b Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy, Xuzhou Medical University, Xuzhou 221004, China;
c School of Life Sciences, Northwestern Polytechnical University, Xi'an 710072, China
Aminopeptidase N (APN, EC 3.4.11.2), known as alanine aminopeptidase CD13 (a zinc-dependent metalloproteinase), is recognized as an exopeptidase that preferentially cleaves N-terminal amino acids from polypeptides with the release of alanine [1]. As an important biological enzyme, APN has been revealed in mammals with various physiological functions [2]. Especially, APN has been explored the close association with tumor invasion, metastasis, and even angiogenesis, which is expected to be a biomarker for tumor diagnosis [3]. Porcine aminopeptidase N was also revealed the related receptor for porcine deltacoronavirus infection [4]. Even, the inhibitors of APN were payed more and more attentions as anti-cancer agents [5-7]. Besides the mammals, APN has been recently revealed in various microorganisms, e.g., Escherichia coli, Streptomyces lividans, which should be adequate to account for the major intracellular peptide turnover capability [8, 9]. As now, the expression of APN in the most important dairy propionibacteria, i.e., Propionibacterium jensenii and Propionibacterium freudenreichii has been confirmed, which played relevant roles in peptide substrate utilization [10]. Thus, the bacterial APN is not only involved in the intracellular peptide metabolism, but also performs an important role in the exogenous peptide metabolism, such as the metabolic function of intestinal bacteria.
Benefit from the advantages of fluorescence technology [11-18], more and more attentions have been paid for the enzyme activity measurement by fluorescence-based detection technique [19-26]. Especially, fluorescent probes and bioluminescent probes were prepared to perform in vitro and in vivo tracking the activity of APN, even APN determination of human urine [27-34]. However, few optical probes have been established for detecting the activity of APN in microorganism. Especially, the identification of microorganism with active APN desired an efficient method, besides of metabolite detection and DNA sequence. Using fluorescent probe as the substrate of APN, the bacterial colonies possessing fluorescence signal indicated active APN, which was measured conveniently as well as protein specially. Therefore, the development of fluorescent molecular tool that can be used to detect APN activity in microorganism has been in high demand.
In the present work, a near-infrared (NIR) fluorescent probe (DDAA) was designed on the basis of 7-hydroxy-9H-(1, 3-dichloro-9, 9-dimethylacridin-2-one (DDAO) and recognition group alanine by the linkage of p-aminophenethyl alcohol. As a well-known NIR fluorophore, DDAO displayed favorable intramolecular charge transfer (ICT) property for probe design, deduced from the phenolic group for turning the electrondonating capability [21, 35-38]. Therefore, the DDAO derivatives showed distinct fluorescence signal by removing the substituted group. The fluorescent probe DDAA was not only used for APN activity assay, but also guided the identification of intestinal microorganism with active APN. As a result, two bacteria strains and one fungus strain were obtained as the target microorganism from human feces, which should be involved in peptide metabolism in human gut.
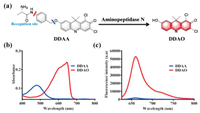
Aminopeptidase N displayed hydrolase activity for the hydrolysis of peptide, with alanine as the key active amino acid. On the basis of the hydrolase activity of APN, alanine was attached to the NIR fluorophore DDAO by the linkage of p-aminophenethyl alcohol. With alanine as the recognition site, the designed fluorescent probe DDAA could be hydrolyzed by APN, and then spontaneously yielded NIR fluorophore DDAO (Fig. 1a). Additionally, HPLC-DAD has been performed to analyze APN enzymatic solution. With DDAA and DDAO as the references, DDAO was observed in enzymatic solution, which confirmed the hydrolysis of DDAA mediated by APN (Fig. S1 in Supporting information). As can be seen in Figs. 1b and c, due to the mechanism of intramolecular charge transfer, DDAA and DDAO are significantly different in absorption and fluorescence spectra. Thus, DDAA can be used as a NIR fluorescent probe for the activity detection of endogenous APN.
|
Download:
|
| Fig. 1. (a) Illustration for the enzymatic hydrolysis of DDAA mediated by APN. (b) Absorbance of DDAA and DDAO. (c) Fluorescence spectra of DDAA and DDAO (λex 600 nm). | |
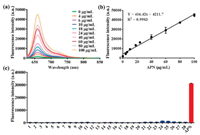
The fluorescence response of DDAA toward APN at different concentrations (0-100 μg/mL) was investigated, with the observation of a sequence of fluorescence spectra (Fig. 2a). A good linear relationship (R2 = 0.9903) was obtained between the fluorescence intensity at 660 nm and APN concentrations (Fig. 2b). Furthermore, the interferences of various species on the fluorescence intensity of DDAA have been investigated in presence of amino acids, metal ions, and biological enzymes. Compared with the APN, weak fluorescence intensity was detected for the co-incubations between DDAA and various species, which indicated good substrate specificity of DDAA toward APN (Fig. 2c). The fluorescence properties of DDAA and DDAO in PBS with different pH values were also evaluated, which suggested the fluorescence stability in PBS ranged from pH 6.0 to 9.0 (Fig. S2 in Supporting information). The enzymatic hydrolysis of DDAA by APN also suggested the optimal pH as 7.4 (Fig. S3 in Supporting information). Along with the good substrate specificity and stability, DDAA was expected to be a NIR fluorescent probe for APN activity determination.
|
Download:
|
| Fig. 2. (a) Fluorescence response of DDAA toward APN with different concentrations (0-100 μg/mL). (b) Linear relationship between fluorescence intensity and APN concentrations. (c) Interference of various species on the fluorescence intensity of APN (1. Ser, 2. Trp, 3. Tyr, 4. Glu, 5. Gly, 6. Arg, 7. Cys, 8. Lys, 9. Gln, 10. GSH, 11. Mn2+, 12. Ca2+, 13. Mg2+, 14. Ni2+, 15. Zn2+, 16. Sn4+, 17. Cu2+, 18. Fe3+, 19. Ba2+, 20. Cr6+, 21. PGP-1, 22. HSA, 23. BSA, 24. CES1c, 25. CES1b, 26. CES-2, 27. Lipase, 28. CYP3A4). | |
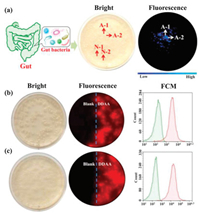
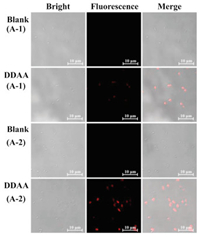
As above mentioned, bacteria from the human gut possessing bioactive APN played a key role in the peptide metabolism. The identification and isolation of target bacteria desired an efficient detected method. DDAA as a NIR fluorescent probe of APN was applied to guide the identification of intestinal bacteria with active APN. Human feces were distributed in saline and cultured in nutrient broth medium on agar plates. When the bacterial colonies were observed on the plates, DDAA was subjected on the colonies for a co-incubation of 4 h. Scanned by fluorescence imager, strong fluorescence signal was displayed for some bacterial colonies, which were picked for further purification (Fig. 3a). As a result, two bacteria were purified and identified to be Escherichia coli strain Y8-2 (A-1) and Shigella sp. CH-31 (A-2) by 16s rDNA sequence. Similarly, APN from Escherichia coli has been crystallized, which was determined to be a broad specificity zinc exopeptidase belonging to aminopeptidase clan MA, family M1 [39, 40]. Using DDAA as the staining, bacterial colonies (A-1, A-2) on agar plates were imaged successfully, indicated the protein expression of APN in these bacteria. Furthermore, flow cytometric analysis was also performed for the bacterial cells stained by DDAA, confirmed the bioapplications of DDAA (Figs. 3b and c). Then confocal laser microscope has been used to record the fluorescence images. When the endogenous APN was sensed by DDAA, the exact fluorescence images were performed for isolated bacterial cell, in comparison with the blank group (Fig. 4). To study the APN dependence of DDAA for bacteria imaging, two control bacteria strains Staphylococcus aureus ssp aureus DSM 20231T (N-1) and Staphylococcus aureus ssp aureus DSM 3463 (N-2) were cultured and stained by DDAA. As a result, no fluorescence signal was observed from agar plates, flow cytometric analysis, and laser confocal microscope (Figs. S4-S7 in Supporting information). Thus, DDAA as a NIR fluorescent probe has been applied to sense endogenous bacterial APN successfully. Additionally, using DDAA as a molecular tool, the target bacteria with active APN could be identified efficiently from mix sample, which was expected to be a useful method for investing the intestinal bacteria.
|
Download:
|
| Fig. 3. (a) Identification of intestinal bacteria with active APN from human feces stained by DDAA. Fluorescence imaging of (b) A-1 (E. coli strain Y8-2) and (c) A-2 (Shigella sp. CH-31) on agar plate together with flow cytometric analysis. | |
|
Download:
|
| Fig. 4. Confocal fluorescence images of bacteria A-1 (E. coli Y8-2) and A-2 (Shigella sp. CH-31) co-incubated with DDAA (50 μmol/L, λex 633/λem 645-690 nm, scale bar: 10 μm). | |
As known that plenty of fungi also existed in gut, APN existed in various intestinal fungi have attracted our interesting. The microbiota from human feces was cultured on martin broth modified medium (MTB) in the presence of antibiotics (penicillin/streptomycin). After the culture of 3 days, various saccharomyces was observed on the agar plate, which was stained by DDAA along with the co-incubation of 4 h. Scanned by fluorescence imager, strong fluorescence signal was recorded for several colonies, which guided the purification of target fungi (Fig. 5a). Finally, one strain of saccharomycete was isolated and identified as Trichosporon asteroids (F-1) by 18s rDNA sequence from these fluorescence colonies. For the isolated T. asteroids (F-1), DDAA was applied for the sensing of endogenous APN and imaging of fungi cells. Under fluorescence imager, strong fluorescence signal was observed for fungi colonies on agar plate (Fig. 5b). Confocal fluorescence imaging of fungi cells also showed fluorescence signal (Fig. 5c and Fig. S8 in Supporting information), then the staining ability of DDAA for fungi F-1 was evaluated by flow cytometric analysis (Fig. 5d), which confirmed the fluorescence of fungi cells in presence of DDAO. On the other hand, a negative saccharomycete strain Pichia occidentalis (NF-1) was cultured and co-incubated with DDAA. As a result, no fluorescence signals were recorded for P. occidentalis in presence of DDAA by fluorescence imager, laser confocal microscope, and flow cytometric analysis (Figs. S9-S11 in Supporting information). Above all, DDAA could be used to sense endogenous APN in fungi, which effectively guided the identification of target fungus with active APN from complex sample successfully.

|
Download:
|
| Fig. 5. (a) Identification of intestinal fungi with active APN from human feces stained by DDAA. (b) Fluorescence imaging of fungus F-1 (T. asteroids) on agar plate together (c) confocal fluorescence images and (d) flow cytometric analysis of F-1 co-incubated with DDAA. | |
APN as an endogenous hydrolase exists in microorganism, besides of generally known mammals. The intestinal microorganism with active APN plays a key role for the peptide metabolism, as well as the intracellular peptide turnover. In the present work, DDAA with alanine as the recognition moiety for APN was designed as a NIR fluorescent probe for APN sensing. Using DDAA, the endogenous APN could be sensed and microorganism could be imaged, which guide the identification of target intestinal microorganism from human feces. Finally, two strains of bacteria (E. coli strain Y8-2 and Shigella sp. CH-31) and one strain of saccharomycete (T. asteroids) were isolated and identified successfully, which were also imaged by DDAA to confirm the biological activity of APN. Thus, the present fluorescent probe of APN as a molecular tool is applied to find the positive gut microorganism for further investigation about intestinal peptide metabolism and intracellular peptide turnover of human gut microbiota.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was supported financially by National Natural Science Foundation of China (Nos. 81872970, 81930112), Dalian Science and Technology Leading Talents Project (No. 2019RD15), Liaoning Provincial Natural Science Foundation (Nos. 20180550761 and 2019-BS-056), and Liaoning Revitalization Talents Program (No. XLYC1907017).
Appendix A. Supplementary dataSupplementary material relatedtothisarticle canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.056.
| [1] |
Y. Luan, W. Xu, Curr. Med. Chem. 14 (2007) 639-647. DOI:10.2174/092986707780059571 |
| [2] |
L. Chen, Y.L. Lin, G.Q. Peng, Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 17966-17971. DOI:10.1073/pnas.1210123109 |
| [3] |
I. Shinya, M. Ryo, T. Rei, et al., Gen. Thoracic Cardiovasc. Surg. 57 (2009) 591-598. DOI:10.1007/s11748-009-0445-x |
| [4] |
X.Y. Zhu, S.D. Liu, X.L. Wang, et al., Emerg. Microbes Infect. 7 (2018) 65. |
| [5] |
C.H. Ma, J.Y. Cao, X.W. Liang, et al., Eur. J. Med. Chem. 108 (2016) 21-27. DOI:10.1016/j.ejmech.2015.11.021 |
| [6] |
S.A. Amin, N. Adhikari, T. Jha, J. Med. Chem. 61 (2018) 6468-6490. DOI:10.1021/acs.jmedchem.7b00782 |
| [7] |
J.L. Ge, Z.F. Wang, Y. Cheng, et al., Aging 12 (2020) 8523-8853. DOI:10.18632/aging.103155 |
| [8] |
M. Murgier, C. Pelissier, A. Laddunski, A. Bernadac, C. Lazdunski, Eur. J. Biochem. 74 (1977) 425-433. DOI:10.1111/j.1432-1033.1977.tb11408.x |
| [9] |
M.J. Butler, Handbook of Proteolytic Enzymes, 2nd ed., Elsevier Ltd., 2004, pp. 322-323.
|
| [10] |
F. Rossi, M. Busetto, S. Torriani, Antonie van Leeuwenhoek 91 (2007) 87-96. |
| [11] |
X. Li, J. Zheng, W. Liu, et al., Chin. Chem. Lett. 31 (2020) 2937-2940. DOI:10.1016/j.cclet.2020.05.043 |
| [12] |
L. Feng, Q.S. Yan, B.J. Zhang, et al., Chem. Commun. 55 (2019) 3548-3551. DOI:10.1039/C9CC00440H |
| [13] |
H. Li, Q. Yao, W. Sun, et al., J. Am. Chem. Soc. 142 (2020) 6381-6389. DOI:10.1021/jacs.0c01365 |
| [14] |
Y. Yang, T. Zhou, M. Jin, et al., J. Am. Chem. Soc. 142 (2020) 1614-1620. DOI:10.1021/jacs.9b12629 |
| [15] |
K. Wang, D. Xi, C. Liu, et al., Chin. Chem. Lett. 31 (2020) 2955-2959. DOI:10.1016/j.cclet.2020.03.064 |
| [16] |
D. Cheng, J. Peng, Y. Lv, et al., J. Am. Chem. Soc. 141 (2019) 6352-6361. DOI:10.1021/jacs.9b01374 |
| [17] |
J. Han, X. Yue, J. Wang, et al., Chin. Chem. Lett. 31 (2020) 1508-1510. DOI:10.1016/j.cclet.2020.01.029 |
| [18] |
J. Ning, W. Wang, G.B. Ge, et al., Angew. Chem. Int. Ed. 58 (2019) 9959-9963. DOI:10.1002/anie.201903683 |
| [19] |
Z. Tian, L. Feng, L. Li, et al., Sens. Actuators B: Chem. 310 (2020) 127872. DOI:10.1016/j.snb.2020.127872 |
| [20] |
P. Wang, H. Yang, C. Liu, et al., Chin. Chem. Lett. 32 (2021) 168-178. DOI:10.1016/j.cclet.2020.11.056 |
| [21] |
L. Feng, Y.L. Yang, X.K. Huo, et al., ACS Sens. 3 (2018) 1727-1734. DOI:10.1021/acssensors.8b00471 |
| [22] |
X. Li, W. Qiu, J. Li, et al., Chem. Sci. 11 (2020) 7292-7301. DOI:10.1039/D0SC01234C |
| [23] |
X.F. Wu, W. Shi, X.H. Li, H.M. Ma, Acc. Chem. Res. 52 (2019) 1892-1904. DOI:10.1021/acs.accounts.9b00214 |
| [24] |
J. Ning, T. Liu, P.P. Dong, et al., J. Am. Chem. Soc. 141 (2019) 13278-13278. DOI:10.1021/jacs.9b07996 |
| [25] |
A. Jiang, G. Chen, J. Xu, et al., Chem. Commun. 55 (2019) 11358-11361. DOI:10.1039/C9CC05759E |
| [26] |
Y. Wang, F. Yu, X. Luo, et al., Chem. Commun. 56 (2020) 4412-4415. DOI:10.1039/D0CC00297F |
| [27] |
H. Chen, H. Wang, X.J. Qin, et al., Chin. Chem. Lett. 26 (2015) 513-516. DOI:10.1016/j.cclet.2015.01.023 |
| [28] |
H.D. Li, Y.Q. Li, Q.C. Yao, et al., Chem. Sci. 10 (2019) 1619-1625. DOI:10.1039/C8SC04685A |
| [29] |
X.Y. He, Y.M. Hu, W. Shi, X.H. Li, H.M. Ma, Chem. Commun. 53 (2017) 9438. DOI:10.1039/C7CC05142E |
| [30] |
L. Chen, W. Sun, J. Li, et al., Org. Biomol. Chem. 11 (2013) 378-382. DOI:10.1039/C2OB26564H |
| [31] |
X.Y. He, Y.H. Xu, W. Shi, H.M. Ma, Anal. Chem. 89 (2017) 3217-3221. DOI:10.1021/acs.analchem.7b00021 |
| [32] |
L.Z. Chen, W. Sun, W.H. Li, et al., Anal. Methods 4 (2012) 2661-2663. DOI:10.1039/c2ay25556a |
| [33] |
J. Li, L.Z. Chen, W.X. Wu, et al., Anal. Chem. 86 (2014) 2747-2751. DOI:10.1021/ac404176x |
| [34] |
Z. Wu, Z. Zhen, J.H. Jiang, G.L. Shen, R.Q. Yu, J. Am. Chem. Soc. 131 (2009) 12325-12332. DOI:10.1021/ja9038054 |
| [35] |
Z.H. Tian, Q.S. Yan, L. Feng, et al., Chem. Commun. 55 (2019) 3951-3954. DOI:10.1039/C9CC01579E |
| [36] |
S.Y. Liu, H. Xiong, J.Q. Yang, et al., ACS Sens. 3 (2018) 2118-2128. DOI:10.1021/acssensors.8b00697 |
| [37] |
T. Duo, K. Robinson, I.R. Greig, et al., J. Am. Chem. Soc. 139 (2017) 15994-15999. DOI:10.1021/jacs.7b09645 |
| [38] |
Z. Wang, W.W. Wang, P.Z. Wang, et al., Anal. Chem. 93 (2021) 3035-3041. DOI:10.1021/acs.analchem.0c05118 |
| [39] |
K. Ito, Y. Nakajima, Y. Onohara, et al., J. Biol. Chem. 281 (2006) 33664-33676. DOI:10.1074/jbc.M605203200 |
| [40] |
M.C. Fournie-Zaluski, H. Poras, B.P. Roques, et al., Acta Crystallogr. D 65 (2009) 814-822. DOI:10.1107/S090744490901779X |
 2021, Vol. 32
2021, Vol. 32 

