b Key Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China;
c CAS Key Laboratory of Cryogenics, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China;
d University of Chinese Academy of Sciences, Beijing 100049, China
Organisms can obtain energy from carbohydrates by glycolysis pathway. Glucose or glycogen undergoes a process similar to fermentation in tissues to form lactic acid or pyruvate. The energy is released to form adenine nucleoside triphosphate (ATP) for tissue utilization [1-3]. Pyruvate kinase (PK) is an important rate limiting enzyme in glycolysis and a key enzyme in abnormal glucose metabolism in tumor cells [4-7]. PK has four isozymes: L, R, M1, M2 (PKL, PKR, PKM1, PKM2). PKM2 is expressed in embryonic tissues, stem cells and tumor cells, which plays an important role in the metabolism and growth of tumor cells [8, 9]. It has been proved that when the cells become cancerous, the expression of PKL, PKR and PKM1 isozymes decreases under the action of the oncogene kinase codon [10]. Meanwhile, the serine and tyrosine sites in PKM2 are phosphorylated. PKM2 is overexpressed in tumor cells. PKM2 occupies a dominant position in tumor cells [11]. Most malignant tumor cells produce ATP through glycolysis even in the presence of sufficient oxygen. Tumor cells show abnormally high glycolysis rate to maintain energy dynamic balance, because the efficiency of ATP production by glycolysis is lower than that of oxidative phosphorylation. This phenomenon is called Warburg effect [7, 12-15]. Warburg effect is necessary for the growth and reproduction of tumor cells. Therefore, PKM2 is an important tumor initiation and development factor, which can affect the occurrence and development of cancer through glycolysis and other ways. Inhibition of PKM2 activity helps to inhibit the growth of tumor cells.
It is well known that metals can change the function of enzymes [16-18]. Some metal ions can effectively inhibit the activity of PKM2. For example, Adalto Bianchini found that copper ions could inhibit PKM2 activity and thus inhibit glycolysis pathway of the killifish Poecilia vivipara [19]. Masataka Yoshino investigated the inhibitory effect of zinc ion on PKM2 and proposed that zinc ion inhibition of PKM2 would reduce glycolysis pathway [20]. However, it is risky to use metal ions as glycolysis inhibitors in vivo. This is because such energy metabolism is a common physiological process of cells, which also exists in normal cells. If metal ions are used as an inhibitor, normal cells will also be damaged, which will bring strong side effects. In fact, one of the important factors leading to zinc cytotoxicity is the inhibition of zinc ions on energy metabolism, especially glycolysis [20]. Therefore, the design of PKM2 inhibitor containing metal ions is more urgent.
Metal Organic Framework (MOF) is a kind of porous materials with periodic three-dimensional network structure, which is formed by self-assembly hybridization of metal ions and organic ligands [21-25]. The nanoscale MOFs have adjustable size. The metal ions in MOFs are surrounded by ligands. It can be transported to tumor site more by passive targeting of nanoscale structure [26]. In this paper, we first found that the nanoscale MOFs containing zinc ions (ZIF-8) had a strong inhibitory effect on PKM2. Moreover, the inhibition ability of ZIF-8 with small size was higher than that of ZIF-8 with large size. ZIF-8 had stronger PKM2 inhibition effect than zinc ion due to the large specific surface area and porosity of MOFs. The 50% inhibiting concentration (IC50) was only one percent of zinc ion. Therefore, ZIF-8 is a novel PKM2 inhibitor, which is expected to be used as a glycolysis inhibitor in cancer therapy.
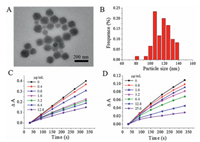
The ZIF-8 used as an inhibitor was synthesized by a room-temperature colloidal chemistry route. The detailed experimental method can be found in Supporting information. The zinc nitrate and 2-methyl imidazole (2-MI) were mixed evenly, and the ZIF-8 was obtained after the mixture standing for 24 h. The ZIF-8 synthesized in this way was called S-ZIF-8. The TEM image of S-ZIF-8 was shown in Fig. 1A. The S-ZIF-8 was a typical polyhedron structure. The maximum population was estimated to be 116 nm according to the size distribution of S-ZIF-8 (Fig. 1B). The XRD patterns of S-ZIF-8 were shown in Fig. S1 (Supporting information). It indicated that S-ZIF-8 was highly crystalline, which demonstrated the formation of ZIF-8 according to the data of the crystal structure [27].
|
Download:
|
| Fig. 1. (A) TEM images and (B) diameter histogram of the S-ZIF-8. The time-dependent ΔA changes of PKM2 (C) and LDH (D) activity detection system with the absence or presence of different concentration of S-ZIF-8. | |
The inhibition of ZIF-8 on PKM2 was investigated by detecting PKM2 activity. The PKM2 catalyzes Phospho(enol) pyruvic acid monopotassium salt (PEP) and Adenosine 5′-diphosphate (ADP) to produce ATP and pyruvate. The Lactate dehydrogenase (LDH) further catalyzes β-Nicotinamide adenine dinucleotide disodium salt (NADH) and pyruvate to produce lactic acid and NAD+. The activity of PKM2 can be reflected by measuring the decrease rate of NADH at 340 nm [28]. This method involves two steps of enzyme reaction. The reaction equation is as follows:
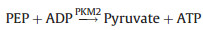
|

|
Because NADH is consumed in the reaction process, the difference (ΔA=A0 − A) between the 340 nm absorption at initial (A0) and different reaction time (A) was used to investigate the reaction rate of PKM2 enzyme. Fig. 1C showed the curves of without or with different amounts of S-ZIF-8 in the enzyme reaction. The relationship between ΔA and reaction time was basically linear without inhibitor. When S-ZIF-8 was added into the enzyme reaction system, the increment of ΔA with time became slower. The slowing down trend was more obvious with the increase of S-ZIF-8 amount. This indicated that S-ZIF-8 could inhibit the PKM2 enzyme reaction. The effects of S-ZIF-8 on LDH activity have also been studied as the measurement of PKM2 activity using an LDH-coupled enzyme reaction. As shown in Fig. 1D, the increment of ΔA with time also became slower when the amount of S-ZIF-8 was increased. So, S-ZIF-8 could also inhibit LDH enzyme. IC50 of inhibitors is an important index of inhibitor performance. The lower the value of IC50, the stronger the ability of inhibitor. The IC50s of S-ZIF-8 to PKM2 and LDH were 3.5 and 7.8 μg/mL, respectively. Therefore, the S-ZIF-8 could inhibit PKM2 enzyme.
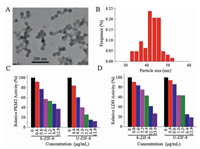
The effect of ZIF-8 synthesized by another method on PKM2 enzyme reaction was studied. The mixture of zinc nitrate and 2-methylimidazole were kept ultrasonic treatment for 40 min. The ZIF-8 synthesized in this way was called U-ZIF-8. U-ZIF-8 had been characterized using TEM (Fig. 2A), and the size distribution of U-ZIF-8 was 42 nm (Fig. 2B), which was much smaller than that of S-ZIF-8.
|
Download:
|
| Fig. 2. (A) TEM images and (B) diameter histogram of the U-ZIF-8. Comparison of S-ZIF-8 and U-ZIF-8 on inhibiting the activity of PKM2 (C) and LDH (D). | |
Further studies showed that U-ZIF-8 also had the ability to inhibit PKM2 activity. With the increase of U-ZIF-8 amount in the enzyme reaction system, the activity of PKM2 decreased step by step (Fig. 2C). It was worth noting that the activity of PKM2 decreased faster with U-ZIF-8 than S-ZIF-8, indicating that U-ZIF-8 had stronger ability to inhibit PKM2. For example, when system containing 3.2 μg/mL ZIF-8, the activity of PKM2 was only 26% of that without nanomaterials. While the activity of PKM2 remained 53% in same amount of S-ZIF-8. The IC50 of U-ZIF-8 on PKM2 was 1.5 μg/mL, less than half of that of S-ZIF-8 (3.5 μg/mL). U-ZIF-8 also showed inhibitory effect on LDH reaction (Fig. 2D). The IC50 of LDH inhibition was about 3.3 μg/mL, also less than half of S-ZIF-8 (7.8 μg/mL). At the same time, the IC50 of U-ZIF-8 on PKM2 was lower than that on LDH, indicating that U-ZIF-8 had inhibitory effect on PKM2.
The mechanism of ZIF-8 inhibiting PKM2 was studied. Firstly, the effected of 2-MI and zinc acetate on the PKM2 enzyme reaction were investigated. As shown in Fig. 3A, the activity of PKM2 was not inhibited when high concentrations of 2-MI (100 or 200 μg/mL) were added, but increased. This may be due to the hydrophobic property of 2-MI, which was beneficial to the unfolding of the PKM2 enzyme molecular. Therefore, 2-MI had no inhibitory effect on PKM2. After adding zinc ions (zinc acetate), PKM2 showed a significant inhibition (Fig. 3B). With the increase of zinc ion concentration, PKM2 activity continued to decrease. Therefore, zinc ions can inhibit PKM2. This is consistent with previous literature [20]. The IC50 of zinc ion on PKM2 was about 150 μg/mL, which was much higher than that of ZIF-8. This may be due to the confinement effect of MOFs. Previous studies have reported that the inhibition of PKM2 by zinc ion is mainly due to the interaction between zinc ion and ADP [20]. The ZnADP complex can be used as competitive or non-competitive enzyme inhibitors. MOFs are organic-inorganic hybrid materials with intramolecular pores formed by self-assembly of organic ligands and metal ions through coordination bonds. It has a large specific surface area and can adsorb various materials from small molecules to large proteins [29, 30]. ADP could be retained in the pores of ZIF-8, which made it easier to form complexes with zinc ions, thus inhibiting the activity of PKM2 (Fig. 3C). The results also showed that U-ZIF-8 had stronger inhibitory effect on PKM2 than S-ZIF-8. Different from the usual assumption, it was not because the U-ZIF-8 has larger specific surface area than S-ZIF-8 due to their smaller size. In fact, the specific surface area of U-ZIF-8 and S-ZIF-8 were 1405 and 1446 m2/g, respectively. The specific surface area of both ZIF-8 was similar because ZIF-8 was porous. The difference of PKM2 inhibition ability of ZIF-8 may be due to the longer pores. When ADP entered the larger size S-ZIF-8, it would affect the subsequent ADP to continue to compound with zinc ions. So, the effective zinc ions compounded with ADP in S-ZIF-8 was less than that in U-ZIF-8 with same weight. The IC50 of U-ZIF-8 was less than that of S-ZIF-8, and it has better inhibition ability. Therefore, ZIF-8 has a high ability to inhibit PKM2 due to its special structure. The IC50 was decreased by 1–2 orders of magnitude compared with that of zinc ion.

|
Download:
|
| Fig. 3. 2-MI (A) or zinc acetate (B) inhibited the activity of PKM2. (C) Scheme of ZIF-8 inhibited the activity of PKM2. | |
In this paper, we investigated the inhibitory effect of ZIF-8 on PKM2, a key enzyme in glycolysis pathway. ZIF-8 had a higher inhibitory effect on PKM2 than zinc ion. IC50 of ZIF-8 can be reduced by nearly two orders of magnitude compared with that of zinc ion. Moreover, the inhibition ability of small size U-ZIF-8 was better than that of large size S-ZIF-8. Therefore, ZIF-8 is expected to become a novel PKM2 inhibitor and multifunctional drug carrier for tumor therapy.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe acknowledge financial support from the National Natural Science Foundation of China (Nos. 61975214, U20A20335, 81630053) and Beijing Natural Science Foundation (Nos. 4202075, 2202057, 7212208).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.081.
| [1] |
N. Hay, Nat. Rev. Cancer 16 (2016) 635-649. DOI:10.1038/nrc.2016.77 |
| [2] |
Y. Wu, J. Wen, H. Li, S. Sun, Y. Xu, Chin. Chem. Lett. 28 (2017) 1916-1924. DOI:10.1016/j.cclet.2017.09.032 |
| [3] |
M. Liu, X. Ren, X. Liu, et al., Chin. Chem. Lett. 31 (2020) 3117-3120. DOI:10.1016/j.cclet.2020.06.024 |
| [4] |
J. Chen, Z. Jiang, B. Wang, Y. Wang, X. Hu, Cancer Lett. 316 (2012) 204-210. DOI:10.1016/j.canlet.2011.10.039 |
| [5] |
S. Masaki, K. Hashimoto, D. Kihara, et al., Biochem. Biophys. Res. Commun. 526 (2020) 973-977. DOI:10.1016/j.bbrc.2020.03.182 |
| [6] |
K. Taniguchi, N. Sugito, M. Kumazaki, et al., Cancer Lett. 363 (2015) 17-27. DOI:10.1016/j.canlet.2015.03.026 |
| [7] |
M.G.V. Heiden, L.C. Cantley, C.B. Thompson, Science 324 (2009) 1029-1033. DOI:10.1126/science.1160809 |
| [8] |
J. Chen, J. Xie, Z. Jiang, et al., Oncogene 30 (2011) 4297-4306. DOI:10.1038/onc.2011.137 |
| [9] |
W.J. Israelsen, M.G.V. Heiden, Semin. Cell Dev. Biol. 43 (2015) 43-51. DOI:10.1016/j.semcdb.2015.08.004 |
| [10] |
X. Chen, S. Chen, D. Yu, Cancer Cell Int. 20 (2020) 523. DOI:10.1109/itoec49072.2020.9141700 |
| [11] |
S. Amin, P. Yang, Z. Li, Biochim. Biophys. Acta Rev. Cancer 1871 (2019) 331-341. DOI:10.1016/j.bbcan.2019.02.003 |
| [12] |
M. Zhou, Y. Zhao, Y. Ding, et al., Mol. Cancer 9 (2010) 33. |
| [13] |
W.H. Koppenol, P.L. Bounds, C.V. Dang, Nat. Rev. Cancer 11 (2011) 325-337. DOI:10.1038/nrc3038 |
| [14] |
M. Upadhyay, J. Samal, M. Kandpal, O.V. Singh, P. Vivekanandan, Pharmacol. Ther. 137 (2013) 318-330. DOI:10.1016/j.pharmthera.2012.11.003 |
| [15] |
H.R. Christofk, M.G.V. Heiden, M.H. Harris, et al., Nature 452 (2008) 230-233. DOI:10.1038/nature06734 |
| [16] |
Y.D. Zebral, Jda.S. Fonseca, M. Roza, et al., Chemosphere 253 (2020) 126631. DOI:10.1016/j.chemosphere.2020.126631 |
| [17] |
Jda.S. Fonseca, Y.D. Zebral, A. Bianchini, Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 240 (2021) 108924. DOI:10.1016/j.cbpc.2020.108924 |
| [18] |
L. Canesi, M. Betti, C. Ciacci, G. Gallo, Gen. Comp. Endocrinol. 122 (2001) 60-66. DOI:10.1006/gcen.2001.7612 |
| [19] |
I.S.A. Anni, Y.D. Zebral, S.B. Afonso, et al., Chemosphere 227 (2019) 580-588. DOI:10.1016/j.chemosphere.2019.04.080 |
| [20] |
K. Murakami, M. Yoshino, Biometals 30 (2017) 335-340. DOI:10.1007/s10534-017-0009-y |
| [21] |
S. Li, L. Tan, X. Meng, Adv. Funct. Mater. 30 (2020) 1908924. DOI:10.1002/adfm.201908924 |
| [22] |
Y. Wang, W. Wu, J. Liu, et al., ACS Nano 13 (2019) 6879-6890. DOI:10.1021/acsnano.9b01665 |
| [23] |
W.H. Chen, M. Vázquez-González, A. Zoabi, R. Abu-Reziq, I. Willner, Nat. Catal. 1 (2018) 689-695. DOI:10.1038/s41929-018-0117-2 |
| [24] |
Y. Wang, L. Yan, K. Dastafkan, et al., Adv. Mater. (2021) 2006351. DOI:10.1002/adma.202006351 |
| [25] |
Y. Zou, W. Zhang, H. Zhou, et al., Chin. Chem. Lett. 30 (2019) 481-484. DOI:10.1016/j.cclet.2018.06.016 |
| [26] |
Y. Ding, H. Xu, C. Xu, et al., Adv. Sci. 7 (2020) 2001060. DOI:10.1002/advs.202001060 |
| [27] |
L. Su, Q. Wu, L. Tan, et al., ACS Appl. Mater. Interfaces 11 (2019) 10520-10531. DOI:10.1021/acsami.8b22177 |
| [28] |
S. Shan, J. Shi, P. Yang, et al., J. Agric. Food Chem. 65 (2017) 8136-8144. DOI:10.1021/acs.jafc.7b02757 |
| [29] |
W. Guo, Z. Chen, L. Tan, et al., Chem. Comm. 56 (2020) 3919-3922. DOI:10.1039/c9cc09712k |
| [30] |
H. Li, X. Lu, Q. Lu, et al., Chem. Comm. 56 (2020) 4724-4727. DOI:10.1039/d0cc00748j |
 2021, Vol. 32
2021, Vol. 32 

