kakade, et al., Sci. Total Environ. 659 (2019) 540-547. DOI:10.1016/j.scitotenv.2018.12.406 |
 2021, Vol. 32
2021, Vol. 32 

