b Key Laboratory of Sensor Analysis of Tumor Marker, Ministry of Education, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, China;
c Science and Technology on Space Physics Laboratory, Beijing 10076, China;
d School of Grain Science and Technology, Jiangsu University of Science and Technology, Zhenjiang 212003, China
All-inorganic cesium lead halide (CsPbX3, X = Cl, Br, I) perovskite quantum dots (QDs) have been widely applied in optoelectronic and photovoltaic devices due to their superior optical/electronic performances [1, 2]. However, the application of these materials in the analysis field has rarely been reported due to the poor stability in the polar solvents [3, 4]. Considering most targets exist in aqueous system, a strong polar solvent, this instability is no doubt a great obstacle. To extend the application area, the most widely applied methods are to enhance the stability through the protection of nanospheres or ligands [5, 6]. But besides complex modification progress, the dispersity of CsPbX3 QDs in water may be affected by the protection layer. Another method is to drive the target molecule to escape from aqueous solution into the non-polar solution containing CsPbX3 QDs. For example, rat brain micro-dialysis solution containing hydrogen sulfide (H2S) was added into to the phosphoric acid solution. H2S gas was released and injected into n-hexane solution to quench the fluorescence of CsPbBr3 QDs [7]. Obviously, this strategy is only feasible for some special targets.
As a novel emitter, the electrochemiluminescence (ECL) performance of perovskite nanocrystals (NCs) has also been explored [8-11]. Zhu's group [11] researched the ECL emission of CsPbBr3 QDs thin films in organic solution, and the study showed that the ECL efficiency was five times higher than the classical Ru(bpy)32+/tri -n-propylamine(TPrA) system. For the application in aqueous system, CsPbBr3 QDs and coreactant are encapsulated into SiO2 matrix, which provides an efficient method to obtain stable ECL emission [12]. Being protected by SiO2 shell, CsPbBr3 QDs are isolated from polar solvents. But the interactions between CsPbBr3 QDs and other environmental factors to regulate the ECL performance are also inevitably affected. For example, the electrical signal is critical to excite ECL emission while SiO2 shell has a low conductivity. The strategy that simultaneously satisfy the requirement of stability, strength and flexible regulation of the ECL biosensing based on CsPbX3 QDs has not been well developed. Bipolar electrode (BPE) technology is a promising tool in electrochemistry, which allows the remote control of electrical field applied to the electrode without the direct connection with power supply [13-15]. Unlike traditional three-electrode system, the electrochemical oxidation and reduction reactions occur at the two ends of the BPE. The cathodic and anodic ends can be immersed into one solution (open BPE) or two physically separated solutions (closed BPE). Because of the unique structure and great flexibility of closed BPE, many researches have been conducted in previous works, which is also an ideal platform for the application of CsPbX3 QDs.
Tetracycline (Tc) is a widely used broad-spectrum antibiotic [16-19]. The excessive use of tetracycline will lead to many residues in animal products, and the remaining of hazardous materials in the food chain is a great threaten to human health [20, 21]. In this work, a novel closed BPE system using the stable and strong ECL performance of all-inorganic perovskite CsPbBr3 QDs has been constructed for sensitive detection of Tc as a proof of concept experiment. Through the electrons conducted by the BPE as the bridge, the quantitative detection of Tc aqueous solution in the cathode reaction cell was successfully achieved by recording the ECL intensity of CsPbBr3 immersed under organic solution at the anode cell of the closed BPE. The ECL emission can be flexibly regulated by environmental factors of both polar and non-polar solvents and the interface status of the BPE, which affords wide space for the further deep research and improvement on the ECL performance of CsPbBr3 QDs.
The detection of the closed BPE is based on the principle of conservation of charge [22-24]. In this work, anode and cathode microcells of the fabricated BPE chip were modified with the luminescent reagent CsPbBr3 QDs and Tc aptamer respectively. CsPbBr3 QDs film in the anode microcell was immersed into organic electrolyte solution while the cathode microcell was filled with PBS aqueous solution. When an enough voltage was applied on the BPE, a stable and strong ECL emission of CsPbBr3 could be excited with the dual enhancement of TPrA and H2O2. But the aptamer in the cathode cell would enhance the steric hindrance of the electrode interface and prevent electron transfer, resulting in a sharp drop in ECL strength [25]. After the addition of Tc molecules, it would bind with the aptamer and the aptamer was detached from the sensing interface, and the ECL signal was restored [26] The change of the ECL intensity was proportional to the concentration of Tc. So the quantitation of targets in the aqueous solution could be obtained through the ECL signal generated in the organic medium, the assembly process was shown in Scheme 1.

|
Download:
|
| Scheme 1. The processes of constructing the ECL aptasensor for tetracycline detection. (A) Schematic diagram of the assembly process of the sensing cell. (B) Schematic diagram of ultrasensitive detection of Tc, and (C) schematic diagram of the BPE with the 3D printed microcell. | |
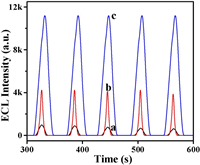
The detailed information about the reagents, apparatus, the preparation of CsPbBr3 QDs, and the fabrication process of BPE are provided in the Supporting information. The well dispersed uniform cubic CsPbBr3 QDs of approximately 10 nm were observed in the transmission electron microscopy (TEM) image. And the solution generated bright green light emission under ultraviolet light, indicating the successful preparation (Fig. S1 in Supporting information).Fig. 1 presents the ECL responses of perovskite QDs modified bipolar electrodes under different experiment conditions. When the anode and cathode microcell were only CsPbBr3 QDs/organic electrolyte solution and PBS solution respectively, the ECL intensity was about 1000 (Fig. 1, curve a). After adding the co-reactant TPrA into the anode microcell, TPrA can be oxidized to TPrA+null and then produced TPrAnull, which can inject electrons into the LUMO of CsPbBr3 QDs to produce anion radicals, which then reacted with the oxidized cation radicals and generated unstable excited substances ([CsPbBr 3]*). The relaxation of excitation state to the ground state produced 5 times enhanced ECL emission (Fig. 1, curve b) [27, 28]. After adding a certain amount of H2O2 in the cathode microcell, the ECL intensity was further enhanced to about 10 times (Fig. 1, curve c). The reduction of H2O2 can promote the electron transport, thus making the oxidation reaction of CsPbBr3 QDs more complete. Through adjusting the environmental factors of both polar and non-polar solvents, the ECL performance was greatly improved [29, 30].
|
Download:
|
| Fig. 1. ECL responses of CsPbBr3 QDs (a), CsPbBr3 QDs/TPrA (b) and CsPbBr3 QDs/TPrA/H2O2 (c). | |
Under optimized experimental conditions (Figs. S2 and S3 in Supporting information), CsPbBr3 QDs showed excellent and stable ECL intensity with the presence of TPrA and H2O2 (Fig. 2A, curve a). When the aptamer was immobilized on the surface of the cathode reaction cell (Fig. 2A, curve b), the ECL intensity decreased sharply. After adding Tc solution (Fig. 2A, curve c), the ECL intensity increased immediately. To study the fixation and detachment of the aptamer, electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) were used to monitor the connection between the aptamer and GO and the process of detachment from the electrode surface after introducing Tc. In Fig. 2B, Ret (electron-transfer resistance) increased significantly from 500 Ω to 3000 Ω after the modification of aptamer, indicating that the aptamer was successfully assembled on the ITO surface through a non-covalent bond connection with GO. Then the impedance decreased after adding Tc solution due to the desorption of the Tc-aptamer complex from the BPE. As shown in Fig. S4 (Supporting information), the current change during the assembly process was opposite to the impedance change. All these results indicated that the proposed ECL aptasensor has been successfully prepared and can be used for the detection of Tc.

|
Download:
|
| Fig. 2. (A) ECL responses of CsPbBr3 QDs/TPrA/H2O2 (a), CsPbBr3 QDs/TPrA/H2O2-Aptamer (b) and CsPbBr3 QDs/TPrA/H2O2-Ampater/Tc (c). (B) EIS spectra of bare BPE (a), Aptamer/BPE (b) and Tc/aptamer/BPE (c). | |
Fig. 3A shows the ECL response of aptamer sensors at different concentrations of Tc solutions. The ECL signal gradually increased with increasing Tc concentration due to the release of more Tc-aptamer complexes. A good linear relationship was obtained between the ECL intensity of the aptasensor and the logarithm of the Tc concentration in the range of 1 nmol/L and 1 mmol/L (R2 = 0.9954) (Fig. 3B). In addition, the detection limit was estimated to be 0.33 nmol/L (S/N = 3). As shown in Fig. S5 (Supporting information), the selectivity, reproducibility and stability of this ECL aptasensing system were explored. After adding 100 nmol/L of various antibiotics solutions (pH 7.4), including ampicillin, oxytetracycline, chloramphenicol, and streptomycin, only Tc caused significant enhancement of ECL intensity, showing the good selectivity and anti-interference ability. No significant signal changes occurred and the calculated relative standard deviation (RSD) was 6.33% under 5 times test, indicating relatively good stability performance. This biosensor was further applied in the detection of milk samples with a good recovery ranging from 98.4%–102.7%, and the RSD were less than 7.6% (Table S1 in Supporting information).

|
Download:
|
| Fig. 3. (A) ECL responses of the aptasensor at different concentrations of Tc: (10−9 → 10-3 mol/L) and (B) the corresponding linear calibration curve for Tc detection. | |
In summary, we explored the flexibly regulated ECL performance of CsPbBr3 QDs based on the closed BPE system. Through adjusting the polar solvent in anode microcell, the non-polar solvent in cathode microcell and the interface status of BPE, a stable and strong ECL from CsPbBr3 QDs was achieved and applied in the detection of Tc in aqueous solution. Compared with traditional methods to overcome the intrinsic instability of perovskite QDs in polar medium, the reported method doesn't need any further surface modifications, has no limitations on the targets and can provide wide development space for further deep research, which may open a new direction for the ECL sensing of CsPbBr3 QDs.
Declaration of competing interestWe declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work.
AcknowledgmentsWe acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21876068, 21705059 and 21675066). Project of Faculty of Agricultural Equipment of Jiangsu University (No. NZXB20200210).
Appendix A. Supplementary dataSupplementarymaterial related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.01.029.
| [1] |
L. Protesescu, S. Yakunin, M.I. Bodnarchuk, et al., Nano Lett. 15 (2015) 3692-3696. DOI:10.1021/nl5048779 |
| [2] |
A. Swarnkar, V.K. Ravi, A. Nag, ACS Energy Lett. 2 (2017) 1089-1098. DOI:10.1021/acsenergylett.7b00191 |
| [3] |
F. Palazon, Q.A. Akkerman, M. Prato, et al., ACS Nano 10 (2016) 1224-1230. DOI:10.1021/acsnano.5b06536 |
| [4] |
M. Saliba, T. Matsui, K. Domanski, et al., Science 354 (2016) 206-209. DOI:10.1126/science.aah5557 |
| [5] |
Y. Cao, W. Zhu, H. Wei, et al., Anal. Chem. 92 (2020) 4123-4130. DOI:10.1021/acs.analchem.0c00070 |
| [6] |
H. Peng, W. Wu, Z. Huang, et al., Electrochem. Commun 111 (2020) 106667. |
| [7] |
C. Chen, Q. Cai, F. Luo, Anal. Chem. 91 (2019) 15915-15921. DOI:10.1021/acs.analchem.9b04387 |
| [8] |
Z. Cai, F. Li, W. Xu, et al., Nano Res. 11 (2018) 1447-1455. DOI:10.1007/s12274-017-1760-7 |
| [9] |
Y. Huang, M. Fang, G. Zou, et al., Nanoscale 8 (2016) 18734-18739. DOI:10.1039/C6NR06456F |
| [10] |
Y. Huang, X. Long, D. Shen, et al., Inorg. Chem. 56 (2017) 10135-10138. DOI:10.1021/acs.inorgchem.7b01515 |
| [11] |
J. Xue, Z. Zhang, F. Zheng, et al., Anal. Chem. 89 (2017) 8212-8216. DOI:10.1021/acs.analchem.7b02291 |
| [12] |
L. Li, Z. Zhang, Y. Chen, et al., Adv. Funct. Mater. 29 (2019) 1902533. DOI:10.1002/adfm.201902533 |
| [13] |
A. Arora, J.C.T. Eijkel, W.E. Morf, et al., Anal. Chem. 73 (2001) 5633-5633. DOI:10.1021/ac011762v |
| [14] |
L. Koefoed, S.U. Pedersen, K. Daasbjerg, Curr. Opin. Electrochem. 2 (2017) 13-17. DOI:10.1016/j.coelec.2017.02.001 |
| [15] |
O. Phuakkong, M. Sentic, H. Li, et al., Langmuir 32 (2016) 12995-13002. DOI:10.1021/acs.langmuir.6b03040 |
| [16] |
M.H. Khan, H. Bae, J.-Y. Jung, J. Hazard. Mater. 181 (2010) 659-665. DOI:10.1016/j.jhazmat.2010.05.063 |
| [17] |
W. Yang, F. Zheng, Y. Lu, et al., Ind. Eng. Chem. Res. 50 (2011) 13892-13898. DOI:10.1021/ie202166g |
| [18] |
L. Zhou, D.J. Li, L. Gai, et al., Sens. Actuator B: Chem. 162 (2012) 201-208. DOI:10.1016/j.snb.2011.12.067 |
| [19] |
X.D. Zhu, Y.J. Wang, R.J. Sun, et al., Chemosphere 92 (2013) 925-932. DOI:10.1016/j.chemosphere.2013.02.066 |
| [20] |
L. Long, Y. Han, X. Yuan, et al., Food Chem. 331 (2020) 127359. DOI:10.1016/j.foodchem.2020.127359 |
| [21] |
J. Qian, H. Cui, X. Lu, et al., Chem. Eng. J. 401 (2020) 126017. DOI:10.1016/j.cej.2020.126017 |
| [22] |
X. Lin, L. Zheng, G. Gao, et al., Anal. Chem. 84 (2012) 7700-7707. DOI:10.1021/ac300875x |
| [23] |
S. Wu, Z. Zhou, L. Xu, et al., Biosens. Bioelectron. 53 (2014) 148-153. DOI:10.1016/j.bios.2013.09.042 |
| [24] |
X. Zhang, C. Chen, J. Li, et al., Anal. Chem. 85 (2013) 5335-5339. DOI:10.1021/ac400805f |
| [25] |
K. Yan, Y. Liu, Y. Yang, et al., Anal. Chem. 87 (2015) 12215-12220. DOI:10.1021/acs.analchem.5b03139 |
| [26] |
H.M. Meng, H. Liu, H. Kuai, et al., Chem. Soc. Rev. 45 (2016) 2583-2602. DOI:10.1039/C5CS00645G |
| [27] |
S. Deng, H. Ju, Analyst 138 (2013) 43-61. DOI:10.1039/C2AN36122A |
| [28] |
X. Tan, B. Zhang, G. Zou, J. Am. Chem. Soc. 139 (2017) 8772-8776. DOI:10.1021/jacs.7b05073 |
| [29] |
L. Bouffier, S. Arbault, A. Kuhn, et al., Anal. Bioanal. Chem. 408 (2016) 7003-7011. DOI:10.1007/s00216-016-9606-9 |
| [30] |
N. Hao, J. Lu, Z. Dai, et al., Electrochem. commun. 108 (2019) 106559. DOI:10.1016/j.elecom.2019.106559 |
 2021, Vol. 32
2021, Vol. 32 


