Tumor-associated carbohydrate antigens (TACAs), which are overexpressed and abundant on various cancer cell surfaces, make effective molecular targets for the exploitation of therapeutic cancer vaccines or cancer immunotherapies [1]. However, they cannot solely elicit robust immune responses sufficient for effective cancer therapy due to a lack of immunogenicity and T cell independence [2]. Traditional methods for overcoming this deficit rely on the covalent coupling of carbohydrate antigens with a carrier protein to form conjugate vaccines, which not only increases immunogenicity but also switches them from T cell-independent to T cell-dependent antigens [3]. However, some inherent limitations of this strategy remain: (1) the carrier protein may competitively suppress the immune response to the carbohydrate antigen; (2) structures of TACA-protein conjugates are heterogeneous and ill-defined, making it is difficult to control their quality; (3) an external adjuvant must be used to make the carbohydrate-protein vaccines effective and this can cause some serious side effects [4].
Efforts to address these drawbacks have explored fully synthetic self-adjuvanting glycoconjugate vaccines [5-7]. In the development of functional conjugate cancer vaccines, an essential issue is the carrier molecule. In the past few decades, several vaccine carriers have been explored. Among them, lipid carrier-based glycoconjugate vaccines are anticipated as potentially useful due to their homogeneous and defined chemical structures, promising immunological activity and lack of immunosuppression. As a result, these vaccines have received considerable attention [8-11].
Lipid A, a natural agonist of toll-like receptor 4 (TLR4), is a potent immunomodulatory agent as an adjuvant for vaccine antigens [12]. However, pro-inflammatory and septic properties limit its medicinal application. To reduce its toxicity, a plethora of lipid A derivatives were designed and investigated. Among them, monophosphoryl lipid A (MPLA), a lipid A derivative with the anomeric phosphate removed, displays satisfactory immunological activity with minimal toxicity, and has been extensively used as a delivery carrier and adjuvant in human vaccine trials for several infectious disease as well as different types of cancer [13-15]. Inspired by these preliminary results of MPLA, a library of novel synthetic glycolipids with simpler configuration and improved activity, called aminoalkyl glucosaminide 4-phosphates (AGPs), were developed [16-18]. One representative example is RC-529 (also called Ribi.529), which could interact with TLR-4 to promote antigen presentation, T-helper activation and T cell mediate immune response. Extensive preclinical studies have proved that RC-529 is as effective as MPLA [19-21]. In addition, it has become a vaccine adjuvant used in humans in phase III clinical trials for hepatitis B and found to be potent and safe [17].
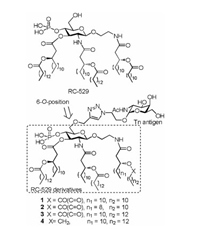
Although, RC-529 has had great success, to the best of our knowledge, it has not yet been used as a glycoconjugate vaccine carrier and self-adjuvant for tumor therapy. As part of our continuing efforts to develop a fully synthetic self-adjuvanting carbohydrate-based cancer vaccine [22, 23], RC-529 was fully synthesized and conjugated with Tn antigen [24], one of the most representative and promising epitopes for cancer vaccine development, to investigate its potential as a carrier for the fully synthetic self-adjuvanting glycoconjugate cancer vaccine. Moreover, three other RC-529 derivatives were also designed and synthesized to probe its structure-activity relationship (Fig. 1).
|
Download:
|
| Fig. 1. Structures of RC-529 and our designed Tn conjugates. | |
In the fully synthetic route for lipid carrier-based glycoconjugate vaccines, a key step is the coupling of carriers with carbohydrate antigens. The crystal structure of lipid A in complex to the TLR4-myeloid differentiation 2 (MD-2) complex indicates that the C6 position of lipid A is exposed [25], and thus could be used for the conjugation site. Moreover, previous studies on MPLA conjugate vaccines have shown that modification at this position did not compromise activity [18, 26]. Therefore, the 6-O-position of RC-529 derivatives was selected as the coupling site in our synthetic design for target glycoconjugates 1-4 (Fig. 1). In addition, the choice of linker is of critical importance to synthetic conjugate vaccines. 1, 2, 3-Triazole was selected as the linker due to its ignored influence to immunological properties [27].
Retrosynthetic analysis of the target conjugates is shown in Scheme 1. The key intermediates are benzyl-protected RC-529 and its derivatives equipped with a propargyl group at the 6-O-position in 5a-5d that can be selectively coupled with Tn using an azide group to form a conjugated 1, 2, 3-triazole through a click reaction; this has the advantages of high yield and mild reaction conditions. On the other hand, deprotection of 5a-5d provides free RC-529 derivatives that can be studied as independent vaccine adjuvants. 5a-5d can be obtained from the common substrate 6. The choice of protecting groups is key during preparation of the conjugate. In our synthetic route, the 2-N-position and the 3, 4-O-positions in 6 were protected with phthalimide, p-methoxybenzyl (PMP) and tert-butyldimethylsilyl (TBS), respectively, which can be regioselectively removed for the next conversion.

|
Download:
|
| Scheme 1. Retrosynthetic analysis of the target conjugates 1-4. | |
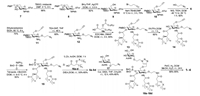
Our research started from the properly protected d-glucosamine derivative 7, prepared according to published procedure in five steps (Scheme 2) [28]. Initially, the 3-O-position was treated with TBSCl to generate the protected intermediate 8. Regioselective opening of the benzylidene ring was accomplished with BH3·THF and AgOTf under room temperature to expose the hydroxyl group at position 6. Compound 9 was then reacted with 2-propynyl bromide in the presence of sodium hydride and tetrabutylammonium bromide (TBAB) to insert a propargyl group for the preparation of a follow-up click reaction. Glycosylation of 10 with 2-azidoethanol was performed under the influence of N-iodosuccinimide (NIS) and trifluoromethanesulfonic acid (TfOH) at -40 ℃ to -30 ℃ to obtain 6 with 82% yield. The doublet signal of H-1 at 5.25 in the 1H NMR spectrum of the product with a coupling constant of 8.4 Hz confirmed β-conformation of the newly generated glycosidic linkage. Treatment of 6 with ethylenediamine in refluxing ethanol removed the phthalimide group at the 2-N-position to give 11. Subsequently, TBS at the 3-O-position of 11 was selectively deprotected by TEA·3HF to generate 12, containing a free amino and a hydroxyl group ready to couple with lipids. The chiral lipids 16a-16d (Scheme 2) were assembled using as published procedures [29] and commercially available (R)-epichlorohydrin as the raw material.
|
Download:
|
| Scheme 2. The synthesis of conjugates 1-4. | |
For lipid installation, the free amino and hydroxyl groups in 12 simultaneously reacted with fatty acid 16c using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI) and 4-dimethylaminopyridine (DMAP) as promoters, to produce 13 with 65% yield. Next, the PMB-protecting group in 13 was removed to afford intermediate 14, which was then phosphorylated at the 4-O-position through the phosphoramidite method in a two-step one-pot manner, delivering the key intermediate 15. This new compound has a singlet peak at δ -2.28 ppm in 31P NMR spectrum and a down-field shift of the H-4 signal in the 1H NMR spectrum, confirming the successful attachment of the phosphate group. Thereafter, the azide group in 15 was reduced to the primary amine by Zn powder and acetic acid, which was employed as a common intermediate for access to all four designed derivatives. Finally, lipids with different chain lengths (16a-16c) or linkages (16d) were installed to the free amino group delivering 5a-5d, which were derivatives of RC-529.
Next, the conjugates of RC-529 derivatives and Tn antigen were assembled. An azide group-equipped Tn derivative 17, which is the same conformation to natural Tn antigen expressed on cancer cell surfaces, was first prepared from N-acetyl-d-galactosamine according to the previously published process [30]. 5a-5d were smoothly coupled with 18 through a click reaction via the catalysis of cuprous iodide (CuI) with DIEA to generate the desired products with a 45%–60% yield. Ultimately, debenzylation of 18a-18d proceeded in the presence of 10% Pd/C under H2 atmosphere to gain the target conjugates 1-4, which were fully characterized using NMR and MS spectrometry.
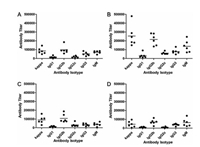
With the desired four conjugates in hand, we turned our attention to their immunological evaluation in female C57BL/6 mice (for details, see Supporting information). As depicted in Fig. 2, the high overall total antibody titers (kappa) of mouse antiserum elicited by target compounds 1-4 on day 38 indicate that each conjugate provoked a strong Tn-specific immune response in the mouse. Notably, high titers of IgG2b antibodies and IgG3 were observed in all four groups, suggesting a T cell-dependent immune response that is desirable for cancer immunotherapy. Higher levels of IgG2b and IgG3 antibodies than corresponding IgG1 levels suggest that a Th1-weighted immune response may be involved. Moreover, the group with conjugate 2 exhibited significantly higher average levels of kappa, IgG2b, IgG2c and IgM antibodies than mice with conjugates 1 and 3, suggesting that an RC-529 derivative with two shorter acyl chains at the 1-O-position may be more effective for immunostimulation. Differences between conjugates 3 and 4 also indicate that the linkage using ester may be more feasible than that using ether in the lipid.
|
Download:
|
| Fig. 2. The titers of total antibody and antibody isotypes in individual antiserum collected from mice immunized with conjugate 1 (A), 2 (B), 3 (C) and 4 (D) on day 38. Each dot represents the result of a mouse, and the horizontal bar represents the average titer of each group. | |
The ability of antisera derived from mice immunized with conjugate 1, 2, 3 or 4 to bind to target cancer cells was explored with fluorescence-activated cell sorting (FACS) technology. The human breast cancer cell line MCF-7, which over-expresses the Tn antigen, was employed in this study. MDA231 cancer cells, which do not express Tn, were used as a negative control. Distinct fluorescent peak shifts to the right were observed in MCF-7 cultured with anti-1, anti-2, anti-3 and anti-4 sera compared to pre-immune sera (Fig. 3A). In contrast, the fluorescent profiles of MDA231 cells treated with pre-immune sera and corresponding antisera did not differ (Fig. 3B). These results reveal that all antibodies induced by conjugates 1-4 could specifically recognize and bind to Tn-associated cancer cells, whereas cells that do not express Tn were unaffected. In addition, the median fluorescence intensity (MFI) of MCF-7 cells treated with antiserum show that the capacity of anti-2 serum binding to cancer cells was higher than anti-1 and anti-3 serum, while the binding capacity of anti-3 serum was higher than anti-4 serum. These results are consistent with the antibody titer analysis and provide further evidence supporting our conclusions.

|
Download:
|
| Fig. 3. FACS assay results of the binding between MCF-7 (A) or MDA231 (B) cancer cells and pre-immune sera, pooled day 38 antisera derived from mice immunized with conjugates 1, 2, 3 and 4, respectively. | |
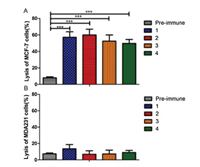
To further investigate the anticancer activities of these target glycoconjugate vaccines carried by RC-529 derivatives, antibody-mediated complement-dependent cytotoxicity (CDC) experiments were performed on the above two cancer cell lines. All four antisera mediated significant lysis of MCF-7 cells compared to the negative control (pre-immune sera, Fig. 4A). In contrast, in MDA231 cancer cells, no differences were observed between pre-immune sera and antisera under the same conditions (Fig. 4B). These results unambiguously demonstrate that all antisera induced by conjugates 1-4 could mediate effective and specific CDC to Tn-expressing tumor cells.
|
Download:
|
| Fig. 4. Results of antibody-mediated CDC to MCF-7 (A) and MDA231 (B) cells, which presented as cell lysis rates. Compared to the result of pre-immune sera, the difference is statistically significant (***P < 0.001). | |
In summary, a facile and efficient method has been established for the synthesis of RC-529 and its derivatives, which are suitable for effective and stable conjugation with carbohydrate antigens. This strategy should be generally appropriate for preparation of other AGP derivatives and their carbohydrate conjugates. Immunological evaluations in mice demonstrate that all four conjugates elicit strong and T cell-dependent immune responses without external adjuvants. All four antisera derived from mice immunized with conjugates of Tn and RC-529 derivatives could specifically recognize, bind to and kill Tn-overexpressing cancer cells while Tn-negative cancer cells are insusceptible. Therefore, RC-529 could be a useful platform for the development of new carbohydrate-based vaccine carriers with self-adjuvanting properties for the treatment of cancer. Moreover, our research suggests that RC-529 derivatives containing shorter acyl chains in the spacer arm exhibit more effective immunostimulatory activities. In addition, linkage of the chiral lipid in the spacer arm also affects the immunology of RC-529. These results provide convincing support that further optimization and additional investigation into RC-529 derivatives as self-adjuvanting cancer treatments are worthwhile.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 81773580, 82003594), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme to Guochao Liao (2019), the Department of education of Guangdong Province, China (No. 2020KZDZX1057), and the Science and Technology Planning Program of Guangzhou City, China (No. 202008040004).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.046.
| [1] |
D. Feng, A. Shaikh, F. Wang, ACS Chem. Biol. 11 (2016) 850-863. DOI:10.1021/acschembio.6b00084 |
| [2] |
M. Wei, Y. Wang, X. Ye, Med. Res. Rev. 38 (2018) 1003-1026. DOI:10.1002/med.21493 |
| [3] |
L.H. Jones, Nat. Chem. 7 (2015) 952-960. DOI:10.1038/nchem.2396 |
| [4] |
R. Astronomo, D. Burton, Nat. Rev. Drug Discov. 9 (2010) 308-324. DOI:10.1038/nrd3012 |
| [5] |
S. Ingale, M. Wolfert, J. Gaekwad, T. Buska, G. Boons, Nat. Chem. Biol. 3 (2007) 663-667. DOI:10.1038/nchembio.2007.25 |
| [6] |
B. Wilkinson, S. Day, L. Malins, V. Apostolopoulos, R. Payne, Angew. Chem. Int. Ed. 50 (2011) 1635-1639. DOI:10.1002/anie.201006115 |
| [7] |
A. Abdel-Aal, D. El-Naggar, M. Zaman, M. Batzloff, I. Toth, J. Med. Chem. 55 (2012) 6968-6974. DOI:10.1021/jm300822g |
| [8] |
W. Li, Y. Li, Chem. Rev. 120 (2020) 11420-11478. DOI:10.1021/acs.chemrev.9b00833 |
| [9] |
Z. Zhou, H. Lin, C. Li, Z. Wu, Chin. Chem. Lett. 29 (2018) 19-26. DOI:10.1016/j.cclet.2017.09.047 |
| [10] |
F. Liao, H. Wang, Y. Dao, et al., Chin. Chem. Lett. 32 (2021) 1575-1579. DOI:10.1016/j.cclet.2020.10.038 |
| [11] |
Z. Zhou, M. Mondal, G. Liao, Z. Guo, Org. Biomol. Chem. 12 (2014) 3238-3245. DOI:10.1039/C4OB00390J |
| [12] |
C. Alving, K. Peachman, M. Rao, S. Reed, Curr. Opin. Immunol. 24 (2012) 310-315. DOI:10.1016/j.coi.2012.03.008 |
| [13] |
V. Mata-Haro, C. Cekic, M. Martin, et al., Science 316 (2007) 1628-1632. DOI:10.1126/science.1138963 |
| [14] |
C. Casella, T. Mitchell, Cell. Mol. Life Sci. 65 (2008) 3231-3240. DOI:10.1007/s00018-008-8228-6 |
| [15] |
G. Liao, Z. Zhou, S. Suryawanshi, M. Mondal, Z. Guo, ACS Cent. Sci. 2 (2016) 210-218. DOI:10.1021/acscentsci.5b00364 |
| [16] |
D. Johnson, C. Sowell, C. Johnson, et al., Bioorg. Med. Chem. Lett. 9 (1999) 2273-2278. DOI:10.1016/S0960-894X(99)00374-1 |
| [17] |
J. Dupont, J. Altclas, A. Lepetic, et al., Vaccine 24 (2006) 7167-7174. DOI:10.1016/j.vaccine.2006.06.053 |
| [18] |
N. Reintjens, E. Tondini, A. Jong, et al., J. Med. Chem. 63 (2020) 11691-11706. DOI:10.1021/acs.jmedchem.0c00851 |
| [19] |
J. Evans, C. Cluff, D. Johnson, M. Lacy, D. HPersing, J. Baldridge, Expert Rev. Vaccines 2 (2003) 219-229. DOI:10.1586/14760584.2.2.219 |
| [20] |
L. Tiberio, L. Fletcher, J.H. Eldridge, D.D. Duncan, Vaccine 22 (2004) 1515-1523. DOI:10.1016/j.vaccine.2003.10.019 |
| [21] |
D. Johnson, Curr. Top. Med. Chem. 8 (2008) 64-79. DOI:10.2174/156802608783378882 |
| [22] |
Z. Zhou, G. Liao, S. Mandal, S. Suryawanshi, Z. Guo, Chem. Sci. 6 (2015) 7112-7121. DOI:10.1039/C5SC01402F |
| [23] |
L. Zeng, Z. Liao, W. Li, et al., Chin. Chem. Lett. 31 (2020) 1162-1164. DOI:10.1016/j.cclet.2019.10.015 |
| [24] |
C. Nativi, F. Papi, S. Roelens, Chem. Commun. (Camb.) 55 (2019) 7729-7736. DOI:10.1039/C9CC02920F |
| [25] |
B. Park, D. Song, H. Kim, et al., Nature 458 (2009) 1191-1195. DOI:10.1038/nature07830 |
| [26] |
L. Wang, S. Feng, S. Wang, et al., J. Org. Chem. 82 (2017) 12085-12096. DOI:10.1021/acs.joc.7b01817 |
| [27] |
Q. Wang, Z. Zhou, S. Tang, Z. Guo, ACS Chem. Biol. 7 (2012) 235-240. DOI:10.1021/cb200358r |
| [28] |
C. Wang, S. Luo, C. Shie, S. Hung, Org. Lett. 4 (2002) 847-849. DOI:10.1021/ol025567u |
| [29] |
Z. Jiang, M. Bach, W. Budzynski, et al., Bioorg. Med. Chem. Lett. 12 (2002) 2193-2196. DOI:10.1016/S0960-894X(02)00362-1 |
| [30] |
Q. Wang, S. Ekanayaka, J. Wu, J. Zhang, Z. Guo, Bioconjugate Chem. 19 (2008) 2060-2067. DOI:10.1021/bc800243f |
 2021, Vol. 32
2021, Vol. 32 

