b Institute of Molecular Aggregation Science of Tianjin University, Tianjin 300072, China;
c Joint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Fuzhou 350207, China
In recent years, the demand for multifunctional materials is increasing. Cocrystal engineering, a method of preparing new materials with unique optical and electrical properties by non-covalent bond interactions, has attracted much attention. For example, organic cocrystal can be applied to organic field effect transistor (OFET) [1-3], photoresponse [4-6], organic light-emitting transistor (OLET) [7], room temperature phosphorescence [8-10], two-photon absorption properties [11-13] and so on [14-16]. Recently, many excellent luminescent crystal materials have been reported to promote the practical application in single components [17], and cocrystal engineering for high-performance luminescent materials has also received great attention and still in its infancy stage [18-24]. However, selecting suitable molecules to prepare cocrystal with novel properties is still a great challenge.
The interactions between different molecules in cocrystal have a nonnegligible effect on the properties of cocrystal, supramolecular interactions in organic cocrystal include charge transfer interactions, π-π interactions, hydrogen bond and halogen bond interactions [25]. Among them, CT interactions are most often found in the cocrystal composed of strong electron-donating donor and easy electron-withdrawing acceptor molecules. By combining different donor and acceptor molecules, its performance can be effectively regulated [22, 26]. Exploring the interactions between molecules of the cocrystals is helpful to understand the relationship between structure and properties in cocrystal system. At present, the most common methods to prepare organic cocrystal include solution method [27, 28], physical vapor transport (PVT) method [29, 30] and mechanochemical preparation [31]. Various preparation methods make it more possible to prepare cocrystal with different morphologies, structures and properties. The solution method has the advantages of simple operation, flexible design and low cost. Here, we designed and prepared a new cocrystal, wherein the TCNB as electron acceptors (A), and phenazine as electron donors (D), shown in Figs. 1a. The surface electrostatic potentials (ESP) of TCNB and phenazine were calculated in order to confirm whether they were suitable for growth cocrystal (Figs. 1b and c). The red regions indicate negative potential, while the blue areas indicate positive potential. The two molecules exhibit complementary electrostatic potential energy, which makes it possible to recognize each other between donor and acceptor molecules, and realize supramolecular self-assembly. Meanwhile, the differences of crystal structure, intermolecular interactions and properties between cocrystal and single component crystals were demonstrated through a variety of spectral measurements and calculations.

|
Download:
|
| Fig. 1. (a) The optical images of TCNB, phenazine powder and PTC crystal with the molecular structures. ESP map of TCNB (b) and phenazine (c). | |
The TCNB-phenazine cocrystal (PTC) can be obtained by slowly volatilizing the acetone solution of TCNB and phenazine (same molar ratio) at room temperature. As shown in Fig. 1a, the TCNB and phenazine crystals are white and yellow, respectively, while the PTCs display slightly darker yellow comparing with their co-formers. Meanwhile, phenazine tends to grow into rod-like crystals in acetone solution and PTC is a large block crystal (Fig. S1 in Supporting information), which implies the successful realization of cocrystallization rather than simple mixing between TCNB and phenazine.
Through the analysis of single crystal X-ray diffraction, PTC is triclinic and belong to the space group P-1 with the cell parameters of a = 7.52Å, b = 7.78Å, c = 8.06Å, α = 74.43°, β = 82.81° and γ = 83.33° (Table S1 in Supporting information). As shown in Fig. 2a and c, the donor and acceptor molecules of PTC are stacked face to face, showing a mixed stacking structure [32]. The intermolecular D-A distance of PTC is 3.341Å (short contact), meaning that there may be CT nature in the cocrystal [33]. It is worth mentioning that the distance between two adjacent molecules in the π-π direction of PTC are shorter than that of donor molecular, while longer than that of acceptor (Fig. S2 in Supporting information). In the meantime, due to the abundant intermolecular -N…HC- interactions of cocrystal (A-A molecules -N…HC- distances of 2.510Å, D-A molecules -N…HC- distances of 2.702Å and 2.676Å) (Fig. 2b), the structure of PTC becomes more stabilization.

|
Download:
|
| Fig. 2. (a-c) Crystal structure of the PTC cocrystal. (d) The calculated growth morphology of PTC cocrystal. | |
On account of the supramolecular interactions in the two different directions (D-A and C-H…N), the cocrystal has three-dimensional block morphologies. The morphology prediction (Fig. 2d) based on Bravais Friedel Donnay Harker (BFDH) model is basically consistent with the experimental results. According to the area ratio of crystal planes (Table S2 in Supporting information), (100), (010) and (001) may be dominant in the formation of bulk crystals. PTC displays different peaks from the constitute components by analyzing the powder X-ray diffractometer (PXRD) pattern (Fig. 3a). And the measured PXRD result of PTC is basically consistent with the data calculated through CIF file.
|
Download:
|
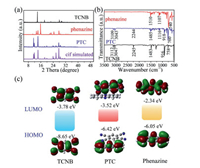
| Fig. 3. (a) PXRD, (b) FTIR and (c) the calculated energy level diagrams and molecular orbital diagrams of TCNB, phenazine and PTC. | |
The spectra of PTC were studied to determine its CT properties. In Fig. 3b, the Fourier transform infrared spectra (FTIR) spectrum of PTC is basically the combination of the TCNB and phenazine. The sharp peaks of cocrystal imply that PTC has excellent crystal quality. The shift of the PTC peak can be attributed to the various interactions between molecules in the cocrystal. For example, in PTC the TCNB 3112 cm-1 band (C-H str) is shifted to 3109 cm-1, 3047 cm-1 (C-H str) is shifted to 3043 cm-1, 2245 cm-1 (CN str) is shifted to 2244 cm-1 [34]. These peaks have different degrees of redshift, indicating that the electron cloud density of benzene ring on TCNB molecule has increased to a certain extent. At the same time, some of the corresponding phenazine peaks in the cocrystal have blue shift as well, which indicates that the CT interactions exist in PTC [35]. From the energy level diagram and molecular orbital diagram obtained by calculation (Fig. 3c), it can be demonstrated that electron clouds in highest occupied molecular orbital (HOMO) of the cocrystal are mainly concentrated on phenazine molecule. While the electron clouds in lowest unoccupied molecular orbital (LUMO) of PTC are concentrated on acceptor. The evident transfer of electron density between donor and acceptor molecules further indicates the existence of CT transition in the cocrystal. And the cocrystal has narrower band gap than the single components.
The interactions between donors and acceptors in PTC cocrystal are further analyzed by calculation. Through the calculation and analysis of the intermolecular forces in the system (Fig. S3 in Supporting information), it is found that the CT interactions between the donor and acceptor molecules are the strongest intermolecular interactions in the PTC cocrystal systems (-49.6 kJ/mol), and they are also stronger than the intermolecular interactions of co-formers. Hirshfeld surface analysis (Figs. 4a and b) and 2D fingerprint plots (Fig. 4c) were performed on PTC to better comprehend the difference of intermolecular interactions in the cocrystal and single components. The white, blue and red regions of Hirshfeld surface mean that the intermolecular distance is equal to, greater than and less than the van der Waals distance, respectively. The red areas on the surface of PTC (Figs. 4a and b) display the -N…HC- between TCNB and phenazine molecules. The 2D fingerprint plot (Fig. 4c) shows the abundant interactions of the cocrystal and their contribution proportion [36]. Fig. 4d is plotted according to the proportion of various interactions, and it can be clearly seen that the π-π interactions of PTC account for 18.0%, and N-H interactions account for the largest proportion (41.3%). At the same time, the Hirshfeld surfaces and fingerprint plots of the co-formers are analyzed (Fig. 4 and Fig. S4 in Supporting information), and it's found that the proportion of C-C interactions and hydrogen bond interactions can be significantly increased through cocrystallization. Furthermore, the outstanding CT nature of cocrystal can be attributed to the largely overlaid π-π stacking between the donor and acceptor molecules in cocrystal system. The changes of intermolecular interactions have a significant effect on the properties and stability of crystal.

|
Download:
|
| Fig. 4. (a, b) Hirshfeld surface (dnorm) of PTC in different directions. (c) Fingerprint plot of PTC. (d) The proportion of different type of intermolecular interactions in PTC. | |
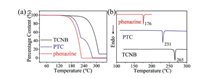
The thermal stabilities of the cocrystal and the single component were compared and analyzed. The smooth curves of thermogravimetric analysis (TGA) curve (Fig. 5a) show that there are no solvent molecules in PTC lattice. And the sublimation temperatures of TCNB and phenazine are 207 ℃ and 148 ℃, respectively. It can be seen that there are two gradients in the TGA curve of PTC. The phenazine component and TCNB component in the cocrystal correspond to the sublimation temperature of 165 ℃ and 229 ℃, respectively. When the temperature reaches 224.5 ℃, the phenazine molecules are completely lost, and the PTC remains 47.8% of the initial mass, which is almost the same as the actual weighted mass ratio of the acceptors and donors in the cocrystal [37, 38]. In general, the sublimation temperature of the single component in cocrystal is higher than that of their single crystals, which indicate that the interactions between molecules in the cocrystal are stronger than that in single component crystal. Differential scanning calorimetry (DSC) diagram demonstrate (Fig. 5b) that the melting points of PTC, TCNB and phenazine are 231 ℃, 265 ℃ and 176 ℃, respectively, meaning the cocrystal has new crystal lattice. And the high-melting-point indicates that PTC has stable structure.
|
Download:
|
| Fig. 5. (a) TGA and (b) DSC spectra of TCNB, phenazine and PTC. | |
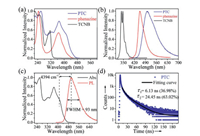
The spectra of PTC were studied to determine its photophysical properties. Fig. 6a exhibits the UV–vis absorption spectrum of PTC, it has a prominent red shift compared with the single component crystals TCNB and phenazine. This is mainly owing to the CT interactions in the cocrystal system [39]. In order to further study the photoluminescence (PL) properties of cocrystals, we have measured its PL spectra (Fig. 6b). The emission peak of PTC appears at 501 nm, and it has apparent red shift compared with single component, which can be attributed to CT nature [34]. Meanwhile, the full width at half-maximum (FWHM) and Stokes shift are 93 nm and 4394 cm-1 (Fig. 6c), respectively, and the Stokes shift of cocrystal is much larger than that of single components (Fig. S5 in Supporting information). The double exponential decay of τ1 = 6.13 ns (36.98%) and τ2 = 24.45 ns (63.02%) were obtained by time-resolved PL measurement (excitation: 375 nm and emission: 501 nm, Fig. 6d), and the fitting constant (χ2) was 1.1267. According to the formula: τ = (B1τ12 + B2τ22)/(B1τ1 + B2τ2), the τ of cocrystal is 17.67 ns (B1 = 518.1725, B2 = 221.4039), which is higher than that of single components (TCNB: 5.10 ns and phenazine: 7.88 ns) (Fig. S6 and Table S3 in Supporting information). In the meantime, the photoluminescence quantum yield (PLQY) of PTC (0.28%) is also different from TCNB (3.40%) and phenazine (0.03%). It's mean that the emission, PLQY and fluorescence lifetime of TCNB and phenazine can be effectively regulated through cocrystallization.
|
Download:
|
| Fig. 6. (a) The absorption and (b) fluorescence spectra of TCNB (black), phenazine (red) and PTC (blue). (c) Normalized absorption and PL spectra of PTC. (d) Time-resolved fluorescence measurements of PTC. | |
In conclusion, a new organic cocrystal of TCNB-phenazine has been obtained by molecular self-assembly. The complementary electrostatic potential energy of donor and acceptor molecules makes the cocrystals have excellent CT interactions. FTIR spectra, UV–vis absorption spectra, fluorescence spectra, DFT and Hirshfeld surfaces calculations show significant differences in stabilities and photophysical behaviors between cocrystal and single components. It provides some suggestions for the preparation of multifunctional cocrystal materials.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThe authors acknowledge financial support from the National Key R & D Program (No. 2017YFA0204503), the National Natural Science Foundation of China (Nos. 51733004, 21875158, 91833306, 51633006).
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.045.
| [1] |
J. Zhang, H. Geng, T.S. Virk, et al., Adv. Mater. 24 (2012) 2603-2607. DOI:10.1002/adma.201200578 |
| [2] |
S. Sato, H. Nikawa, S. Seki, et al., Angew. Chem. Int. Ed. 51 (2012) 1589-1591. DOI:10.1002/anie.201106912 |
| [3] |
T. Wakahara, P. D'Angelo, K. Miyazawa, et al., J. Am. Chem. Soc. 134 (2012) 7204-7206. DOI:10.1021/ja211951v |
| [4] |
Y. Wang, Y. Li, W.G. Zhu, et al., Nanoscale 8 (2016) 14920-14924. DOI:10.1039/C6NR05001H |
| [5] |
H.D. Wu, Y. Xiao, F.X. Wang, G.B. Pan, J. Nanosci. Nanotechnol. 14 (2014) 4097-4100. DOI:10.1166/jnn.2014.9037 |
| [6] |
S. Khan, Z.A. Alothman, M. Mohammad, et al., Polyhedron 180 (2020) 114454. DOI:10.1016/j.poly.2020.114454 |
| [7] |
S.K. Park, J.H. Kim, T. Ohto, et al., Adv. Mater. 29 (2017) 1701346. DOI:10.1002/adma.201701346 |
| [8] |
B. Zhou, D.P. Yan, Adv. Funct. Mater. 29 (2019) 1807599. DOI:10.1002/adfm.201807599 |
| [9] |
B. Zhou, Q.H. Zhao, L.C. Tang, D.P. Yan, Chem. Commun. 56 (2020) 7698-7701. DOI:10.1039/D0CC02730H |
| [10] |
Y.S. Wang, J. Yang, Y.X. Gong, et al., SmartMat 1 (2020) e1006. |
| [11] |
L.J. Sun, W.G. Zhu, W. Wang, et al., Angew. Chem. Int. Ed. 56 (2017) 7831-7835. DOI:10.1002/anie.201703439 |
| [12] |
C.H. Dai, Z.X. Wei, Z.Y. Chen, et al., Adv. Opt. Mater. 7 (2019) 1900838. DOI:10.1002/adom.201900838 |
| [13] |
Y. Wang, H. Wu, P.H. Li, et al., Nat. Commun. 11 (2020) 4633. DOI:10.1038/s41467-020-18431-7 |
| [14] |
G.F. Liu, J. Liu, X. Ye, et al., Angew. Chem. Int. Ed. 56 (2017) 198-202. DOI:10.1002/anie.201609667 |
| [15] |
B. Li, L. Cui, C.J. Li, Angew. Chem. Int. Ed. 59 (2020) 22012-22016. DOI:10.1002/anie.202010802 |
| [16] |
S.Z. Li, D.P. Yan, Adv. Opt. Mater. 6 (2018) 1800445. DOI:10.1002/adom.201800445 |
| [17] |
B. Lu, S.Y. Liu, D.P. Yan, Chin. Chem. Lett. 30 (2019) 1908-1922. DOI:10.1016/j.cclet.2019.09.012 |
| [18] |
Y.L. Lu, Y.Q. Tang, H.Y. Lin, et al., Chin. Chem. Lett. 29 (2018) 1541-1543. DOI:10.1016/j.cclet.2017.12.021 |
| [19] |
S.Z. Li, B. Lu, X.Y. Fang, D.P. Yan, Angew. Chem. Int. Ed. 59 (2020) 22623-22630. DOI:10.1002/anie.202009714 |
| [20] |
Y.L. Lei, Y. Jin, D.Y. Zhou, et al., Adv. Mater. 24 (2012) 5345-5351. DOI:10.1002/adma.201201493 |
| [21] |
C.F. Feng, S. Li, X.X. Xiao, et al., Adv. Opt. Mater. 7 (2019) 1900767. DOI:10.1002/adom.201900767 |
| [22] |
D.P. Yan, A. Delori, G.O. Lloyd, et al., Angew. Chem. Int. Ed. 50 (2011) 12483-12486. DOI:10.1002/anie.201106391 |
| [23] |
S. Oh, S.K. Park, B.H. Jhun, et al., J. Phys. Chem. C 124 (2020) 20377-20387. DOI:10.1021/acs.jpcc.0c04977 |
| [24] |
T. Ono, Y. Hisaeda, J. Mater. Chem. C 7 (2019) 2829-2842. DOI:10.1039/C8TC06165C |
| [25] |
L.J. Sun, Y. Wang, F.X. Yang, X.T. Zhang, W.P. Hu, Adv. Mater. 31 (2019) 1902328. DOI:10.1002/adma.201902328 |
| [26] |
J.L. Han, D. Yang, X. Jin, et al., Angew. Chem. Int. Ed. 58 (2019) 7013-7019. DOI:10.1002/anie.201902090 |
| [27] |
H. Jiang, P. Hu, J. Ye, et al., J. Mater. Chem. C 6 (2018) 1884-1902. DOI:10.1039/C7TC04982J |
| [28] |
K. Liu, Y.L. Lei, H.B. Fu, Chem. Mater. 32 (2020) 5162-5172. DOI:10.1021/acs.chemmater.0c01184 |
| [29] |
H.T. Black, D.F. Perepichka, Angew. Chem. Int. Ed. 53 (2014) 2138-2142. DOI:10.1002/anie.201310902 |
| [30] |
H. Jiang, C. Kloc, MRS Bull. 38 (2013) 28-33. DOI:10.1557/mrs.2012.308 |
| [31] |
Y.J. Huang, Q.Y. Gong, J. Ge, et al., ACS Nano 14 (2020) 15962-15972. DOI:10.1021/acsnano.0c07416 |
| [32] |
L.J. Sun, W.G. Zhu, F.X. Yang, et al., Phys. Chem. Chem. Phys. 20 (2018) 6009-6023. DOI:10.1039/C7CP07167A |
| [33] |
W.G. Zhu, R.H. Zheng, X.L. Fu, et al., Angew. Chem. Int. Ed. 54 (2015) 6785-6789. DOI:10.1002/anie.201501414 |
| [34] |
Y. Wang, W.G. Zhu, W.N. Du, et al., Angew. Chem. Int. Ed. 57 (2018) 3963-3967. DOI:10.1002/anie.201712949 |
| [35] |
L.J. Sun, W.J. Hua, Y. Liu, et al., Angew. Chem. Int. Ed. 58 (2019) 11311-11316. DOI:10.1002/anie.201904427 |
| [36] |
M.A. Spackman, J.J. McKinnon, CrystEngComm 4 (2002) 378-392. DOI:10.1039/B203191B |
| [37] |
R. Li, J.F. Li, Y.J. Sun, X.T. Zhang, W.P. Hu, ChemPlusChem 84 (2019) 1245-1248. DOI:10.1002/cplu.201900197 |
| [38] |
R. Usman, A. Khan, H. Sun, M. Wang, J. Solid State Chem. 266 (2018) 112-120. DOI:10.1016/j.jssc.2018.07.009 |
| [39] |
S. Tian, Z.M. Huang, J.H. Tan, et al., ACS Energy Lett. 5 (2020) 2698-2705. DOI:10.1021/acsenergylett.0c01466 |
 2021, Vol. 32
2021, Vol. 32 

