b Key Laboratory of Cosmetic, China National Light Industry, Beijing Technology and Business University, Beijing 100048, China
Piezochromic luminescent (PCL) materials have inspiring extensive application prospects in mechanosensors and photoelectronic devices because pressure exists everywhere in nature [1-4]. Their optical signal changes are mainly through the regulation of the stacking of molecules, while molecular structure is maintained [5-9]. Generally, molecular stacking modes are determined by the weak intermolecular interactions such as π–π interaction and hydrogen bonding [10-13]. Thus, the phase transition in solids often occurs as a result of such weak driving forces as shear or grinding stresses, which makes it easy to achieve significant changes in optical properties [14, 15]. However, the most current PCL materials have problems such as aggregation-caused quenching (ACQ) effect, easily affected by the external environment and poor self-recovery ability due to existence in the form of powder [16, 17, 18]. Therefore, there is still a challenge to design an effective PCL material.
Recently, PCL materials based on organic complexes [19-22], molecular assemblies [23, 24] and aggregation-induced emission luminogens [25-27] have made great research progress, but there are still some problems such as complicated synthesis steps. By contrast, carbon dots (CDs) as a new type of luminescent materials have a very simple synthesis method, accompanied by excellent optical properties and good biocompatibility [28-31], indicating it is a superior candidate for PCL material. However, most CDs in the powder state will probably produce ACQ effect, and their molecular structure will be destroyed under high pressure, resulting in fluorescence quenching [32]. To address this problem, it is critical to find a matrix combined with CDs. In this regard, the two-dimensional layered double hydroxides (LDHs) show its unique advantages. The rigid and confined space of LDH can disperse chromophores, which significantly improves the luminescent efficiency of chromophores [33, 34]. Thus, the CDs-LDHs composites were fabricated by combining the intercalation and hydrothermal method.
In order to achieve the device of PCL, the CDs-LDHs/polyvinyl alcohol (PVA) composite film was prepared by solvent evaporation. Moreover, dual emissions (fluorescence and phosphorescence) behavior of CDs can be obtained. When performing pressure sensing, the CDs-LDHs/PVA film shows a linear correlation with the pressure of MPa in terms of fluorescence and phosphorescence. The gradual increase in fluorescence intensity with the increase of pressure is attributed to the tight and order packing of CDs in interlayer of LDHs [35]. Nevertheless, the gradual decrease in phosphorescence intensity is caused by destroyed interaction force between CDs-LDHs composite material and PVA under external pressure. The PCL material with dual optical signal greatly improves the sensitivity of the sensor, which will open up a new window for advanced PCL sensors.
Firstly, to study the morphology of CDs in the interlayer of LDHs, solid CDs-LDHs were dispersed in hydrochloric acid solution to etch away the LDHs (expressed as CDs@hae) (Fig. S1 in Supporting information). The transmission electron microscopy (TEM) image of the CDs@hae shows the uniform spherical particles (Fig. 1a), and average particle diameter is about 2.90 nm (Fig. 1b). The lattice fringe spacing of the CDs@hae is about 0.23 nm, which coincides with the (100) crystal plane in graphite, indicating the crystalline core of the CDs is a graphite structure (Fig. 1c). The atomic force microscopy (AFM) image shows the CDs@hae has a thickness of ~1 nm, corresponding to a single layer of CDs (Fig. 1d). In contrast, pure CDs were obtained by direct hydrothermal AS under the same conditions (expressed as CDs@dc), TEM image shows that the CDs@dc is also uniform spherical particles with narrow particle size distribution of ~3.4 nm (Figs. 1e and f). A lattice plane with 0.33 nm was observed in the high resolution TEM (HRTEM), which coincided with the (002) plane of graphite (Fig. 1g). AFM image shows that the CDs@dc has a thickness of ~3 nm, corresponding to 3–6 CD layers (Fig. 1h). These results indicate that the confined microenvironment of LDHs promotes the formation of a single layer of CDs with a small and uniform particle size, which provides a prerequisite for determining the CD structure and interpreting the structure-property relationship [36].

|
Download:
|
| Fig. 1. (a) TEM image, (b) particle size distribution, (c) HRTEM image and (d) AFM image (inset: height profile along the white line) of the CDs@hae. (e) TEM image, (f) particle size distribution, (g) HRTEM image and (h) AFM image (inset: height profile along the white line) of the CDs@dc. | |
In addition, the X-ray diffraction (XRD) pattern of the MgAl-NO3-LDHs powder shows an obvious (003), (006) and (009) reflection peaks, attributed to typical NO3-LDHs (Fig. S2 in Supporting information). The d003 basal spacing is 8.8Å, which is in good agreement with that of MgAl-NO3-LDHs [37]. The intercalation of the AS into MgAl-NO3-LDHs increases the basal spacing from d = 8.8Å–15.3Å, indicating the molecule was successfully intercalated into LDHs. The surface defect states of the CDs determine their luminescent properties. Fig. 2a shows two Raman peaks of the CDs-LDHs at approximately 1310 and 1600 cm–1, which correspond to D and G bands of carbon with an intensity ratio (ID/IG) of 0.973 [38], indicating that the formation of graphene structure in the LDHs gallery. In addition, ATR-FTIR of the CDs-LDHs powder shows peaks of C=O group at 1633 cm−1 (Fig. 2b), indicating the appearance of CD luminescent group. X-ray photoelectron spectroscopy (XPS) was performed to further investigate the surface functional groups of CDs. A main peak centered at 399.5 eV is showed in the N 1 s spectrum, which is attributed to pyrrolic nitrogen instead of the formation of graphitic nitrogen and pyridinic nitrogen (Fig. S3 in Supporting information) [39]. The high-resolution C 1 s XPS spectrum of the CDs-LDHs shows two peaks corresponding to C=C (284.8 eV) and C=O (289 eV) bonds, respectively (Fig. 2c) [40]. The peak at 7–8 ppm in1H NMR corresponds to the aromatic ring hydrogen, indicating that the existence of aromatic carbon in the CDs@hae (Fig. S4 in Supporting information). The effects of LDHs on CDs can be studied using the cyclic voltammogram through a standard three-electrode system (Fig. S5 in Supporting information). By comparing the LUMO of the CDs-LDHs with the CDs@hae in Fig. 2d, it is concluded that the valence electrons of the CDs@hae can be stabilized by the inert LDHs layer, resulting in enhanced luminescence.

|
Download:
|
| Fig. 2. (a) Raman spectrum, (b) ATR-FTIR spectra, (c) C 1 s XPS spectra of the CDs-LDHs. (d) Band edge placement of the CDs-LDHs and the CDs@hae. | |
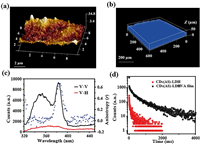
Atomic force microscopy (AFM) image of the CDs-LDHs/PVA film exhibits a homogeneous and flat surface with a small root-mean-square roughness of ~7.6 nm (Fig. 3a). 3D fluorescence confocal microscopy (600 × 600 μm) shows that the film has a uniform surface and fluorescence emission intensity (Fig. 3b), indicating the CDs-LDHs/PVA films are uniform in different sizes. Compared to the CDs-LDHs powder, the Raman spectra of the film shows that the value of the ID/IG is increased from 0.973 to 1.029, indicating the presence of PVA facilitates the expression sp3 carbon on the surface (luminescence-related defects) (Fig. S6 in Supporting information). The polarized fluorescence spectra has been applied to evaluate the alignment and orientation [41]. The anisotropic value (r) of the CDs-LDHs/PVA film is 0.60, which is larger than the powder (Fig. S7 in Supporting information), indicating that film is more orderly and arranged than the CDs-LDHs powder (Fig. 3c). Luminescence properties of the CDs-LDHs powder are described in detail in the supporting information (Figs. S8, S9 and Table S1 in Supporting information). The CDs-LDHs powder shows intense fluorescence and phosphorescence emission at 355 and 500 nm, respectively (Fig. S9b). At the same time, the excitation-emission two-dimensional plots show the absence of fluorescence and phosphorescence excitation dependence. This is because the CDs are uniform in size and the illuminating group is the only one (Fig. S10 in Supporting information). Additionally, the phosphorescence quantum yield of the CDs-LDHs/PVA film is 2.47% (Table S2 in Supporting information), and the phosphorescent lifetime of the CDs-LDHs/PVA film (316.20 ms) is longer than that of the CDs-LDHs powder (158.55 ms) (Fig. 3d and Table S3 in Supporting information). The fluorescent lifetime of the CDs-LDHs/PVA film is 1.38 ns (Fig. S11 and Table S4 in Supporting information). The CDs-LDHs/PVA film shows intense fluorescence and phosphorescence emission at 355 and 450 nm, respectively (Fig. S12 in Supporting information), indicating film has excellent dual emission performance.
|
Download:
|
| Fig. 3. (a) AFM image, (b) 3D fluorescence image and (c) polarized fluorescence profiles in the V–V, V–H modes and anisotropic value (r) of the CDs-LDHs/PVA film. (d) Time-resolved decays phosphorescence of the CDs-LDHs powder and the CDs-LDHs/PVA film. | |
The as-prepared CDs-LDHs/PVA film is applicated for pressure sensing. Its phosphorescence intensity decreases from 0 s to 15 s at 10 MPa, and reaches a stable state at 12 s. (Fig. 4a). Therefore, in the following study, the pressure reaction time is fixed to 12 s. As the pressure increases, the phosphorescence intensity of film decreases, but the fluorescence intensity increases regularly (Figs. 4b and c). Both the intensity of fluorescence and phosphorescence have a good linear relationship with pressure with the goodness of fit (Figs. S13 and S14 in Supporting information). The change of phosphorescence emission under pressure indicates that the interaction force between CDs-LDHs composite and PVA is destroyed by external pressure, resulting in inefficient intersystem crossing conversion and weak phosphorescence. The change of fluorescence emission under pressure supports the contraction-expansion transformation of the interlayer region under external pressure. Under external pressure, the non-radiative transition of CDs is suppressed and the array is more orderly. Moreover, the reversibility experiment shows the original phosphorescence intensity of the film can be restored to almost 87% by alternating treatment in pressure of 10 MPa and heating for 3 min (Fig. 4d). Therefore, the change in interaction force can be regarded as the main cause of the difference in phosphorescence. To develop potential luminescence sensor devices, light stability is an important factor. Thus, the stability of the CDs-LDHs/PVA film was examined by irradiation under UV light and phosphorescence mode irradiation for 1 h, respectively. The intensity of fluorescence and phosphorescence of the irradiated film was reduced to 77% and 83% of its original value, respectively (Fig. S15a in Supporting information). Therefore, the limitation problem of the optical material with poor stability in practical applications is solved effectively.

|
Download:
|
| Fig. 4. Phosphorescence emission spectra of (a) the CDs-LDHs/PVA film in the presence of 10 MPa pressure at various response time. (b) Phosphorescence and (c) fluorescence emission spectra of the CDs-LDHs/PVA film in the presence of various external pressures. (d) Reversibility of phosphorescence intensity of the CDs-LDHs/PVA film (Inset: the digital photographs of the film by alternating treatment in pressure under UV illumination at 365 nm). | |
The pressure-enhanced luminescence mechanism was explored by monitoring the changes of the luminescence lifetime during the contraction-expansion cycle. Fluorescence and phosphorescence lifetime of the CDs-LDHs/PVA film were studied at the original, compressed and recovered state, respectively. The fluorescence lifetime of the original CDs-LDHs/PVA film is 1.38 ns, and then applying a pressure of 10 MPa, the lifetime is improved to 1.58 ns. Finally, the lifetime is recovered to 1.41 ns with a simple heat at 50 ℃ for 3 min (Table S5 in Supporting information). The change could be ascribed to that the CD movement is restricted and the arrangement was more orderly under external pressure. Meanwhile, the phosphorescence lifetime of the CDs-LDHs/PVA film goes through a contraction-expansion cycle, from 316.20 ms to 272.45 ms and finally returns to 320.68 ms (Table S6 in Supporting information). The decreased phosphorescence lifetime originates that external pressure induces the changes of the molecule stacking and hydrogen bond interaction.
In conclusion, based on the luminescence property of CDs, the confinement effect of LDHs and the abundant hydrogen bonds of PVA, the CDs-LDHs/PVA film with adjustable size, good flexibility and dual luminescence characteristics were prepared by solvent evaporation method. The problems such as ACQ phenomenon, complicated preparation steps and the quenching of fluorescence under high pressure were solved. Excellent dual emission performance and interaction were researched systematically. In the pressure response test, with the increase of pressure, fluorescence intensity of the film enhances regularly resulted from the non-radiative transition of CDs is suppressed and the array is more orderly; the phosphorescence intensity decrease regularly is attributed to the destroyed interaction force between CDs-LDHs composite and PVA at external pressure. The dual emission performance shows a good linear relationship between intensity and pressure. Therefore, the dual emission film with great sensitivity, stability and reversibility boost the sensitivity of pressure-sensing.
Declaration of competing interestThe manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. The authors declare no competing financial interest.
AcknowledgmentsThis work was supported by the National Key R & D Program of China (No. 2019YFC1906100), the National Natural Science Foundation of China (Nos. 21571014 and 21521005), the Beijing Municipal Natural Science Foundation (No. 2172044), the Open Research Fund Program of Key Laboratory of Cosmetic, China National Light Industry, Beijing Technology and Business University (No. KLC-2019-ZD1), and the Fundamental Research Funds for the Central Universities (No. 12060093063).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.12.035.
| [1] |
G. Xiao, X. Yang, X. Zhang, et al., J. Am. Chem. Soc. 137 (2015) 10297-10303. DOI:10.1021/jacs.5b05629 |
| [2] |
T. Gong, Q. Sui, P. Li, et al., Small 15 (2019) 1803648. |
| [3] |
G.V. Biesold-McGee, S. Liang, B. Brettmann, et al., Angew. Chem. Int. Ed. 60 (2021) 9772-9788. DOI:10.1002/anie.202008395 |
| [4] |
Z. Wang, Z. Ma, Y. Wang, et al., Adv. Mater. 27 (2015) 6469-6474. DOI:10.1002/adma.201503424 |
| [5] |
Y. Sagara, S. Yamane, M. Mitani, et al., Adv. Mater. 28 (2016) 1073-1095. DOI:10.1002/adma.201502589 |
| [6] |
B. He, Z. Chang, Y. Jiang, et al., Dyes Pigm. 106 (2014) 87-93. DOI:10.1016/j.dyepig.2014.02.026 |
| [7] |
Z. Wu, S. Mo, L. Tan, et al., Small 14 (2018) 1802524. DOI:10.1002/smll.201802524 |
| [8] |
Q. Qi, J. Qian, X. Tan, et al., Adv. Funct. Mater. 25 (2015) 4005-4010. DOI:10.1002/adfm.201501224 |
| [9] |
C. Weng, N. Fan, T. Xu, et al., Chin. Chem. Lett. 31 (2020) 1490-1498. DOI:10.1016/j.cclet.2019.11.009 |
| [10] |
B. Li, K. Seth, B. Niu, et al., Angew. Chem. Int. Ed. 57 (2018) 3401-3405. DOI:10.1002/anie.201713357 |
| [11] |
W. Yu, M. Shevtsov, X. Chen, et al., Chin. Chem. Lett. 31 (2020) 1366-1374. DOI:10.1016/j.cclet.2020.02.036 |
| [12] |
Y. Yang, X. Yang, X. Fang, et al., Adv. Sci. 5 (2018) 1801187. DOI:10.1002/advs.201801187 |
| [13] |
Z. Wang, F. Yu, W. Chen, et al., Angew. Chem. Int. Ed. 59 (2020) 17580-17586. DOI:10.1002/anie.202005933 |
| [14] |
G. Guan, M. Wu, M.Y. Han, Adv. Funct. Mater. 30 (2019) 1903439. |
| [15] |
Z. Man, Z. Lv, Z. Xu, et al., Adv. Funct. Mater 30 (2020) 2000105. DOI:10.1002/adfm.202000105 |
| [16] |
M. Zhang, Y. Li, K. Gao, et al., Dyes Pigm. 173 (2020) 107928. DOI:10.1016/j.dyepig.2019.107928 |
| [17] |
M. Yang, I.S. Park, Y. Miyashita, et al., Angew. Chem. Int. Ed. 132 (2020) 2-9. DOI:10.1002/ange.201914768 |
| [18] |
J. Cao, W. Sun, J. Fan, Chin. Chem. Lett. 31 (2020) 1402-1405. DOI:10.1016/j.cclet.2019.10.006 |
| [19] |
Y. Qi, N. Ding, Z. Wang, et al., ACS Appl. Mater. Interfaces 11 (2019) 8676-8684. DOI:10.1021/acsami.8b21617 |
| [20] |
T. Seki, N. Tokodai, S. Omagari, et al., J. Am. Chem. Soc. 139 (2017) 6514-6517. DOI:10.1021/jacs.7b00587 |
| [21] |
T. Seki, Y. Takamatsu, H. Ito, J. Am. Chem. Soc. 138 (2016) 6252-6260. DOI:10.1021/jacs.6b02409 |
| [22] |
B.Y.W. Wong, H.L. Wong, Y.C. Wong, et al., Chem. Sci. 8 (2017) 6936-6946. DOI:10.1039/C7SC02410J |
| [23] |
L. Bai, P. Bose, Q. Gao, et al., J. Am. Chem. Soc. 139 (2016) 436-441. |
| [24] |
Y. Liu, Q. Zeng, B. Zou, et al., Angew. Chem. Int. Ed. 57 (2018) 15670-15674. DOI:10.1002/anie.201810149 |
| [25] |
W. Zhao, Z. He, Q. Peng, et al., Nat. Commun. 9 (2018) 3044. DOI:10.1038/s41467-018-05476-y |
| [26] |
G. Weng, S. Thanneeru, J. He, He J. , Adv. Mater. 30 (2018) 1706526. DOI:10.1002/adma.201706526 |
| [27] |
C. Calvino, A. Guha, C. Weder, et al., Adv. Mater 30 (2018) 1704603. DOI:10.1002/adma.201704603 |
| [28] |
M. Han, S. Zhu, S. Lu, et al., Nano Today 19 (2018) 201-218. DOI:10.1016/j.nantod.2018.02.008 |
| [29] |
J.B. Brito, T.M.H. Costa, F.S. Rodembusch, et al., Carbon 91 (2015) 234-240. DOI:10.1016/j.carbon.2015.04.062 |
| [30] |
M.L. Liu, B.B. Chen, C.M. Li, et al., Green Chem. 21 (2019) 449-471. DOI:10.1039/C8GC02736F |
| [31] |
S. Lu, G. Xiao, L. Sui, et al., Angew. Chem. Int. Ed. 56 (2017) 6187-6191. DOI:10.1002/anie.201700757 |
| [32] |
Q. Wang, S. Zhang, B. Wang, et al., Nanoscale Horiz. 4 (2019) 1227-1231. DOI:10.1039/C9NH00287A |
| [33] |
Taviot-Guého C., V. Prévot, C. Forano, et al., Adv. Funct. Mater. 28 (2018) 1703868. DOI:10.1002/adfm.201703868 |
| [34] |
L.Q. Bai, N. Xue, X.R. Wang, et al., Nanoscale 9 (2017) 6658-6664. DOI:10.1039/C6NR09648D |
| [35] |
L. Zhang, J. Ge, C. Lu, et al., Sens. Actuators B:Chem. 268 (2018) 519-528. DOI:10.1016/j.snb.2018.04.145 |
| [36] |
L. Bai, N. Xue, Y. Zhao, et al., Nano Res. 11 (2018) 2034-2045. DOI:10.1007/s12274-017-1820-z |
| [37] |
X. Kong, X. Wang, H. Cheng, et al., J. Mater. Chem. 7 (2019) 230-236. |
| [38] |
S. Zhang, Y. Zhang, W. Jiang, et al., Carbon 107 (2016) 162-170. DOI:10.1016/j.carbon.2016.05.056 |
| [39] |
W. Liu, R. Liang, Y. Lin, Nanoscale 12 (2020) 7888-7894. DOI:10.1039/D0NR00272K |
| [40] |
C. Shi, J. Yao, X. Wang, et al., J. Mater. Chem. C 7 (2019) 14170-14180. DOI:10.1039/C9TC04277F |
| [41] |
Z. Xia, N. Xue, W. Shi, et al., J. Phys. Chem. C 123 (2019) 30536-30544. DOI:10.1021/acs.jpcc.9b08488 |
 2021, Vol. 32
2021, Vol. 32 


