b University of Science and Technology of China, Hefei 230026, China;
c Department of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun 130024, China
In nature, most marine mollusks, such as jellyfishes known to exist in a hydrogel state, have excellent dynamic control over color of bioluminescence for mutual communication, camouflage and even reproduction [1, 2]. The switchable bioluminescence phenomena have inspired scientists to integrate color-shifting functionality in gel system, resulting in tunable fluorescent polymeric gels [3-5]. Popular synthetic strategy is to introduce fluorogens as building blocks into the hydrogel matrix, including fluorescent proteins [6], organic fluorophores [7, 8], lanthanide complexes [9, 10] and luminescent nanoparticles [11, 12]. Among these hydrogels, lanthanide-doped ones are widely explored towards the design of smart stimuli-responsive advanced functional materials due to their unique dynamic metal coordination-controlled photoluminescence [13-15]. For example, Chen et al. [4] have fabricated light-emitting metallogels based on lanthanide metal-ligand coordination, where color can be switched in the presence of acid and base for real-time monitoring.
Among luminescence materials, white-light-emitting (WLE) materials performing versatile tunability of fluorescence to stimuli have drawn great attention for the applications in sensors and luminescent devices [16]. In general, WLE are composed of the three primary (red, green and blue) colors at the entire visible wavelength [17]. Recently, lanthanide ions Eu3+ and Tb3+ serving as photoluminescent emitters have been introduced into gel with red and green emission, respectively. Wang et al. [18] doped lanthanide ions (Eu3+, Tb3+, Eu3+/Tb3+) into alginate/PAAm polymer networks to obtain tunable fluorescence colors (red, green, yellow) hydrogels. So far, lanthanide complexes doped Eu3+ and Tb3+ can realize multiple emission from red to green color emission. However, few lanthanide ions introduced in gel can realize blue characteristic emission to easily balance combination for white light emission. Most reported lanthanide-based WLE gels need to introduce another complex blue chromophore, for example, Tang et al. [19] combined another blue (or cyan) chromophore (aggregation-induced emission block copolymer network) with lanthanide-doped polymer network to prepare a WLE ion gel. In our previous work, we have shown that the Zn-enaminitrile coordination yielded a blue color emission [20]. Here, we envision doping simply metal ions (Zn2+, Eu3+, Tb3+) into the gel matrix to realize the white light emission, overcoming the deficiency of lanthanide for full-color emission.
Given stimuli-responsive materials, lanthanide complexes are sensitive to stimuli owing to dynamic noncovalent coordination [21-23]. Nonetheless, most of the current lanthanide-doped supramolecular hydrogels have poor mechanical strength [24-26]. So far, the preparation of lanthanide-doped fluorescent hydrogels with stimuli-responsiveness property and high strength character remains challenging [27, 28]. We sought to design a new hydrogel model induced WLE through metal-coordination interaction, which together with hydrogen-bonding and ionic interaction can form the interpenetrating network (IPN) dual-polymer gel system to overcome the mechanical strength limitation.
Herein, we employ PMAA and PANMAM with enaminitrile receptors as a matrix dual-polymer network. Through doping with lanthanide ions (Eu3+ and Tb3+) and zinc ion, robust interpenetrating gel network can be formed through the multiple-interactions, e.g., hydrogen bonding, ionic interaction and metal ion coordination (Scheme 1). White light emission can be realized by tuning ions stoichiometry. In addition, with the distinct metal-ligand coordination mechanism, the WLE hydrogels possess reversible stimuli-responsive properties, leading to potential applications in information display and security.

|
Download:
|
| Scheme 1. Schematic Illustration for the preparation process and network architecture of tunable fluorescent gel. | |
Our synthesis of PANMAM started with the substitution reaction of (E)-3-ethoxy-2-(pyridin-2-yl)acrylonitrile with N-boc-ethylenediamine under Ar. After deprotection and purification, enaminitrile molecules PAN-1 (1H NMR, Fig. S1 in Supporting information) with the primary amine groups (-NH2) were subsequently grafted onto an active poly(N-methacryloxysuccinimide) (PNMAS) via the quantitative amidation reaction. Both PNMAS and PMAA were prepared by free radical polymerization initiated by azobisisobutyronitrile (AIBN). PANMAM and PMAA can form cross-linked gel network (Fig. S2 in Supporting information) through the inter- and intramolecular hydrogen bond. The chemical structure of the utilized polymers and the blending procedure are outlined in Scheme 1. The molecular weight as determined by gel permeation chromatography (GPC) are 395 kDa and 482 kDa for PANMAM and PMAA, respectively (Fig. S3 in Supporting information).
The preparation process of the polymeric gel with tunable emission is presented in Scheme 1. Firstly, the solutions of PANMAM and PMAA-Zn2+/Eu3+/Tb3+ were mixed under stirring to give an integrated composite through the multiple-interactions between PANMAM and PMAA-Zn2+/Eu3+/Tb3+. After sedimentation and centrifugation, the precipitates were collected and naturally evaporated to form the gel networks.
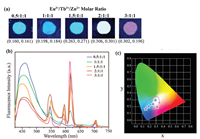
Given that the blue, red or green responses under UV irradiation (350 nm) were observed after doping Zn2+, Eu3+ or Tb3+, respectively (Fig. S4 in Supporting information), we perform the investigation in tuning the emission color of hydrogels by varying the stoichiometry of the three metal ions. Variation of the molar ratio of three ions led to a series of multicolor fluorescent hydrogels under UV irradiation (Fig. 1a). The detailed composition of each gel is presented in Table S1 (Supporting information). The fluorescence emission spectrum (Fig. 1b) indicated that the red emission intensity at 618 nm increased at the loss of the blue emission intensity at 460 nm as a function of the molar ratio of Eu3+: Tb3+: Zn2+. Furthermore, the Commission International de L'Eclairage (CIE) coordinates (Fig. 1c) demonstrates that the fluorescence colors of hydrogels changed from cyan to white to magenta in accordance with photographs in Fig. 1a. All the gels are transparent under daylight (Fig. S5 in Supporting information). Particularly, the gel exhibits an intense white-light emission (CIE coordinates (0.306, 0.301)) at the Eu3+: Tb3+: Zn2+ molar ratio of 2:1:1. The straightforward color control of gels offers a simple strategy to broad-spectrum emission polymer materials.
|
Download:
|
| Fig. 1. Fluorescence tuning: (a) Photographs of the hydrogels with different ion molar ratio under UV irradiation; (b) emission spectra of the hydrogels (λex = 350 nm) and (c) the corresponding change in the CIE coordinates. | |
The FT-IR spectra of PMAA, PANMAM and WLE gel have shown in Fig. S6 (Supporting information). Pristine PANMAM has a typical stretching vibration band of C≡N at 2195 cm−1, which is in line with that in WLE gel occurring at 2199 cm−1. Moreover, two characteristic bands appear at 1693 and 1667 cm−1 corresponding to the stretching vibration modes of the C = O ester carbonyl group and C = O amide carbonyl group from pristine PMAA and PANMAM. After the formation of WLE gel, the C = O stretching vibration band shifted to 1644 cm−1. We attribute to the presence of metal-ligand coordination, ionic interaction, and hydrogen-bonding interaction with the C = O functionality.
Next the microstructure of WLE gel was investigated through cryo-scanning electronic microscopy (Cryo-SEM). As shown in Fig. S7 (Supporting information), a typical porous network structure can be observed in the WLE gel. Photostability is one of the key properties in view of the practicability. We examined the photostability of WLE gel at ambient air. There is no significant change in the fluorescence emission spectrum after one week of storage (Fig. S8 in Supporting information), indicating the good photostability.
Mechanical property as a key parameter for polymeric gels, is of scientific significance in practical applications. High-strength gels can adapt to various complex environments. Thus, we tested the storage modulus (G′) and the loss modulus (G′′) of WLE gel and single-component (PANMAM) gel over a wide frequency range. As shown in Fig. S9 (Supporting information), both WLE gel and PANMAM gel exhibit constantly higher G′ value than G′′ value, behaving as quasi-solid states, corroborating the formation of hybrid gel and PANMAM gel [29]. It is noteworthy that WLE gel has a modulus value (G′ and G′′) between 104 Pa and 106 Pa, which is about two orders of magnitude higher than PANMAM gel, indicating the stronger hydrogen-bonding/coordination interaction [30].
To further study the mechanical properties of WLE gel, we tested the tensile performance of WLE gel. Meanwhile, tensile strength for single-component (PANMAM) gel was also tested for comparison. The tensile breaking stress σb is 0.53 MPa, and the Young's modulus E is 0.12 MPa for WLE gel. Noteworthy, WLE gel is stretchable, reaching a tensile breaking strain εb of 133%. In contrast, single-component (PANMAM) gel has low tensile mechanical strength and toughness (0.26 MPa and 72%, respectively), which is demonstrated in Fig. 2. Apparently, the hybrid gel (WLE gel) exhibits much higher mechanical strength as examined by dynamic rheology and tensile tests. The mechanical properties of the as prepared hybrid gel are superior to most of luminescent supramolecular hydrogels [19, 31], which facilitates its practical application.

|
Download:
|
| Fig. 2. Mechanical properties: (a) Tensile stress-strain curves and (b) corresponding mechanical parameters. | |
In general, WLE gel materials with the character of multiple emission will lead to versatile tunability of luminescent colors in responsiveness to environmental stimulation. Successively, we sought to probe the change of luminescent color when subjected to external stimuli. Under acidic condition, WLE gel underwent a rapid white to blue fluorescent color transition (inset of Fig. S10 in Supporting information). It is evident as shown in Fig. S10 that the disappearance/significant decrease of the red emission peak at 618 nm and green emission peak at 550 nm is caused by the treatment of acid, leaving a blue color band at 460 nm (Fig. S10). We attribute this stimuli-responsive property to the different ion responsive mechanisms between zinc and lanthanide ions. The ligands in the polymer network coordinate with lanthanide ions like an "antenna", which always absorb and transfer energy to lanthanide ions. However, the interaction is easily destroyed by acid, quenching the characteristic emission of lanthanide [32-34]. On the contrary, the zinc ion coordination induced blue-light-emission is evidently unaffected when subject to acid or base [20]. Subsequently, the addition of base to neutralize the acid triggers the recovery of the white light emission (Fig. S10), indicating the reversible acidity/alkalinity stimuli-responsive nature of the WLE gel. Notably, we tested the reversible chromisms of WLE gel using simultaneously aqueous HCl or trifluoroacetic acid (TFA) vapor as acid stimulus and aqueous NaOH or triethylamine (TEA) vapor as base stimulus. Similar results were obtained, suggesting the potential applications of WLE gel in solution and volatile detection.
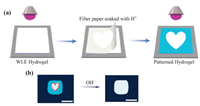
On the basis of the above established stimuli-responsive luminescent behavior, we attempted to investigate the patternability of WLE gel for its application in information display and security. The ion transfer printing strategy [35, 36] was further developed to produce patterns upon WLE gel. As depicted in Fig. 3a, cellulose filter paper was soaked with H+ solution and placed onto the surface of WLE gel for 10 s. The H+ ions spontaneously diffused from cellulose filter paper to the coverage of WLE gel matrix, leading to a quick transition from white to blue emission. After the paper being peeled off, the pattern was prepared as expected. The processed WLE gel is transparent and no pattern can be observed with naked eyes under daylight. Pattern is displayed under UV irradiation due to the distinct contrast of fluorescent colors. For example, we used heart-shaped filter paper to fabricate fluorescence patterning on WLE gel via the method as described above (Fig. 3). Outline of heart-shaped pattern is clear (red dotted line in Fig. 3), showing the local tunability and patternability of WLE gel. Interestingly, the fluorescent pattern can be facilely erased by OH− ions (or TEA vapor, Fig. S11 in Supporting information) because of the reversible acidity/alkalinity stimuli-responsive nature of WLE gel. These results demonstrate that the patterned WLE gel can be used in message passing and information security.
|
Download:
|
| Fig. 3. (a) Illustration of the ion transfer printing strategy to produce fluorescence patterning on WLE gel. (b) Photograph of heart-shaped pattern hydrogel under UV irradiation. Scale bar = 1 cm. | |
In summary, we have proposed a model design for the fabrication of multicolor fluorescent hydrogels. The fluorescence tuning is enabled by a simple modulation of metal ion stoichiometry (Zn2+, Eu3+, Tb3+) to multi-spectrum fluorescence emission of hydrogels. Taking the advantages of hydrogen-bonding, ionic and coordination interaction, the prepared WLE gel turns out to possess favorable mechanical properties. Furthermore, we have demonstrated the highly reversible acidity/alkalinity stimuli-responsive behavior of WLE gel when it is exposed to solution or vapor stimuli. Finally, by combining the stimuli-responsive property and the locally tunable fluorescent behavior, we have successfully developed patterned hydrogels, which might be ideal materials for information security. The established strategy may provide new insights for the design of multicolor fluorescent materials, and further widen the path for advanced functional fluorescent materials.
Declaration of competing interestThe authors report no competing interest.
AcknowledgmentsThe financial support of this work by the National Natural Science Foundation of China (No. 51973026) and the Jilin Provincial Education Departments (No. JJKH20201169KJ) is greatly acknowledged.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.059.
| [1] |
E.A. Widder, Science 328 (2010) 704-708. DOI:10.1126/science.1174269 |
| [2] |
S.A. Morin, R.F. Shepherd, S.W. Kwok, et al., Science 337 (2012) 828-832. DOI:10.1126/science.1222149 |
| [3] |
H. Wei, N. Shi, J. Zhang, et al., Chem. Commun. 50 (2014) 9333-9335. DOI:10.1039/C4CC04000G |
| [4] |
W. Lu, S. Wei, H. S, et al., Aggregate 2 (2021) e37. |
| [5] |
Y. Yao, Y. Wang, Z. Li, H. Li, Langmuir 31 (2015) 12736-12741. DOI:10.1021/acs.langmuir.5b03102 |
| [6] |
M.D. Weber, L. Niklaus, M. Proeschel, et al., Adv. Mater. 27 (2015) 5493-5498. DOI:10.1002/adma.201502349 |
| [7] |
P. Wei, Z. Li, J.X. Zhang, et al., Chem. Mater. 31 (2019) 1092-1100. DOI:10.1021/acs.chemmater.8b04909 |
| [8] |
X. Ji, Z. Li, X. Liu, et al., Adv. Mater. 31 (2019) 1902365-1902372. DOI:10.1002/adma.201902365 |
| [9] |
W. Lu, C. Ma, D. Zhang, et al., J. Phys. Chem. C 122 (2018) 9499-9506. |
| [10] |
K. Meng, C. Yao, Q. Ma, et al., Adv. Sci. 6 (2019) 1802112. DOI:10.1002/advs.201802112 |
| [11] |
Q. Li, Y. Zhang, C. Wang, D.A. Weitz, S. Chen, Adv. Mater. 30 (2018). |
| [12] |
S. Wei, Z. Li, W. Lu, et al., Angew. Chem. 132 (2020) 2-19. DOI:10.1002/ange.201914768 |
| [13] |
K. Binnemans, Chem. Rev. 109 (2009) 4283-4374. |
| [14] |
D. Ananias, F.A.A. Paz, D.S. Yufit, L.D. Carlos, J. Rocha, J. Am, Chem. Soc. 46 (2015) 3051-3058. |
| [15] |
P. Falcaro, P.D.S. Furukawa, Angew. Chem. Int. Ed. 51 (2012) 8431-8433. DOI:10.1002/anie.201203719 |
| [16] |
V.K. Praveen, C. Ranjith, N. Armaroli, Angew. Chem. Int. Ed. 53 (2014) 365-368. DOI:10.1002/anie.201306787 |
| [17] |
G. He, D. Guo, C. He, X. Zhang, X. Zhao, Angew. Chem. 121 (2009) 6248-6251. DOI:10.1002/ange.200901266 |
| [18] |
F. Zhou, Z.G. Xu, J.H. Suo, et al., Macromol. Rapid Commun. 36 (2015) 465-471. DOI:10.1002/marc.201400630 |
| [19] |
Z. Tang, X. Lyu, L. Luo, Z. Shen, X.H. Fan, ACS Appl. Mater. Interfaces 12 (2020) 45420-45428. DOI:10.1021/acsami.0c15656 |
| [20] |
S. Chen, T. Sun, Z. Xie, D. Dong, N. Zhang, Polym. Chem. 11 (2020) 6650-6657. DOI:10.1039/D0PY01054E |
| [21] |
Y. Wang, S. Liu, Y. Yang, et al., ACS Appl. Mater. Interfaces 7 (2015) 4415-4422. DOI:10.1021/am5089346 |
| [22] |
X. Chen, Y. Wang, R. Chai, Y. Xu, B. Liu, ACS Appl. Mater. Interfaces 9 (2017) 13554-13563. DOI:10.1021/acsami.7b02679 |
| [23] |
P.P. Neelakandan, M. Hariharan, D.A. Ramaiah, J. Am. Chem. Soc. 128 (2006) 11334-11335. DOI:10.1021/ja062651m |
| [24] |
S.K. Samanta, S. Bhattacharya, J. Mater. Chem. 22 (2012) 25277-25287. DOI:10.1039/c2jm35012b |
| [25] |
C. Vijayakumar, V.K. Praveen, A. Ajayaghosh, Adv. Mater. 21 (2010) 2059-2063. |
| [26] |
G. Weng, S. Thanneeru, J. He, Adv. Mater. 30 (2018) 1706526. DOI:10.1002/adma.201706526 |
| [27] |
M. Martínez-Calvo, O. Kotova, M.E. Möbius, et al., J. Am. Chem. Soc. 137 (2015) 1983-1992. DOI:10.1021/ja511799n |
| [28] |
Z. Li, G. Wang, Y. Wang, H. Li, Angew. Chem. Int. Ed. 57 (2018) 2194-2198. DOI:10.1002/anie.201712670 |
| [29] |
Y. Xiong, Z. Chen, H. Wang, et al., Chem. Commun. 52 (2016) 14157-14160. DOI:10.1039/C6CC08513J |
| [30] |
X. Yu, Z. Wang, Y. Li, et al., Inorg. Chem. 56 (2017) 7512-7518. DOI:10.1021/acs.inorgchem.7b01031 |
| [31] |
S. Bhattacharya, S.K. Samanta, Chemistry 18 (2012) 16632-16641. DOI:10.1002/chem.201201940 |
| [32] |
M. Kawa, J.M. Fréchet, Chem. Mater. 10 (1998) 286-296. DOI:10.1021/cm970441q |
| [33] |
N. Sabbatini, M. Guardigli, Guardigli, J.M. Guardigli, Coord. Chem. Rev. 123 (1993) 201-228. DOI:10.1016/0010-8545(93)85056-A |
| [34] |
Z. Liu, W. He, Z. Guo, Chem. Soc. Rev. 42 (2013) 1568-1600. DOI:10.1039/c2cs35363f |
| [35] |
P. Li, D. Zhang, Y. Zhang, et al., ACS Macro Lett. 8 (2019) 937-942. DOI:10.1021/acsmacrolett.9b00337 |
| [36] |
X. Le, H. Shang, H. Yan, et al., Angew. Chem. Int. Ed. 60 (2021) 3640-3646. DOI:10.1002/anie.202011645 |
 2021, Vol. 32
2021, Vol. 32 

