Enzymes are involved as efficient biocatalysts in many physiological processes and reactions in multicellular organisms [1]. Nonetheless, natural enzymes have several drawbacks that severely hinder their development and application; they are unstable (easily denatured), expensive, and extremely sensitive to the external conditions, which affect their catalytic activities [2-4]. Thus, artificial enzymes, especially combined with nanotechnology, that can maintain excellent catalytic activities and overcome these drawbacks have attracted considerable interest. Since Pasquato et al. introduced triazacyclononane-functionalized gold nanoparticles (NPs) with phosphoesterase-like activity in 2004, and Yan et al. reported Fe3O4 NPs with inherent peroxidase-like characteristics in 2007, nanozymes containing diverse artificial nanomaterials that possess inherent enzyme-like characteristics have garnered considerable attention [5, 6]. Compared with natural enzymes, nanozymes are favorable because they are low cost, produced in large scale, and possess remarkable catalytic activities. Hence, chemists have become very interested in nanozyme technology as an emerging artificial enzyme field to link nanotechnology and biomedical applications, rather than just being a concept [7].
As the most diverse emerging class of nanomaterials, carbon dots (CDs) have become widely known because of their remarkable biocompatibility, excellent photophysical characteristics, low cytotoxicity, inexpensive precursors, stability, and economic preparation [8-14]. Various CD-based nanocomposites, including carbon nanodots, carbon quantum dots, graphene quantum dots, and other element-doped CDs, have been developed and widely used in biosensing, photocatalysis, theranostics, and biomedical applications [15-23]. In addition, considerable research funding for CDs nanozyme investigations has been awarded in recent years owing to their intrinsic excellent catalytic characteristics. The environmental favorability of CDs nanozymes is the greatest advantage over semiconductor NPs and organometallic nanozymes which include heavy metals and are not suit for practical biomedical research [24].
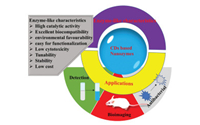
Although a variety of remarkable and comprehensive reviews on nanozymes have been reported, no review focusing on CDs nanozymes has been published [25-30]. In this review, we summarize newly developed CDs nanozymes, as well as their corresponding catalytic characteristics and biomedical applications (Scheme 1). We hope that this review will aid in a better understanding of nanozymes and provide references for future design of CDs nanozymes, advancing the combination of the nanozyme and nanotechnology fields.
|
Download:
|
| Scheme 1. Schematic illustration of CDs nanozymes and their corresponding enzyme-like activities and applications. | |
2. Classification of CDs nanozymes
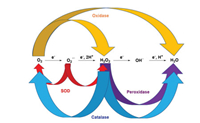
Newly reported CDs-based redox nanozymes can be divided into four catalytic types: peroxidase, oxidase, catalase, and superoxide dismutase (SOD). According to the different substrates and products after the enzymatic reaction, these four types of nanozymes are classified in Scheme 2 [4]. Some substrates that are widely used in nanozyme biosensors [28, 29] are 3, 3′, 5, 5′-tetramethylbenzidine (TMB), o-phenylenediamine (OPD), 2, 2′-azino-bis(3-ethylbenzothizoline-6-sulfonic acid) (ABTS), and Amplex Red (AR) [30-32]. TMB, OPD, and ABTS are colorless, and they can be oxidized to blue (TMBox, λabs = 652 nm), yellow (phenazine-2, 3-diamine (OPDox), λabs = 564 nm), and green (ABTSox, λabs = 420 nm) oxidation products by peroxidases, respectively. AR is colorless and nonfluorescent, and it can be oxidized to resorufin (red under room light and yellow emission at 585 nm), which can be further oxidized to resazurin (purple under room light and nonfluorescent).
|
Download:
|
| Scheme 2. Schematic diagram of different catalytic reactions using peroxidase, oxidase, SOD, and catalase. Reproduced with permission [4]. Copyright 2019, Royal Society of Chemistry. | |
2.1. CDs peroxidase
Peroxidases can catalyze oxidation of peroxides (H2O2 and some organic hydroperoxides), which are transformed into water. In 2014, Shamsipur et al. reported carbon quantum dots (CDs-1) that possess peroxidase activity to monitor the glutathione (GSH) level in human blood [31]. They used Na2EDTA·2H2O as the raw material, which was heated at 400 ℃ under argon for 2 h in a tube furnace to give CDs-1 possessing peroxidase activity (Fig. 1). CDs-1 can be used as the peroxidase to catalyze the reaction of TMB to TMBox with H2O2, accompanied by a color change from colorless to blue. There was almost no reaction between TMB I and II and H2O2 without CDs-1. Upon addition of CDs-1, three obvious emission peaks were observed at 370, 450, and 653 nm. They suggested that the electrons of the amino group of TMB were transferred to CDs-1, which increased the rate of electron transfer from CDs-1 to H2O2, finally catalyzing the reaction of H2O2 with TMB. The absorption intensity at 653 nm was significantly weaker when GSH was added. The absorption intensity showed a good linear relationship with the GSH concentration in the range of 0–7 mmol/L, and the detection limit was 3 mmol/L. Moreover, the nonthiol compound showed little interference in detection of GSH because the sulfhydryl moiety possesses remarkable hydrogen-donating power. Because other biothiols, such as cysteine (Cys) and homocysteine (Hcy), existed in low concentrations in the organism, they could be ignored when the blood was diluted 1000 times, indicating that CDs-1 can be used for specifically monitoring the GSH level in human blood.

|
Download:
|
| Fig. 1. (A) Synthesis process and proposed mechanism of CDs-1 reacting with TMB I and II in the presence of H2O2, respectively, and then reacting with GSH. (B) Absorbance of TMBox with increasing GSH. Copied with permission [31]. Copyright 2014, Elsevier. | |
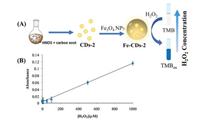
Yousefinejad et al. developed novel Fe-CDs-2 as a peroxidase mimic to detect H2O2 at the nanomolar level [32]. Fe-CDs-2 was synthesized by integrating CDs-2 and Fe3O4 NPs at a ratio of 4:1 under acidic conditions, and CDs-2 was prepared using carbon soot and nitric acid as precursors (Fig. 2). Transmission electron microscopy images showed that Fe-CDs-2 was a hemisphere with a size of about 10 nm. Fe-CDs-2 was capable of catalyzing oxidation of TMB to TMBox with H2O2, accompanied by a color change from colorless to blue. TMBox exhibited higher absorbance with Fe-CDs-2 than with CDs-2 and Fe3O4 NPs alone under the same conditions. In the presence of TMB and Fe-CDs-2, the absorbance showed a remarkable linear relationship with the H2O2 concentration from 10 nmol/L to 1 mmol/L, accompanied by an extremely low detection limit of 1 nmol/L.
|
Download:
|
| Fig. 2. (A) Synthesis route of Fe-CDs-2 and Fe-CDs-2 catalysis of TMB oxidation by H2O2. (B) Absorbance of TMBox at 653 nm with the increasing H2O2 concentration (μM: μmol/L). Copied with permission [32]. Copyright 2017, Elsevier. | |
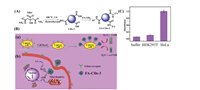
Guo et al. reported folic acid (FA)-CDs-3 with peroxidase activity for detection and treatment of cancer cells [33]. FA-CDs-3 was synthesized by combining Fe3(PO4)2·8H2O with CDs-3, which were synthesized using ammonium citrate and FA-NH2 as raw materials (Fig. 3). Because of overexpression of FA receptors on the surface of tumor cell membranes, FA is a remarkable targeting ligand that can target various tumor cells. FA-CDs-3 is capable of catalyzing oxidation of TMB by H2O2, indicating its peroxidase activity. When HeLa cells and normal HEK293T cells were incubated with FA-CDs-3 for 2 h, washed with phosphate-buffered saline (PBS) to remove the unattached FA-CDs-3, and then treated with TMB and H2O2, a blue color change occurred in the solution with HeLa cells, while there was little change in the solution with HEK293T cells, indicating that FA-CDs-3 can target HeLa cells. Moreover, FA-CDs-3 catalyzed exogenous H2O2 to produce ·OH, which disrupted the redox balance in the cancer cells and induced apoptosis of the cancer cells. Although ascorbic acid (AA) is commonly used to induce apoptosis, a large dose of AA is needed to induce cells to produce H2O2 and generate numerous reactive oxygen species (ROS) through oxidative stress to induce cell death. When HeLa cells were co-incubated with 0.5 mmol/L AA and 50 μg/mL FA-CDs-3 for 48 h, 67% of the HeLa cells showed apoptosis. By contrast, normal HEK293T cells incubated with high doses of FA-CDs-3 and AA exhibited only 20% cytotoxicity, suggesting that FA-CDs-3 has the potential to specifically detect and treat cancer cells.
|
Download:
|
| Fig. 3. (A) Synthesis route of FA-CDs-3. (B) Schematic diagram of FA-CDs-3 for (a) detecting and (b) treating cancer cells. (C) Absorbance of different solutions (buffer, HEK293T, and HeLa cells) at 652 nm. Reproduced with permission [33]. Copyright 2019, Elsevier. | |
Shi et al. developed two types of surface-functionalized CDs (CDs-4.1 and CDs-4.2) with peroxidase activity to explore how the surface structure influences the catalytic performance of CDs [34]. CDs-4 was synthesized by the hydrothermal method using citric acid and thiourea as raw materials (Fig. 4). Different surface modifications were then performed to obtain positively charged poly(ethyleneimine)-modified CDs (CDs-4.1) and negatively charged citric acid-modified CDs (CDs-4.2). CDs-4, CDs-4.1, and CDs-4.2 catalyzed oxidation of TMB and ABTS to their corresponding oxidation products using H2O2. The activity of CDs-4.1 in catalyzing ABTS and the activity of CDs-4.2 in catalyzing TMB were higher than those of CDs-4, indicating that CDs-4.1 and CDs-4.2 showed excellent peroxidase-like activity. Moreover, when catalytic oxidation of TMB was performed, the absorbance value of CDs-4.2 at 652 nm was higher than that of CDs-4, and the absorbance of CDs-4.1 was the weakest. This might be because of the relatively strong affinity between the two amino groups of TMB and the negatively charged surface of CDs-4.2, whereas the affinity between the positively charged surface of CDs-4.1 and TMB is relatively weak. For ABTS containing two sulfonic groups, the catalytic activity of CDs-4.2 for ABTS was less than that for TMB, while the catalytic activity was the opposite for CDs-4.1. In addition, surface-functionalized CDs-4.1 and CDs-4.2 did not change the catalytic mechanism, which was that the CDs catalyzed H2O2 to produce ·OH, while surface modification enhanced electron transfer.

|
Download:
|
| Fig. 4. (A) Synthesis routes of CDs-4, CDs-4.1, and CDs-4.2. (B) Absorbance of TMBox (652 nm) and ABTSox (416 nm) under different CDs: (a)CDs-4, (b) CDs-4.1, and (c) CDs-4.2. Reproduced with permission [34]. Copyright 2019, Elsevier. | |
Zhao et al. reported novel CDs-5 with excellent peroxidase activity to detect the cholesterol content in human serum [35]. CDs-5 was prepared by a one-step solvothermal method using MoS2 and ethanol as raw materials (Fig. 5). Co-doping of Mo and S improved the yield and peroxidase activity of the CDs. In the presence of H2O2, CDs-5 catalyzed oxidation of TMB to TMBox, which showed two characteristic absorption peaks at 369 and 652 nm. Interestingly, cholesterol enzymes catalyzed cholesterol and generated H2O2, which promoted oxidation of TMB without exogenous H2O2. The absorbance at 652 nm showed a good linear relationship with the cholesterol concentration in the range 0.01–0.6 mmol/L, and the detection limit was 7 μmol/L. CDs-5 showed high stability and almost no change in the catalytic activity after 20 days, and it was successfully applied to detect the cholesterol content in human serum.

|
Download:
|
| Fig. 5. (A) Synthesis route of CDs-5. (B) Mechanism of cholesterol detection by applying cholesterol and CDs-5. (C) (a) Absorbance of TMB for cholesterol detection and (b) calibration curves of (a) (mM: mmol/L). Copied with permission [35]. Copyright 2019, Royal Society of Chemistry. | |
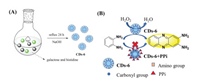
Chen et al. reported CDs-6 with peroxidase activity to selectively detect the pyrophosphate ion (PPi) concentration in human urine [36]. They used galactose and histidine, which were refluxed in NaOH to give CDs-6 (Fig. 6). CDs-6 catalyzed oxidation of TMB, OPD, and AR in the presence of H2O2, which was confirmed by the appearance of the corresponding absorption peaks at 652, 450, and 585 nm, respectively. When the above system was treated with the PPi, the absorption peak disappeared. When horseradish peroxidase (HRP) was used to catalyze oxidation of TMB and AR, the absorbance showed a negligible change after addition of the PPi. These phenomena can be attributed to the interaction between the amino and carboxyl groups on the surface of CDs-6 and the PPi, resulting in inhibition of electron transfer and interruption of the catalytic reaction. Thus, CDs-6 can detect the PPi, and the detection limit was calculated to be 4.29 nmol/L. Furthermore, a portable kit was prepared by setting CDs-6 and OPD on paper. The color of the paper turned pale with increasing PPi concentration, and the detection limit was about 39.2 nmol/L, which is 500 times lower than the PPi concentration in healthy human urine [37-39].
|
Download:
|
| Fig. 6. (A) Synthesis of CDs-6. (B) The sensing mechanism of PPi by its inhibition effect toward the peroxidase activity of CDs-6. | |
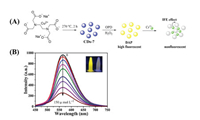
Li et al. reported copper-doped CDs (CDs-7) as a peroxidase mimic to detect chromium ions [Cr(Ⅲ)] [40]. CDs-7 was synthesized from Na2[Cu(EDTA)], which was heated at 270 ℃ in a tube furnace for 2 h under N2 (Fig. 7). In the presence of CDs-7, OPD was oxidized in DAP by H2O2, which emitted yellow fluorescence at 564 nm under excitation of 421 nm. When Cr(Ⅲ) was added, a new absorption peak at 400 nm appeared and the fluorescence intensity of the generated DAP immediately decreased. The new absorption peak showed good overlap with excitation of DAP, which was absorbed by Cr(Ⅲ), resulting in fluorescence quenching through the well-known inner filter effect (IFE) mechanism [41-43]. The fluorescence intensity of DAP showed a remarkable linear relationship with the Cr(Ⅲ) concentration in the range 5–150 μmol/L, accompanied by an extremely low detection limit of 0.12 μmol/L. Finally, because Cr(Ⅵ) can be reduced to Cr(Ⅲ) by using ABTS, CDs-7 was successfully used to directly detect Cr(Ⅲ) and total Cr in water samples. The concentration of Cr(Ⅵ) can then be indirectly calculated, which provides a novel and effective approach for environmental detection of Cr(Ⅲ) and Cr(Ⅵ).
|
Download:
|
| Fig. 7. (A) Synthesis and detection mechanism of CDs-7 with peroxidase activity for Cr(Ⅲ). (B) Fluorescence intensity of DAP with increasing Cr(Ⅲ) concentration. Copied with permission [40]. Copyright 2020, Elsevier. | |
Wang et al. reported new iodine-containing CDs (CDs-8) with peroxidase-like and antibacterial activities through activating H2O2 under room light [44]. CDs-8 was prepared by heating iohexol at 180 ℃ for 8 h in an autoclave (Fig. 8). CDs-8 catalyzed oxidation of TMB to TMBox by H2O2, accompanied by appearance of a new absorption peak at 652 nm and a color change from colorless to blue. There was little antibacterial activity when CDs-8 or H2O2 alone was added to Staphylococcus aureus and Escherichia coli. However, when CDs-8 and H2O2 were added together, the bacterial shape changed from rod-shaped to wrinkled, and the cell membrane integrity was destroyed by the generated hydroxyl radicals (·OH), resulting in bacterial death. Furthermore, the antibacterial activity was significantly influenced by the ratio of CDs-8 to H2O2 and whether they were exposed to room light. In addition, CDs-8 successfully accelerated wound healing even though the exogenous H2O2 concentration was extremely low (0.07 mmol/L), indicating that CDs-8 can be used as an antibacterial agent and for wound healing.

|
Download:
|
| Fig. 8. Mechanism of CDs-8 with antibacterial activity in the presence of H2O2. | |
Ibarbia et al. reported a graphene oxide quantum dot (CDs-9) based hydrogel functionalized by the cross-linkable 3-(triethoxysilyl)propyl methacrylate (MPS) group (CDs-9-MPS-A50coS50) to photodegrade rhodamine B (RhB) [45]. CDs-9 was prepared by refluxing a mixture of XC-72 carbon black and HNO3 aqueous solution for 72 h (Fig. 9). A mixture of CDs-9 and MPS, as a cross-linker, was stirred in ultrapure water for 24 h to obtain CDs-9-MPS. CDs-9-MPS showed high peroxidase ability with 45% decomposition of RhB in the dark and 92% decomposition of RhB in room light. When H2O2 and CDs-9-MPS were added, the absorbance of RhB at 554 nm was quenched, along with a color change from pink to pale orange. The degradation mechanism is that the·OH radicals generated from H2O2 in the process of photodegradation show high oxidation activity for RhB. Furthermore, CDs-9-MPS was incorporated into the poly[2-(methacryloyloxy)ethyl]trimethylammonium]-co-(3-sulfopropylmethacrylate) 50:50 (A50coS50) hydrogel to obtain the CDs-9-MPS-A50coS50 hydrogel. The CDs-9-MPS-A50coS50 hydrogel showed a similar trend for degradation of RhB in the dark to the hydrogel-free sample. The 2.5% CDs-9-MPS-A50coS50 hydrogel showed 10% degradation for RhB after 6 h, which was slightly higher than that of the hydrogel-free sample (4%). When irradiated by visible light, decomposition of RhB by the 2.5% CDs-9-MPS-A50coS50 hydrogel increased from 20% to 50%, while that of the hydrogel-free sample was only enhanced from 4% to 20%, indicating the remarkable photocatalytic activity of the 2.5% CDs-9-MPS-A50coS50 hydrogel. In addition, all of the RhB was completely degraded by the 2.5% CDs-9-MPS-A50coS50 hydrogel after 24 h, and the hydrogel showed excellent long-term stability in water, which means that the CDs-9-MPS-A50coS50 hydrogel is a promise material for degradation of organic dyes in aqueous solution.

|
Download:
|
| Fig. 9. (A) Synthesis of CDs-9-MPS-A50coS50. (B) Degradation of RhB by H2O2 using (a) CDs-9 and (b) CDs-9-MPS in the dark and under room light. Reproduced with permission [45]. Copyright 2020, Elsevier. | |
Li et al. reported CDs (CDs-10-Cu) that stabilized Cu4O3 as nanozymes with high stability and catalytic activities (dual oxidase- and peroxidase-like activities) [46]. CDs-10-Cu was obtained by hydrothermal reaction using CDs-10 and CuCl as raw materials, and Cu was prepared by the same approach using CuCl as the raw material (Fig. 10). CDs-10-Cu catalyzed oxidation of OPD to OPDox and showed higher catalytic activity than Cu4O3 both in the dark and under light. The Vmax value of CDs-10-Cu was 20.93 μmol L-1 min-1 in the dark, which was 2.55-times higher than that of Cu4O3 (8.22 μmol L-1 min-1) from the kinetic parameters. Furthermore, CDs-10-Cu also catalyzed oxidation of dopamine (DA) to oxidative dopamine (DAox). The Vmax values of CDs-10-Cu were 4.21 and 6.67 μmol L-1 min-1 under dark and light conditions, which were higher than those of Cu4O3 (2.02 and 3.85 μmol L-1 min-1). The above results indicated that CDs-10-Cu possesses excellent enzyme activity because of its rich Cu2+/Cu+ redox couples. The reaction mechanism is that CDs-10-Cu reduces absorbed O2 to ·O2- or H2O2, along with oxidation of Cu+ to Cu2+. Owing to coordination of Cu2+ to the amino units of CDs-10, Cu2+ can accept the delocalized electrons from CDs-10 to revert to Cu+. The catalytic activities were significantly affected by the pH, temperature, and other oxidizing agents.

|
Download:
|
| Fig. 10. (A) Catalytic reaction mechanism of. (B) (a) Enzyme-like activities of under different influencing factors at the same pH and (b) enzyme-like activities of at different pH values. Copied with permission [46]. Copyright 2020, Elsevier. | |
Li et al. reported a type of Fe3C/N-doped nanozyme (CDs-11) to efficiently heal wounds by suppressing bacterial infection [47]. CDs-11 was prepared through an electrochemical strategy using FeSO4·H2O and histidine (His) as the electrolyte (Fig. 11). The anode and cathode were a graphite rod and a Pt sheet, respectively, and the electrolysis reaction was completed by stirring for 8 h to obtain grey-black CDs-11. In the presence of CDs-11, H2O2 produced ·OH, oxidizing colorless TMB to a blue product (TMBox) in vitro. CDs-11 exhibited the best peroxidase-like activity at 50 ℃ in the temperature range 25–70 ℃, and at pH 4.0 in the pH range of 1.0–12.0. The Vmax value from the Lineweaver–Burk equation was higher than those of other nanozymes, suggesting its outstanding catalytic activity. In further studies, E. coli and S. aureus were treated with PBS, H2O2, CDs-11, and CDs-11 + H2O2. When incubated with CDs-11 + H2O2, bacterial colonies were hardly observed compared with other control groups. Scanning electron microscopy (SEM) confirmed that the cell morphology was rough and damaged when treated with only CDs-11 + H2O2. The E. coli cells treated with CDs-11 + H2O2 were observed to be damaged by a confocal microscope. Furthermore, when Sprague-Dawley rats with a bacterial infection by S. aureus were treated with CDs-11 + H2O2, the wounds gradually healed after 6 days and fewer bacterial colonies were detected compared with other groups treated with PBS, H2O2, and CDs-11. The above results showed that CDs-11 can accelerate wound healing because it catalyzes oxidation of hydrogen peroxide to ·OH.

|
Download:
|
| Fig. 11. (A) Synthesis of CDs-11. (B) Oxidation and antibacterial mechanisms of CDs-11 (TMB as the substrate). (C) (a) Wound changes of rats treated with PBS, H2O2, CDs-11, and CDs-11 + H2O2, (b) changes of the wound area, and (c) number of different bacterial colonies. Reproduced with permission [47]. Copyright 2020, Elsevier. | |
Liang et al. reported enzyme-like CDs-12 that inhibits bacterial infections through destroying the biofilms of the bacteria [48]. First, citric acid and formamide were added to an autoclave and heated at 180 ℃ for about 12 h to obtain the CD precursor, and then CDs-12 was obtained by integrating the CDs with Pt NPs (Fig. 12). CDs-12 efficiently catalyzed H2O2 oxidation of TMB, OPD, and ABTS to TMBox, OPDox, and ABTSox, respectively. Owing to its excellent properties, CDs-12 can also be used as a potent disinfection nanoagent. When spherical-like S. aureus (MRSA) and rod-shaped E. coli were treated with CDs-12 in the presence of H2O2, SEM showed that the bacterial membrane became rough and was damaged. The mechanism is that the ·OH generated from H2O2 destroys the bacterial colonies by cleavage of the proteins and nucleic acids, causing bacterial death. The antibacterial effect was more obvious for higher concentration of hydrogen peroxide. In further studies, when MRSA-infected mice were treated with CDs-12, the wounds showed excellent healing after 7 days compared with the control group. Note that from the inflammation triggered around the wound, the produced endogenous H2O2 participated in the reaction without additional H2O2.

|
Download:
|
| Fig. 12. (A) Process of biofilm eradication mediated by CDs-12. (B) Changes of the wound infected by MRSA treated with CDs-12 during 7 days. Copied with permission [48]. Copyright 2020, Wiley Publishing Group. | |
Su et al. reported CDs (Hemin-CDs-13) with peroxidase-like activity for colorimetric and fluorometric sensing of hydrogen peroxide, glucose, and xanthine [49]. CDs-13 was prepared using citric acid and urea, which were dissolved in dimethylformamide and transferred into an autoclave at 160 ℃ for 6 h (Fig. 13). The synthesized CDs-13 emitted a strong fluorescence signal at 540 nm under excitation of 480 nm. The obtained CDs-13 was then self-assembled with hemin by stirring for 7 h at room temperature to form Hemin-CDs-13. The peroxidase-like activity of CDs-13, hemin, and Hemin-CDs-13 was compared. Hemin showed much less activity owing to it poor water solubility and CDs-13 showed no catalytic activity. Thus, introduction of CDs-13 efficiently increased dispersion. The generated Hemin-CDs-13 exhibited high peroxidase-like activity, and 4-aminoantipyrine (4-AAP) and phenol were oxidized to the red quinoneimine dye by H2O2, accompanied by fluorescence quenching of Hemin-CDs-13. In further H2O2-detection studies, the absorbance of the quinoneimine dye at 505 nm was enhanced and the fluorescence intensity of Hemin-CDs-13 at 540 nm decreased with increasing concentration of H2O2 in the range 0.17–333 μmol/L. In addition, the solution color gradually darkened from light red. The detection limits of the fluorescent and colorimetric assays were 0.15 and 0.11 μmol/L, respectively. In the presence of glucose or xanthine, similar experimental phenomena to H2O2 were observed. This was attributed to glucose and xanthine being able to generate H2O2 under oxidation of glucose oxidase (GOx) and xanthine oxidase (XOD). In general, Hemin-CDs-13 can be applied to detect the species related to the H2O2-generation reaction.

|
Download:
|
| Fig. 13. (A) Synthesis route of CDs-13 and Hemin-CDs-13. (B) Mechanism of oxidation of 4-AAP and phenol by H2O2 catalyzed by Hemin-CDs-13. | |
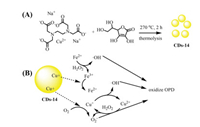
Yang et al. reported CDs-14 nanozymes with dual oxidase- and peroxidase-like activities in a neutral environment [50]. CDs-14 was prepared from Na2[Cu(EDTA)] and AA, which were heated at 270 ℃ for 2 h in a quartz glass tube to give CDs-14 with yellow fluorescence (Fig. 14). The as-prepared CDs-14 catalyzed oxidation of OPD to OPDox by H2O2 and O2. The oxidation mechanism is that Cu+ from CDs-14 reduces H2O2 and O2 to generate ·OH and ·O2-, respectively, which further oxidize OPD to OPDox. With the assistance of ferrous ions, the catalytic activity of CDs-14 exhibited excellent enhancement owing to generation of ·OH and ·O2-, which were decomposed by H2O2 under the reaction of ferrous ions. The lower Km value and higher Vmax value suggested that the ferrous ions play a crucial role in the catalytic process. Notably, the fluorescence of CDs-14 + Fe2+/H2O2 was only quenched in the presence of Cr(Ⅲ) ions. Further studies showed that the Cr(Ⅲ) ions were transformed to CrO2-, which was further oxidized to CrO42- by H2O2. The detection limit for Cr(Ⅲ) was 36 nmol/L. The excellent selectivity and sensitivity of CDs-14/H2O2 indicate that it can be applied to detect Cr(Ⅲ) in real water samples.
|
Download:
|
| Fig. 14. (A) Synthesis process of CDs-14. (B) Mechanism of oxidation of OPD by CDs-14 with the aid of Fe2+. | |
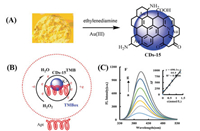
Li et al. reported a nanozyme (CDs-15) that can release DNAzyme to enhance its catalytic efficiency in the presence of K+ [51]. CDs-15 was prepared from corn starch and ethylenediamine (EDA) by the microwave method at 140 ℃ for 10 min (Fig. 15). When CDs-15 was linked with an aptamer (Apt), Apt-CDs-15 quenched the fluorescence of CDs-15, which was attributed to inhibition of the catalytic activity. Apt-CDs-15 generated DNAzyme with hemin (HM) to improve the catalytic activity of CDs-15 in the presence of K+. CDs-15 and DNAzyme co-catalyzed generation of TMBox with a fluorescence signal at 403 nm and an absorption band at 563 nm in the presence of H2O2. The fluorescence intensity of TMBox gradually increased with increasing concentration of Apt-CDs-15 with K+. Moreover, the low Km value and high Vmax value suggested that Apt-CDs-15 possesses excellent affinity and high catalytic activity. The catalytic mechanism can be attributed to the rich π electrons on the CDs-15 surface. The rich electrons promote electron transfer in the redox process of TMB and H2O2. The DNAzyme produced from Apt-CDs-15 with K+ can synergistically catalyze oxidation of TMB owing to the π-π electrons and accelerated redox reaction between TMB and H2O2. CDs-15 was also used to detect ultra-trace K+, and the detection limit was 0.024 nmol/L.
|
Download:
|
| Fig. 15. (A) Synthesis process of CDs-15. (B) Oxidation mechanism of TMB in the presence of Apt-CDs-15 and K+. (C) Fluorescence intensity of TMBox with increasing K+ concentration. Reproduced with permission [51]. Copyright 2020, Elsevier. | |
Oxidase plays a catalytic role in the oxidation process in the presence of oxygen, which is finally turned into H2O2, H2O, or ·O2-. Li et al. reported Cu with light-boosting enzyme-like activity and high reaction rate [52]. CDs-16 was prepared by mixing a solution containing coal pitch powder, formic acid, and H2O2, which were further reacted with copper(II) acetylacetonate (Cu(acac)2) to give Cu (Fig. 16). OPD was oxidized to OPDox in the presence of Cu under light irradiation. Under visible light, the photogenerated holes and electrons participated in oxidation of OPD. The electrons on the surface reacted with O2 to generate ·O2- radicals, and then combined with H+ to form ·OH. The photogenerated holes promoted generation of electrons to activate the N-H bond in OPD through accelerating the process of the self-oxidation reaction of Cu2O. The kinetic parameter (Km) of Cu for OPD was 3.94 mmol/L, which is lower than those of reported mimic peroxidases, such as Cu@Cu2O aerogels and ZnFe2O4 (Km = 8.88 and 22.6 mmol/L, respectively) [53, 54], suggesting that Cu possessed better binding affinity to OPD. The Vmax (9.47 μmol L-1 min-1) was higher than that of the above nanocomposites and Cu2O, indicating that Cu possessed higher catalytic activity for OPD. Furthermore, Cu with additional CDs-16 showed higher activity for OPD than Cu2O, which was attributed to CDs-16 facilitating photogenerated carrier transfer.

|
Download:
|
| Fig. 16. (A) Synthesis route of. (B) Oxidation mechanism of TMB in the presence of. | |
Zhang et al. developed phosphorescent CDs (CDs-17) with oxidase activity for conversion of triplet oxygen (3O2) to singlet oxygen (1O2) [55]. CDs-17 was prepared by hydrothermal polymerization using citric acid and EDA as raw materials (Fig. 17). TMB was oxidized by CDs-17 in several seconds under 365 nm because CDs-17 can catalyze formation of singlet oxygen, which can oxidize TMB. The phosphorescence intensity of CDs-17 was related to the singlet oxygen emission and photosensitizing activity. Interestingly, the phosphorescence intensity increased with increasing reaction temperature because the CDs synthesized at higher temperature possessed higher nitrogen content.E. coli and Salmonella were incubated with CDs-17 and phloxine B (PB, a photosensitizer) under dark and light conditions. For CDs-17 in light, the two types of bacteria showed apparent growth inhibition, and the corresponding inhibition efficiency was nearly twice that of PB, indicating that CDs-17 possesses remarkable antibacterial activity and can be used in photodynamic antibacterial chemotherapy.

|
Download:
|
| Fig. 17. (A) Synthesis route and photosensitization mechanism of CDs-17 for bacteria. (B) Bacterial inhibition by CDs-17 and PB under dark and light conditions. Reproduced with permission [55]. Copyright 2015, American Chemical Society. | |
Ren et al. reported novel CDs doped with copper (CDs-18) as an oxidase mimic for monitoring the level of hydroquinone (HQ) [56]. CDs-18 was synthesized by the hydrothermal method using Cu(NO3)2 and poly(methacrylic acid) sodium salt as raw materials (Fig. 18). CDs-18 emitted blue fluorescence at 460 nm and exhibited remarkable photostability and stability in a wide pH range (3.0–13.5).p-Phenylenediamine (PPD) is colorless, and it changed to brown in 30 min with addition of CDs-18, which catalyzed oxidation of PPD and generated a clear absorption peak at 495 nm. CDs-18 also showed better temperature stability than laccase, and the optimum pH was 8.5. When polyacrylamide (PAM+) was added to CDs-18, PPD and the oxidation product of PPD on the surface of CDs-18 could be removed by flocculation between PAM+ and CDs-18. In addition, CDs-18 reacted with HQ with a color change from colorless to yellow in 10 min. In carbonate buffer (pH 9.2), the fluorescence intensity at 460 nm decreased with increasing HQ concentration, and the relative fluorescence quenching (I/I0) showed a remarkable linear relationship with the HQ concentration in the range 0.05–2 mmol/L. The detection limit was 1 μmol/L, which is much lower than the maximum level specified by the Environmental Protection Agency of 3 μmol/L.

|
Download:
|
| Fig. 18. (A) Schematic illustration of CDs-18 reacting with PPD. (B) Fluorescence intensity change of CDs-18 with increasing HQ concentration (mM: mmol/L) in carbonate buffer. Copied with permission [56]. Copyright 2018, Royal Society of Chemistry. | |
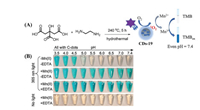
Zhang et al. developed Mn(Ⅱ)-containing CDs (CDs-19) as a photo-oxidation nanozyme to improve the catalytic effect of CDs under physiological conditions (pH 7.4) and resolve the pH limitations [57]. Under irradiation of 365 nm light, the TMB solution was colorless, and upon addition of CDs-19, the solution was only blue at pH < 5. When Mn(Ⅱ) was added to the above system, the solution was blue even at physiological pH (Fig. 19). The mechanism is that singlet oxygen is generated from CDs-19 under light-oxidized Mn(Ⅱ), and the oxidation product Mn(Ⅲ) as a mediator further oxidizes TMB. Among common divalent metals, only Mn(Ⅱ) was able to catalyze oxidation of TMB. Because Mn(Ⅲ)/EDTA has high stability, when CDs-19 was treated with EDTA, TMB was oxidized within 10 s. Furthermore, the amount of Mn(Ⅱ) in the CDs was positively correlated with the absorbance of TMB at 652 nm, and the detection limit of Mn(Ⅱ) was 5 nmol/L. Based on this strategy, many nanozymes could be explored for use in biosensing and nanotechnology at neutral pH.
|
Download:
|
| Fig. 19. (A) Synthesis route of CDs-19 and mechanism of Mn(Ⅱ) oxidation, which can increase the photo-oxidation characteristic of CDs-19 under physiological conditions (pH 7.4). (B) Images of TMB oxidized by CDs-19 with and without Mn(Ⅱ) and EDTA under 365 nm light and different pH conditions. Copied with permission [57]. Copyright 2019, American Chemical Society. | |
Honarasa et al. developed CDs-20 with oxidase activity for detection of trace Fe2+ [58]. CDs-20 was synthesized by mixing KMnO4 and CDs, which were prepared from candle dust using nitric acid (Fig. 20). CDs-20 catalyzed oxidation of TMB to TMBox by oxygen, leading to appearance of the characteristic absorption peak at 655 nm. In addition, with increasing oxygen concentration, the blue color of the solution deepened. The increase of the absorbance at 655 nm proved that oxygen, as an oxidant, participated in oxidation of TMB and was reduced to H2O. Notably, when Fe2+ was added to the solution, the absorbance at 655 nm decreased and the color became weaker, which was attributed to competitive oxidation of Fe2+ and TMB. Moreover, addition of other metal ions, such as Co2+, Ni2+, and Mn2+, had little effect on the absorbance at 655 nm because Fe3+/Fe2+ has the lowest reduction potential among Co3+/Co2+, Ni2O/Ni2+, and Mn3+/Mn2+. The absorbance at 655 nm showed a good linear relationship with the Fe2+ concentration in the range 0.03–0.83 μmol/L, and the detection limit of Fe2+ was 0.03 μmol/L.

|
Download:
|
| Fig. 20. (A) Synthesis route of CDs-20. (B) Schematic illustration of the mechanisms of oxidation of TMB and sensing of Fe2+ by CDs-20. | |
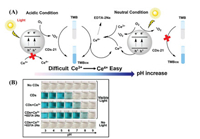
Li et al. reported CDs-21 with the pH-dependent photo-oxidation characteristic, which was driven by Ce3+ [59]. In the presence of oxygen, CDs-21 oxidized colorless TMB to blue TMBox at the natural pH under visible light (Fig. 21). The Km value of CDs-21-Ce + EDTA·2Na became lower than that of CDs-21 with increasing pH, suggesting that CDs-21-Ce + EDTA·2Na has better affinity for TMB than CDs-21. The 1O2 generated from CDs-21 under light oxidized TMB to TMBox under acidic conditions. Conversely, under the neutral pH condition, 1O2, which was photo-generated by CDs-21-Ce + EDTA·2Na under illumination, oxidized Ce3+ to Ce4+ because the Ce3+/Ce4+ potential decreases with increasing pH, and TMB was then further oxidized by Ce4+. Furthermore, the oxidation product Ce4+ was the key contributor to oxidation of TMB in a pH neutral environment, and it was stabilized by EDTA·2Na to prevent generation of cerium hydroxide.
|
Download:
|
| Fig. 21. (A) Mechanism of oxidation of TMB by CDs-21-Ce3+ + EDTA·2Na in room light. (B) Color changes of TMB under different conditions. Copied with permission [59]. Copyright 2020, Elsevier. | |
SOD can transform superoxide radicals to O2 and H2O2 through a disproportionated reaction and then alleviate the oxidative stress caused by cell metabolism. Sun et al. reported graphene oxide quantum dots (CDs-22) to alleviate ethanol-induced cellular metabolic disturbances [60]. CDs-22 was purchased from XFNANO Materials (Nanjing, China) and showed good dual catalase- and SOD-like activities (Fig. 22). CDs-22 increased the activities of intracellular alcohol dehydrogenase and aldehyde dehydrogenase, accelerating conversion of ethanol to acetaldehyde and acetic acid. When the ethanol level in the cells was relatively high, the cell activity, as well as the membrane integrity, was reduced. Upon addition of CDs-22, the cell viability and integrity of the membrane were enhanced by nearly 80% compared with the control values, and the excessive autophagy of the endothelial cells caused by ethanol was reduced. Therefore, CDs-22 was capable of maintaining the integrity of the membrane and reducing the number of damaged endocytes, which were generated to protect the cells from the cell viability decrease caused by ethanol. In addition, when the cells were treated with ethanol, the concentrations of ROS and malondialdehyde (MDA) increased by about 67% and 117% respectively. Upon addition of CDs-22, the ROS and MDA levels decreased and showed negligible differences with the levels of the control group. This suggested that CDs-22 can reduce the oxidative stress caused by ethanol, and it showed a better alcohol detoxification effect than GSH. Moreover, CDs-22 can also regenerate ethanol-destroyed cells through repairing ethanol-destroyed proteins. Thus, CDs-22 can reduce ethanol-induced oxidative stress and promote ethanol and lipid metabolism to increase ethanol detoxification.

|
Download:
|
| Fig. 22. Proposed mechanism and enzyme-like activities of CDs-22 for ethanol. (B) (a) Viability and (b) integrity of cells treated with ethanol and CDs-22 + ethanol, respectively. Reproduced with permission [60]. Copyright 2017, American Chemical Society. | |
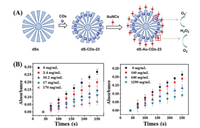
Zhao et al. reported a nanozyme (dS-Au-CDs-23) with dual SOD- and HRP-like activities [61]. dS-Au-CDs-23 was prepared by loading SOD-like CDs-23 into the interior surface of dendritic silica spheres (dSs), and HRP-like GSH–Au NPs were linked to the dS surface (Fig. 23). CDs-23 was synthesized from a mixture of citric acid and EDA by the hydrothermal method. Owing to the wide radial channel in the surface of the dSs, CDs-23 could be loaded into the inner surface to form dS-CDs-23, maintaining the fluorescence signal at 450 nm. The fluorescence intensity of dS-CDs-23 was enhanced with increasing concentration, and the fluorescence quenching effect of CDs-23 was eliminated owing to the distribution in dS-CDs-23. Moreover, the fluorescence of dS-CDs-23 was stronger for higher content of loaded CDs-23, indicating that CDs-23 plays an important role in the fluorescence. dS-CDs-23 prevented accumulation of the colored intermediates produced by self-oxidization of pyrogallol owing to its SOD-like activity. The typical absorbance at 325 nm can be used to evaluate the activity of dS-CDs-23, and it decreased with increasing concentration of CDs, suggesting its high SOD activity. The dSs showed no obvious change in the absorbance at 325 nm with pyrogallol, indicating that the SOD-like activity was indeed from CDs-23 in dS-CDs-23. The GSH-Au NPs also catalyzed oxidation of TMB to TMBox in the presence of H2O2 because of catalyzed generation of ·OH from H2O2 owing to their HRP-like activity. However, the dS-Au-CDs-23 loaded GSH-Au NPs also showed excellent HRP-like activity. In conclusion, dS-Au-CDs-23 exhibits dual SOD- and HRP-like catalytic activities and improves the fluorescence quenching effect of CDs-23.
|
Download:
|
| Fig. 23. (A) Synthesis process of dS-Au-CDs-23 and oxidation mechanism of dS-Au-CDs-23 with dual SOD- and HRP-like catalytic activities. (B) Changes of the absorption peak at 325 nm with increasing concentration of CDs-23 and dS-Au-CDs-23, respectively. Copied with permission [61]. Copyright 2020, Royal Society of Chemistry. | |
Catalase can efficiently convert H2O2 to H2O and O2. Various nanozymes and nanoparticles with peroxidase- or SOD-like activities possess catalase-mimicking characteristics. Passi et al. reported a theranostic nanozyme (CDs-24) to simultaneously image and remove ROS [62]. CDs-24 was self-assembled using a positively charged CD precursor, cationic cerium oxide NPs (CeNPs), and silk fibroin NPs (SFSNPs) loaded with the antioxidant drug sulforaphane. CDs-24 eliminated the active oxide produced in response to the oxidative stress in the organism (Fig. 24). The CD precursor was prepared from mixed polyethylenimine (PEI) and mulberry leaves, which were heated at 200 ℃ for 4 h. The CD precursor with green fluorescence strengthened the antioxidant potential of CDs-24 and promoted imaging. In an acidic environment, the sulforaphane released from the SFSNPs activated the antioxidant response element responsible for antioxidant gene expression in the nucleus. The ROS were removed under the reaction of CeNPs containing Ce3+ and Ce4+. When A549 cells were incubated with nonfluorescent DCFH-DA, green fluorescence was observed at 535 nm owing to DCF, which was generated by oxidation of ROS. The cells treated with H2O2 showed brighter green fluorescence with DCFH-DA than the above control group. However, when CDs-24 was added, the green fluorescence from the cells gradually decreased with increasing concentration of CDs-24. No fluorescence signal of DCF was detected for only the 100 μg/mL CDs-24-treated cells, suggesting that CDs-24 can efficiently remove ROS in response to oxidative stress.

|
Download:
|
| Fig. 24. (A) Synthesis of the CD precursor and CDs-24. (B) Images of A549 cells: (a) blank sample, (b) treated with H2O2, and (c–e) treated with 20, 60, and 100 μg/mL CDs-24, respectively. Reproduced with permission [62]. Copyright 2020, Elsevier. | |
Dehvari et al. developed peroxidase-active CDs (CDs-25) to scavenge for free radicals [63]. CDs-25 was synthesized by the microwave hydrothermal method using citric acid trisodium salt, N-acetyl-l-cysteine, manganese acetate, and Na2S as raw materials (Fig. 25). When TMB was treated with H2O2 and Fe2+, the solution changed from colorless to blue, and the characteristic absorption peak of ox-TMB appeared at 652 nm, which was because formation of ·OH catalyzed oxidation of TMB. When CDs-25 was added to the system, the absorbance at 652 nm significantly decreased, which was attributed to removal of ·OH by CDs-25. In addition, CDs-25 also exhibited high scavenging capacity for reactive nitrogen species, and 100 μg/mL CDs-25 showed a 98% scavenging effect for ONOO-. When the surface of CDs-25 was conjugated with hyaluronic acid (HA), the water solubility of HA-CDs-25 significantly improved owing to the increase of surface negative functional groups. When B16F1 cells were incubated with 2′, 7′-dichlorofluorescin diacetate (DCFH-DA), DCFH-DA was oxidized to green fluorescent dichlorofluorescein (DCF) in the presence of ROS. Upon addition of H2O2, the green fluorescence was significantly enhanced. When HA-CDs-25 was added, the green fluorescence signal weakened. When the B16F1 cells were treated with 500 μg/mL HA-CDs-25 and 0.2 mmol/L H2O2, the green fluorescence signal was almost not observed. These results suggested that HA-CDs-25 can scavenge for ROS in cells.

|
Download:
|
| Fig. 25. (A) Synthesis route of HA-CDs-25 and the corresponding mechanism for reducing ROS. (B) Images of B16F1 cells incubated with DCFH-DA, and then with addition of H2O2 (mM: mmol/L) and HA-CDs-25. Reproduced with permission [63]. Copyright 2020, Elsevier. | |
Wang et al. developed a dual-signal indicator (CDs-26) with catalase-like activity to detect iodide ions (I-) in urine by a dual-readout method that can enhance the selectivity and sensitivity, and reduce environmental interference [64]. CDs-26 was synthesized by hydrothermal reaction at 180 ℃ for 4 h using ammonium citrate and o-aminobenzoic acid (Fig. 26). CDs-26 emitted bright-blue fluorescence at 450 nm. CDs-26 could not catalyze oxidation of OPD by H2O2 without I-, but OPD was immediately oxidized by H2O2 in the presence of I-, and it then transformed to OPDox, accompanied by generation of a yellow color under room light. In addition, the emission intensities at 450 and 565 nm showed a decrease and an increase, respectively, which were attributed to fluorescence resonance energy transfer (FRET). The ratio of the fluorescence at 450 and 565 nm (F565/F450) showed remarkable linearity with the I- concentration in the range 0.09–50 μmol/L, and the detection limit was 0.06 μmol/L. Although Br- showed slight interference compared with other ions, the concentration of I- in urine is much higher than that of Br- [65, 66], suggesting its high selectivity for I- when used in analysis of real urine samples.

|
Download:
|
| Fig. 26. Synthesis route and proposed mechanism of CDs-26 for sensing I- in the presence of OPD and H2O2. | |
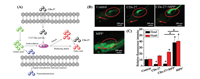
Ren et al. reported CDs (CDs-27) with catalase-like and antioxidant activities that can efficiently decrease the ROS level and inhibit neurotoxicity [67]. They purchased CDs-27 from XFNANO (XF042, China). When the 1-methyl-4-phenyl-pyridinium (MPP+) ion, which can induce oxidative stress, was added to PC12 cells, the α-synuclein level immediately increased (Fig. 27). When CDs-27 was added, the α-synuclein level decreased, indicating that it inhibited the neurotoxicity in vitro through the expression block of α-synuclein. In vivo, CDs-27 could inhibit the neurotoxicity through regulating the metabolism and inhibiting biosynthesis of the important components of the living organism. The corresponding mechanism is shown in Fig. 27. The same phenomenon was observed in zebrafish, and the fluorescence intensity of the head was much lower than that of heart, suggesting that CDs-27 was mainly located in the brain to reduce neurotoxicity.
|
Download:
|
| Fig. 27. (A) Schematic of CDs-27 protecting PC12 cells and larval zebrafish from neurotoxicity and the underlying mechanism. (B) Heads and hearts of zebrafish larvae stained with DCFH-DA. The red arrows indicate the heads and the red ellipses indicate the hearts. (C) Relative fluorescence intensity of (B). Reproduced with permission [67]. Copyright 2020, Wiley Publishing Group. | |
CDs nanozymes are superior to natural enzymes and other organometallic nanozymes, and they are gradually becoming a research hotspot because of their low cost, stability, environmental favorability, easy of functionalization, outstanding photophysical characteristics, and catalytic activities. Herein, we have reviewed the recent progress in development of CDs nanozymes. Nanozymes have promising prospects in monitoring reactive biological species, theranostics, and tissue imaging. Despite the excellent advances in CDs nanozymes, some critical issues and challenges still need to be resolved through combined efforts.
(1) The structure–activity relationship needs to be determined to realize rational design of CDs nanozymes [68]. Exploring the relationship between the structure of CDs, catalytic sites, and catalytic characteristics should be paid more attention to provide guidance for synthesizing CDs nanozymes with remarkable catalytic performance instead of using the traditional trial-and-error method. We presume that the special groups on the surface of CDs act as the catalytic sites, although further investigation is required. The kinetics and thermodynamics of nanozymes should also be taken into consideration.
(2) The catalytic mechanisms and structure–activity relationship of CDs nanozymes need to be further understood through computational chemistry [69, 70]. Because the structure of CDs is extremely complex, more efficient theoretical methods should be used. For example, density functional theory can predict the electronic structure and density.
(3) The number of types of nanozymes should be increased. This could be accomplished by searching for novel catalytic sites or activities in natural enzymes and then synthesizing analogues that are finally incorporated into CDs or by using CDs as the precursor [71, 72]. For example, previously reported 1, 4-dihydronicotinamide adenine dinucleotide (NADH) oxidase and peroxidase-mimicking nanozymes have mainly been organometallic nanozymes, which are toxic to some extent [73, 74]. It would advance the NADH and nanozyme fields if CDs nanozymes with NADH oxidase and peroxidase-like activities were developed owing to their low toxicity and excellent biocompatibility.
(4) Systematically evaluating the efficiency and biocompatibility is extremely important for promoting translational applications of the nanozyme field. For instance, researchers should pay attention to the cellular fate, toxicity, immunogenicity, and therapeutic effect of nanozymes.
In summary, combining the remarkable photophysical properties, water solubility, and biocompatibility of CDs with the remarkable catalytic activities, stability, and low-cost of nanozymes, CDs nanozymes have greatly advanced co-development of the artificial enzyme, CD, and nanotechnology fields. CDs nanozymes have promising prospects in biosensing, photocatalysis, theranostics, biomedicine, and catalytic therapy.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21974125, 21708035), the Collaborative Innovation Project of Zhengzhou (Zhengzhou University) (No. 18XTZX12002), and the 111 Project (No. D20003).
| [1] |
M. Liang, X. Yan, Acc. Chem. Res. 52 (2019) 2190-2200. DOI:10.1021/acs.accounts.9b00140 |
| [2] |
X. Zhang, S. Lin, S. Liu, et al., Coord. Chem. Rev. 429 (2021) 213652. DOI:10.1016/j.ccr.2020.213652 |
| [3] |
P. Li, C. Wu, Y. Xu, et al., TrAC Trends Anal. Chem. 131 (2020) 116007. DOI:10.1016/j.trac.2020.116007 |
| [4] |
D. Jiang, D. Ni, Z.T. Rosenkrans, et al., Chem. Soc. Rev. 48 (2019) 3683-3704. DOI:10.1039/C8CS00718G |
| [5] |
L. Gao, J. Zhuang, L. Nie, et al., Nat. Nanotechnol. 2 (2007) 577-583. DOI:10.1038/nnano.2007.260 |
| [6] |
F. Manea, F.B. Houillon, L. Pasquato, P. Scrimin, Angew. Chem. Int. Ed. 43 (2004) 6165-6169. DOI:10.1002/anie.200460649 |
| [7] |
J. Wu, X. Wang, Q. Wang, et al., Chem. Soc. Rev. 48 (2019) 1004-1076. DOI:10.1039/C8CS00457A |
| [8] |
H. Qin, Y. Sun, X. Geng, et al., Anal. Chim. Acta. 1106 (2020) 207-215. DOI:10.1016/j.aca.2020.02.002 |
| [9] |
X. Geng, Y. Sun, Y. Guo, et al., Anal. Chem. 92 (2020) 7940-7946. DOI:10.1021/acs.analchem.0c01335 |
| [10] |
X. Shi, H. Meng, Y. Sun, et al., Small 15 (2019) 1901507. DOI:10.1002/smll.201901507 |
| [11] |
C. Long, Z. Jiang, J. Shangguan, et al., Chem. Eng. J. 406 (2021) 126848. DOI:10.1016/j.cej.2020.126848 |
| [12] |
S. Lu, L. Sui, J. Liu, et al., Adv. Mater. 29 (2017) 1603443. DOI:10.1002/adma.201603443 |
| [13] |
H. Song, X. Liu, B. Wang, Z. Tang, S. Lu, Sci. Bull. 64 (2019) 1788-1794. DOI:10.1016/j.scib.2019.10.006 |
| [14] |
H. Liu, Y. Sun, Z. Li, et al., Chin. Chem. Lett. 30 (2019) 1647-1651. DOI:10.1016/j.cclet.2019.06.012 |
| [15] |
Y. Sun, H. Qin, X. Geng, et al., ACS Appl. Mater. Interfaces. 12 (2020) 31738-31744. DOI:10.1021/acsami.0c05005 |
| [16] |
C. Xia, S. Zhu, T. Feng, M. Yang, B. Yang, Adv. Sci. 6 (2019) 1901316. DOI:10.1002/advs.201901316 |
| [17] |
M.L. Liu, B.B. Chen, C.M. Li, C.Z. Huang, Green. Chem. 21 (2019) 449-471. DOI:10.1039/C8GC02736F |
| [18] |
X. Geng, Y. Sun, Z. Li, et al., Small 15 (2019) 1901517. DOI:10.1002/smll.201901517 |
| [19] |
H. Liu, Y. Sun, Z. Li, et al., Nanoscale 11 (2019) 8458-8463. DOI:10.1039/C9NR01678C |
| [20] |
W. Li, Y. Liu, B. Wang, et al., Chin. Chem. Lett. 30 (2019) 2323-2327. DOI:10.1016/j.cclet.2019.06.040 |
| [21] |
B. Wang, J. Li, Z. Tang, B. Yang, S. Lu, Sci. Bull. 64 (2019) 1285-1292. DOI:10.1016/j.scib.2019.07.021 |
| [22] |
J. Fan, P.K. Chu, Small 6 (2010) 2080-2098. DOI:10.1002/smll.201000543 |
| [23] |
J. Wu, S. Li, H. Wei, Chem. Commun. 54 (2018) 6520-6530. DOI:10.1039/C8CC01202D |
| [24] |
Y. Zhou, B. Liu, R. Yang, J. Liu, Bioconjugate Chem. 28 (2017) 2903-2909. DOI:10.1021/acs.bioconjchem.7b00673 |
| [25] |
J. Wu, S. Li, H. Wei, Nanoscale Horiz. 3 (2018) 367-382. DOI:10.1039/C8NH00070K |
| [26] |
Y. Li, W. Zhu, J. Li, H. Chu, Colloid. Surf. B. 198 (2021) 111465. DOI:10.1016/j.colsurfb.2020.111465 |
| [27] |
A. Kumar, S. Das, P. Munusamy, et al., Environ. Sci. Nano 1 (2014) 516-532. DOI:10.1039/C4EN00052H |
| [28] |
B. Unnikrishnan, C.W. Lien, H.W. Chu, C.C. Huang, J. Hazard. Mater. 401 (2021) 123397. DOI:10.1016/j.jhazmat.2020.123397 |
| [29] |
A. Hasan, N.M.Q. Nanakali, A. Salihi, et al., Talanta 215 (2020) 120939. DOI:10.1016/j.talanta.2020.120939 |
| [30] |
W. Wang, S. Gunasekaran, TrAC Trends Anal. Chem. 126 (2020) 115841. DOI:10.1016/j.trac.2020.115841 |
| [31] |
M. Shamsipur, A. Safavi, Z. Mohammadpour, Sens. Actuators B: Chem. 199 (2014) 463-469. DOI:10.1016/j.snb.2014.04.006 |
| [32] |
S. Yousefinejad, H. Rasti, M. Hajebi, et al., Sens. Actuators B: Chem. 247 (2017) 691-696. DOI:10.1016/j.snb.2017.02.145 |
| [33] |
J. Guo, Y. Wang, M. Zhao, Sens. Actuators B: Chem. 297 (2019) 126739. DOI:10.1016/j.snb.2019.126739 |
| [34] |
J. Shi, T. Yin, W. Shen, Colloid. Surfaces. B 178 (2019) 163-169. DOI:10.1016/j.colsurfb.2019.03.012 |
| [35] |
L. Zhao, Z. Wu, G. Liu, et al., J. Mater. Chem. B 7 (2019) 7042-7051. |
| [36] |
C.Y. Chen, Y.Z. Tan, P.H. Hsieh, et al., ACS Sensors 5 (2020) 1314-1324. DOI:10.1021/acssensors.9b02486 |
| [37] |
Y. Liu, Q. Zhou, Y. Yuan, Y. Wu, Carbon 115 (2017) 550-560. DOI:10.1016/j.carbon.2017.01.035 |
| [38] |
F. Wang, C. Zhang, Q. Xue, H. Li, Y. Xian, Biosens. Bioelectron. 95 (2017) 21-26. DOI:10.1016/j.bios.2017.04.010 |
| [39] |
N. Shao, H. Wang, X. Gao, R. Yang, W. Chan, Anal. Chem. 82 (2010) 4628-4636. DOI:10.1021/ac1008089 |
| [40] |
Q. Li, D. Yang, Y. Yang, Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 244 (2021) 118882. DOI:10.1016/j.saa.2020.118882 |
| [41] |
H.W. Yang, P. Xu, B. Ding, et al., Eur. J. Inorg. Chem. 48 (2019) 5077-5084. |
| [42] |
R. Amini, E. Rahimpour, A. Jouyban, Anal. Chim. Acta 1117 (2020) 9-17. DOI:10.1016/j.aca.2020.04.001 |
| [43] |
Y. Guo, L. Zhang, S. Zhang, et al., Biosens. Bioelectron. 63 (2015) 61-71. DOI:10.1016/j.bios.2014.07.018 |
| [44] |
X. Wang, Y. Lu, K. Hua, D. Yang, Y. Yang, Anal. Bioanal. Chem. 413 (2021) 1373-1382. DOI:10.1007/s00216-020-03100-x |
| [45] |
A. Ibarbia, L. Sánchez-Abella, L. Lezama, H.J. Grande, V. Ruiz, Appl. Surf. Sci. 527 (2020) 146937. DOI:10.1016/j.apsusc.2020.146937 |
| [46] |
F. Li, Q. Chang, N. Li, et al., Chem. Eng. J. 394 (2020) 125045. DOI:10.1016/j.cej.2020.125045 |
| [47] |
Y. Li, W. Ma, J. Sun, et al., Carbon. 159 (2020) 149-160. DOI:10.1016/j.carbon.2019.11.093 |
| [48] |
M. Liang, Y. Wang, K. Ma, et al., Small. 16 (2020) 2002348. DOI:10.1002/smll.202002348 |
| [49] |
L. Su, Y. Cai, L. Wang, et al., Microchim. Acta. 187 (2020) 132. DOI:10.1007/s00604-019-4103-4 |
| [50] |
D. Yang, Q. Li, S.K. Tammina, Z. Gao, Y. Yang, Sens. Actuators B: Chem. 319 (2020) 128273. DOI:10.1016/j.snb.2020.128273 |
| [51] |
C. Li, Q. Liu, X. Wang, Y. Luo, Z. Jiang, Microchem. J. 159 (2020) 105508. DOI:10.1016/j.microc.2020.105508 |
| [52] |
F. Li, N. Li, C. Xue, et al., Chem. Eng. J. 382 (2020) 122484. DOI:10.1016/j.cej.2019.122484 |
| [53] |
F. Vetr, Z. Moradi-Shoeili, S. Özkar, Appl. Organomet. Chem. 32 (2018) e4465. DOI:10.1002/aoc.4465 |
| [54] |
P. Ling, Q. Zhang, T. Cao, F. Gao, Angew. Chem. Int. Ed. 57 (2018) 6819-6824. DOI:10.1002/anie.201801369 |
| [55] |
J. Zhang, X. Lu, D. Tang, et al., ACS Appl. Mater. Interfaces 10 (2018) 40808-40814. DOI:10.1021/acsami.8b15318 |
| [56] |
X. Ren, J. Liu, J. Ren, F. Tang, X. Meng, Nanoscale 7 (2015) 19641-19646. DOI:10.1039/C5NR04685H |
| [57] |
J. Zhang, S. Wu, X. Lu, P. Wu, J. Liu, Nano Lett. 19 (2019) 3214-3220. DOI:10.1021/acs.nanolett.9b00725 |
| [58] |
F. Honarasa, F. Peyravi, H. Amirian, J. Iran. Chem. Soc. 17 (2020) 507-512. DOI:10.1007/s13738-019-01787-z |
| [59] |
S. Li, E. Pang, C. Gao, et al., Chem. Eng. J. 397 (2020) 125471. DOI:10.1016/j.cej.2020.125471 |
| [60] |
A. Sun, L. Mu, X. Hu, ACS Appl. Mater. Interfaces. 9 (2017) 12241-12252. DOI:10.1021/acsami.7b00306 |
| [61] |
L. Zhao, X. Ren, J. Zhang, et al., New J. Chem. 44 (2020) 1988-1992. DOI:10.1039/C9NJ05655F |
| [62] |
M. Passi, V. Kumar, G. Packirisamy, Mater. Sci. Eng. C 107 (2020) 110255. DOI:10.1016/j.msec.2019.110255 |
| [63] |
K. Dehvari, S.H. Chiu, J.S. Lin, et al., Acta Biomater. 114 (2020) 343-357. DOI:10.1016/j.actbio.2020.07.022 |
| [64] |
H. Wang, Q. Lu, Y. Liu, et al., Sens. Actuators B: Chem. 250 (2017) 429-435. DOI:10.1016/j.snb.2017.04.117 |
| [65] |
R.E. Cuenca, W.J. Pories, J. Bray, Biol. Trace Elem. Res. 16 (1988) 151-154. DOI:10.1007/BF02797099 |
| [66] |
Y. Fuse, N. Saito, T. Tsuchiya, Y. Shishiba, M. Irie, Thyroid 17 (2007) 145-155. DOI:10.1089/thy.2006.0209 |
| [67] |
C. Ren, X. Hu, Q. Zhou, Adv. Sci. 5 (2018) 1700595. DOI:10.1002/advs.201700595 |
| [68] |
B. Jiang, D. Duan, L. Gao, et al., Nat. Protoc. 13 (2018) 1506-1520. DOI:10.1038/s41596-018-0001-1 |
| [69] |
H. Wang, K. Wan, X. Shi, Adv. Mater. 31 (2019) 1805368. DOI:10.1002/adma.201805368 |
| [70] |
M.K. Nazeeruddin, F.D. Angelis, S. Fantacci, et al., J. Am. Chem. Soc. 127 (2005) 16835-16847. DOI:10.1021/ja052467l |
| [71] |
Z. Zhang, Y. Liu, X. Zhang, J. Liu, Nano Lett. 17 (2017) 7926-7931. DOI:10.1021/acs.nanolett.7b04298 |
| [72] |
E. Golub, H.B. Albada, W.C. Liao, Y. Biniuri, I. Willner, J. Am. Chem. Soc. 138 (2016) 164-172. DOI:10.1021/jacs.5b09457 |
| [73] |
L. He, Y. Li, Q. Wu, et al., ACS Appl. Mater. Interfaces 11 (2019) 29158-29166. DOI:10.1021/acsami.9b09283 |
| [74] |
Y. Shen, L. Liang, S. Zhang, et al., Nanoscale 10 (2018) 1622-1630. DOI:10.1039/C7NR08636A |
 2021, Vol. 32
2021, Vol. 32 

