b Environmental Process Modelling Centre, Nanyang Environment and Water Research Institute, Nanyang Technological University, Singapore 637141, Singapore;
c School of Mechanical and Aerospace Engineering, Nanyang Technological University, Singapore 639798, Singapore;
d Department of Environmental Science and Engineering, Wuhan University, Wuhan 430079, China;
e Henan Institute of Advanced Technology, Zhengzhou University, Zhengzhou 450052, China
The increasing activities of textile, printing, dyeing, and pharmaceutical industries have led to the discharge of large quantities of wastewater into the aquatic environment [1-3]. Such wastewater is highly stable, contains high concentrations of organic pollutants, and is highly non-biodegradable. It also possesses characteristics of high teratogenicity, carcinogenicity, and biotoxicity, which are greatly detrimental to human health and local ecosystems [4, 5]. However, existing water treatment strategies, including physical, chemical, and biological methods, exhibit drawbacks and limitations such as (1) merely changing the phases of pollutants instead of completely degrading them (e.g., adsorption), (2) exhibiting a high operating cost and low degradation efficiency (e.g., chemical oxidation), and (3) demonstrating a general ineffectiveness in degrading toxic and non-biodegradable pollutants (e.g., biological oxidation) [6]. Therefore, developing novel, cost-effective, and efficient technologies for wastewater treatment is a vital imperative.
Recently, an emerging advanced oxidation process (AOP) pertaining to sulfate radicals (SO4•‒) has attracted extensive interest in the field of environmental remediation owing to its low cost, high efficiency, and wide application range [7-9]. SO4•‒ possesses a higher redox potential (E0=2.5–3.1 V) and a longer half-life (30−40 μs) than hydroxyl radicals (•OH) produced in Fenton processes, and it is also capable of significantly mineralizing organic pollutants [10]. SO4•‒ can be generated by peroxymonosulfate (PMS) and persulfate (PDS) with the incorporation of alkalines, heat, light, ultrasound, carbon materials, and transition metal ions [11-13]. Compared with PDS, PMS exhibits an asymmetric structure and a longer superoxide O-O bond, which increases its probability of being activated [14]. Cobalt ions (Co2+) have proved to be the most effective candidate for activating PMS. However, they are difficult to recover and can potentially result in secondary pollution [15]. Thus, it is of paramount importance to develop efficient and reusable cobalt-based heterogeneous catalysts for PMS activation.
Spinel-type oxides have received much attention from researchers due to the synergy between their constituent metal atoms, excellent chemical and thermal stability, and high mechanical resistance [16]. The cobalt aluminate (CoAl2O4) spinel, known as Thenard's blue, is extensively employed as a color filter, pigment layer, as well as an adsorbent and a catalyst for pollutant removal due to its acid-base resistance, excellent chemical stability, good optical properties, and high heat resistance [17-19]. Some spinel-type oxides, such as CoFe2O4 [20], CuCo2O4 [21], and NiCo2O4 [22], have demonstrated high efficiency in activating PMS for pollutant degradation. Thus, it is postulated that CoAl2O4 can also function as a PMS activator for the treatment of organic pollutants.
Herein, mesoporous CoAl2O4 with a spinel structure was synthesized by a combustion method, and it was used for the degradation of organic contaminants through PMS activation for the first time. The effects of catalyst loading, PMS dosage, initial pH, and common ions on the catalytic performance were evaluated systematically. Meanwhile, the stability and general applicability of the catalyst were also investigated, and the dominant active species and degradation mechanism in the catalytic degradation system were analyzed.
Analytical reagent-grade chemicals of cobalt nitrate hexahydrate, aluminum nitrate nonahydrate, and triethanolamine were obtained from Sinopharm Chemical Co., Ltd. All the other chemical reagents were of analytical grade. CoAl2O4 was prepared through a combustion method based on existing literature [23]. First, 10 mmol/L of cobalt nitrate and 20 mmol/L of aluminum nitrate were added to 30 mL of deionized water, respectively. Then, the two solutions were added to a beaker and stirred for 30 min. Afterwards, 12 mL of triethanolamine was introduced to the solution as a fuel with a 2:1 molar ratio of triethanolamine to the total metal ions. A rose-colored flocculent precipitate was then formed, and HCl (12 mol/L) was added dropwise to the mixture until a clear rose-colored solution was obtained. The solution was further heated at 100 ℃ to remove excess water. Finally, the obtained product was converted to mesoporous CoAl2O4 by thermal decomposition at 500 ℃ for 3 h. Co3O4 was prepared by a similar procedure, in which aluminum nitrate was not added.
For comparison, a CoAl2O4 sample was also synthesized by a precipitation-calcination method. Briefly, 20 mmol/L of aluminum nitrate was added dropwise to 10 mmol/L of cobalt nitrate solution under magnetic stirring. Afterwards, the solution pH was adjusted to 10 by adding NaOH. The precipitate was washed with deionized water and heated at 100 ℃ for 2 h. Finally, the residue was calcinated at 500 ℃ for 3 h and designated as CoAl2O4-p. The characterization details of the catalysts and the experimental procedures are provided in Supporting information.
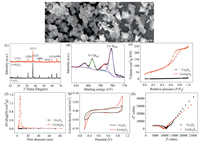
Figs. 1a and b reveal the typical scanning electron microscopy (SEM) images of Co3O4 and CoAl2O4. Co3O4 exhibits an octahedral morphology in high yield. The average particle size was measured to be approximately 2 μm. As for CoAl2O4, such an octahedral morphology was partly destroyed due to the Al atoms occupying the octahedral coordination sites.
|
Download:
|
| Fig. 1. SEM images of (a) Co3O4 and (b) CoAl2O4, (c) XRD patterns of Co3O4 and CoAl2O4, (d) Co 2p XPS spectrum of CoAl2O4, (e) N2 adsorption-desorption isotherm curves, (f) pore size distributions, CV (g) and EIS Nyquist (h) plots of Co3O4 and CoAl2O4. | |
The crystal structures of CoAl2O4 and Co3O4 were characterized by X-ray diffraction (XRD), as illustrated in Fig. 1c. The XRD peaks of CoAl2O4 and Co3O4 are identical to those of the cubic spinel phase of CoAl2O4 (JCPDS No. 44-0160) and Co3O4 (JCPDS No. 42-1467), respectively [24]. The positions of the XRD peaks are almost identical since CoAl2O4 and Co3O4 share the same spinel cubic (Fd3m) crystallographic structure [25].
X-ray photoelectron spectroscopy (XPS) was performed to verify the formation of pure CoAl2O4. As displayed in Fig. 1d, the high-resolution spectrum of Co 2p is deconvoluted into two peaks centered at 781.0 and 796.7 eV, which correspond to Co 2p3/2 and Co 2p1/2, respectively. Satellite peaks are also visible at around 786.5 and 803.1 eV [26]. The energy separation between the spin-orbit doublets is 15.7 eV, which is characteristic of Co2+. Moreover, no peak can be observed in the vicinity of 780 eV, thereby indicating the absence of the Co3O4 phase [25].
As shown in Fig. 1e, the N2 sorption isotherm of CoAl2O4 corresponds to a typical Ⅳ isotherm with a H3 hysteresis loop, which indicates the presence of mesopores in CoAl2O4 [27]. In contrast, no hysteresis loop can be observed in the N2 sorption isotherm of Co3O4, which indicates a non-mesoporous structure. These results can be further confirmed by the Barrett-Joyner-Halenda (BJH) pore diameter distribution, as demonstrated in Fig. 1f. Moreover, the specific surface area of CoAl2O4 (216.2 m2/g) is 28 times higher than that of Co3O4 (7.7 m2/g). Thus, CoAl2O4 was expected to provide more active sites that facilitate adsorption and reaction processes.
Electrochemical tests were conducted to evaluate the charge transfer capacities of Co3O4 and CoAl2O4. As shown in Fig. 1g, the cyclic voltammetry (CV) curve of CoAl2O4 exhibits a larger loop area than that of Co3O4, indicating a superior electron transfer capability. Meanwhile, the electrochemical impedance spectroscopy (EIS) Nyquist plots of CoAl2O4 correspond to a smaller charge transfer resistance than that of Co3O4 (Fig. 1h), suggesting a more efficient electron transfer in CoAl2O4.
The catalytic activities of the as-obtained catalysts were assessed by the oxidative degradation of RhB through PMS activation. As shown in Fig. 2a, in 40 min, CoAl2O4 adsorbed a negligible amount of RhB, and only 4.3% of RhB was degraded by PMS. In the presence of PMS, 18.1% of RhB was degraded by Co3O4 after 40 min, while CoAl2O4 degraded 99.5% of RhB, which was significantly higher than CoAl2O4-p (53.5%). Meanwhile, the degradation rate constant (fitted by applying pseudo-first-order kinetics) of RhB by CoAl2O4/PMS (0.178 min−1) was calculated to be 89.0 and 10.5 times higher than those of CoAl2O4-p/PMS (0.017 min−1) and Co3O4/PMS (0.002 min−1), respectively. The superior catalytic performance of CoAl2O4 can be attributed to its large surface area, mesoporous structure, and decent electron transfer capability. Moreover, CoAl2O4 exhibited a better catalytic performance for PMS activation than many other spinel-type oxides (Table 1) [20-22, 28-31].

|
Download:
|
| Fig. 2. (a) Degradation of RhB by different systems, effects of (b) CoAl2O4 dosage, (c) PMS concentration, and (d) solution pH on the degradation of RhB in the CoAl2O4/PMS system. | |
|
|
Table 1 Comparison of CoAl2O4 with other spinel-type catalysts in references. |
To explore the effect of CoAl2O4 dosage, PMS concentration, and solution pH on the reaction rate, a series of experiments were performed to obtain the optimal reaction conditions. As shown in Fig. 2b, the increase of the CoAl2O4 dosage provided more active sites, which led to the rapid degradation of RhB [32]. The increase of the PMS concentration from 0.05 g/L to 0.10 g/L also accelerated the degradation of RhB (Fig. 2c), while the continued increase of the PMS concentration up to 0.15 g/L did not significantly impact the degradation rate due to the self-quenching of generated reactive species [33]. In general, PMS based AOPs are highly dependent on the solution pH. In this work, the optimal pH value was determined to be between 5.0 and 7.0, in which 99.0% of RhB was degraded within 20 min (Fig. 2d). At a low pH of 3.0, H2SO5 would be the predominant species instead of HSO5− [34]. At a high pH of 9.0, the excess OH− ions would scavenge SO4•−, leading to a low RhB degradation efficiency [35].
Anions, such as Cl−, HCO3−, and CO32−, are ubiquitous in most wastewater. Therefore, their effects on the degradation of RhB in the CoAl2O4/PMS system were evaluated from a practical point of view. As shown in Fig. 3a, the RhB degradation rate of the system increased in the presence of Cl−. According to existing literature, Cl− may directly react with PMS to generate HClO that can promote the degradation of pollutants [36, 37]. HCO3− and CO32− are generally employed as radical scavengers in AOPs. Thus, they were expected to demonstrate an inhibiting effect on the degradation of RhB [38]. Moreover, HCO3− and CO32− contributed to the increase of the solution pH, which also suppressed RhB degradation, as confirmed by the aforementioned pH study. The catalytic performance of CoAl2O4 for activating PMS to degrade typical dyes (cationic dye-MB, anionic dye-MO) and antibiotics (TCH) was examined. As illustrated in Fig. 3b, more than 90.0% of these pollutants were degraded, thereby establishing the general applicability of CoAl2O4.

|
Download:
|
| Fig. 3. (a) Effect of anions on the degradation of RhB in the CoAl2O4/PMS system. (b) Degradation of different contaminants in the CoAl2O4/PMS system. (c) Recycling experiments for RhB degradation in the CoAl2O4/PMS system. (d) XRD patterns of CoAl2O4 before and after the reaction. (e) Comparison of reaction rates under different quenching conditions, and (f) EPR spectra of DMPO-•OH and DMPO-SO4•‒ adducts in different systems. | |
The reusability and stability of catalysts are crucial in practical applications. As shown in Fig. 3c, CoAl2O4 demonstrated excellent catalytic performance in each cycle, and more than 94.0% of RhB was degraded by the sixth run. The minor deactivation of the catalyst can be attributed to the surface of CoAl2O4 being corroded during the reaction [39]. The concentration of the leached cobalt ions in the CoAl2O4/PMS system was determined to be 0.41 mg/L, which is lower than that in the Co3O4/PMS system (0.56 mg/L) and is below the concentration limit of cobalt ions (1 mg/L) according to the Environmental Quality Standard of Surface Water (GB3838-2002) issued in China. After RhB degradation, the main characteristic XRD peaks of CoAl2O4 remained unchanged (Fig. 3d). The results reveal the excellent reusability and stability of CoAl2O4 for PMS activation to degrade aqueous organic pollutants.
The existence of different ROS and their contributions to RhB degradation were further explored by radical quenching experiments and electron paramagnetic resonance (EPR) tests. As demonstrated in Fig. 3e, when 0.1 mol/L of tert-butyl alcohol (TBA) was introduced to the reaction solution, the degradation of RhB was slightly inhibited and the degradation rate decreased from 0.178 min−1 to 0.102 min−1. In contrast, an addition of 0.1 mol/L of ethanol (EtOH) remarkably reduced the degradation rate to 0.026 min−1. These results establish that SO4•‒ worked as the predominant ROS during the activation of PMS by CoAl2O4. EPR measurements utilizing DMPO as a spin-trapping agent were conducted to verify the generation of SO4•‒ and •OH. As illustrated in Fig. 3f, the signals of DMPO-•OH and DMPO-SO4•‒ can be observed. Moreover, the intensity of such signals from CoAl2O4 was much higher than that of Co3O4, indicating that a larger number of SO4•‒ and •OH were generated in the CoAl2O4/PMS system [40].
In summary, a mesoporous CoAl2O4 spinel that is highly efficient for the degradation of RhB, MB, MO, and TCH was prepared through a combustion approach. The as-obtained CoAl2O4 was discovered to be extremely efficient in activating PMS, as evident from its RhB degradation rate of 0.178 min−1, which is significantly higher than those of CoAl2O4-p (0.017 min−1), Co3O4 (0.002 min−1), and many other spinel-type catalysts used for pollutant degradation. The presence of HCO3− and CO32− significantly suppressed the degradation of RhB, whereas the addition of Cl− slightly promoted it. Radical quenching experiments and EPR tests revealed that both SO4•‒ and •OH were present in the CoAl2O4/PMS system, and SO4•‒ was the dominant ROS. The excellent catalytic performance of the CoAl2O4 spinel can be ascribed to its large surface area, mesoporous structure, and good electrical conductivity, which facilitate the generation of ROS for RhB removal. The excellent reusability and adaptability of the CoAl2O4 spinel render it a suitable candidate for pollutant degradation in PMS-based AOPs.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work received financial support from the National Natural Science Foundation of China (No. 51604194), China Scholarship Council (No. 201808420137), and Nanyang Environment and Water Research Institute (Core Fund), Nanyang Technological University, Singapore.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2021.01.019.
| [1] |
J. Liu, X.C. Zhang, Q. Zhong, et al., J. Mater. Chem. A 8 (2020) 4083-4090. DOI:10.1039/C9TA13724F |
| [2] |
Y.D. Guo, C.X. Li, Z.H. Gong, et al., J. Hazard. Mater 397 (2020) 122580. DOI:10.1016/j.jhazmat.2020.122580 |
| [3] |
Q.K. Chen, L. Chen, J.J. Qi, et al., Chin. Chem. Lett. 30 (2019) 1214-1218. DOI:10.1016/j.cclet.2019.03.002 |
| [4] |
Z.P. Wen, J. Lu, Y.L. Zhang, et al., Chem. Eng. J 391 (2020) 123578. DOI:10.1016/j.cej.2019.123578 |
| [5] |
S. Guo, W. Yang, L.M. You, et al., Chem. Eng. J 393 (2020) 124758. DOI:10.1016/j.cej.2020.124758 |
| [6] |
S. Guo, G.K. Zhang, J.Q. Wang, J. Colloid Interface Sci. 433 (2014) 1-8. DOI:10.1016/j.jcis.2014.07.017 |
| [7] |
M.M. Du, Q.Y. Yi, J.H. Ji, et al., Chin. Chem. Lett. 31 (2020) 2803-2808. DOI:10.1016/j.cclet.2020.08.002 |
| [8] |
A. Jawad, K. Zhan, H.B. Wang, et al., Environ. Sci. Technol. 54 (2020) 2476-2488. DOI:10.1021/acs.est.9b04696 |
| [9] |
C. Li, W. Yang, S. Guo, et al., Funct. Mater. Lett 12 (2019) 1950083. DOI:10.1142/S1793604719500838 |
| [10] |
X.Y. Wu, W.H. Zhao, Y.H. Huang, G.K. Zhang, Chem. Eng. J 381 (2020) 122768. DOI:10.1016/j.cej.2019.122768 |
| [11] |
H. Tang, Z. Dai, X.D. Xie, Z.P. Wen, R. Chen, Chem. Eng. J. 356 (2019) 472-482. DOI:10.1016/j.cej.2018.09.066 |
| [12] |
F. Rehman, M. Sayed, J.A. Khan, et al., J. Hazard. Mater. 337 (2018) 506-514. |
| [13] |
X.G. Duan, H.Q. Sun, J. Kang, et al., ACS Catal. 5 (2015) 4629-4636. DOI:10.1021/acscatal.5b00774 |
| [14] |
S. Guo, Z.X. Yang, H.L. Zhang, et al., J. Mater. Sci. Technol. 62 (2021) 34-43. DOI:10.1016/j.jmst.2020.05.057 |
| [15] |
J.L. Zhu, J. Wang, C. Shan, et al., Chem. Eng. J 375 (2019) 122009. DOI:10.1016/j.cej.2019.122009 |
| [16] |
Q. Zhao, Z.H. Yan, C.C. Chen, J. Chen, Chem. Rev. 117 (2017) 10121-10211. DOI:10.1021/acs.chemrev.7b00051 |
| [17] |
T. Wu, S. Sun, J. Song, et al., Nat. Catal. 2 (2019) 763-772. DOI:10.1038/s41929-019-0325-4 |
| [18] |
S. Khademolhoseini, R. Talebi, J. Mater. Sci. Mater. El. 27 (2016) 2938-2943. DOI:10.1007/s10854-015-4113-x |
| [19] |
T. Gholami, M. Salavati-Niasari, S. Varshoy, Int. J. Hydrogen Energy 41 (2016) 9418-9426. DOI:10.1016/j.ijhydene.2016.03.144 |
| [20] |
J. Li, M.J. Xu, G. Yao, B. Lai, Chem. Eng. J. 348 (2018) 1012-1024. DOI:10.1016/j.cej.2018.05.032 |
| [21] |
Y.P. Wang, H.D. Ji, W. Liu, et al., ACS Appl. Mater. Interfaces 12 (2020) 20522-20535. DOI:10.1021/acsami.0c03481 |
| [22] |
X.K. Tian, C. Tian, Y.L. Nie, et al., Chem. Eng. J. 331 (2018) 144-151. DOI:10.1016/j.cej.2017.08.115 |
| [23] |
W. Li, J. Li, J. Guo, J. Eur. Ceram. Soc. 23 (2003) 2289-2295. DOI:10.1016/S0955-2219(03)00081-5 |
| [24] |
A.J. Reynoso, U. Iriarte-Velasco, M.A. Gutiérrez-Ortiz, J.L. Ayastuy, Catal. Today 367 (2021) 278-289. DOI:10.1016/j.cattod.2020.03.039 |
| [25] |
C. MÁlvarez-Docio, J.J. Reinosa, A.D. Campo, J.F. Fernandez, J. Alloys Compd. 779 (2019) 244-254. DOI:10.1016/j.jallcom.2018.11.263 |
| [26] |
X.L. Duan, M. Pan, F.P. Yu, D.R. Yuan, J. Alloys Compd. 509 (2011) 1079-1083. DOI:10.1016/j.jallcom.2010.09.199 |
| [27] |
J.B. Xi, Q.J. Wang, J. Liu, et al., J. Catal. 359 (2018) 233-241. DOI:10.1016/j.jcat.2018.01.003 |
| [28] |
J. Deng, M.Y. Xu, C.G. Qiu, et al., Appl. Surf. Sci. 459 (2018) 138-147. DOI:10.1016/j.apsusc.2018.07.198 |
| [29] |
Y.P. Wang, F. Li, T.S. Xue, et al., Environ. Sci. Pollut. Res. 25 (2018) 4419-4434. DOI:10.1007/s11356-017-0773-9 |
| [30] |
W.Y. Zhang, Y. Su, X.M. Zhang, Y. Yang, X.H. Guo, RSC Adv. 6 (2016) 64626-64633. DOI:10.1039/C6RA12706A |
| [31] |
P. Niu, C.H. Li, C.X. Jia, D.Q. Wang, S.W. Liu, J. Sol-Gel Sci. Technol. 93 (2020) 419-427. DOI:10.1007/s10971-019-05118-9 |
| [32] |
Y. Liu, H.G. Guo, Y.L. Zhang, et al., Chem. Eng. J. 356 (2019) 717-726. DOI:10.1016/j.cej.2018.09.086 |
| [33] |
P.D. Hu, M.C. Long, X. Bai, et al., J. Hazard. Mater. 332 (2017) 195-204. DOI:10.1016/j.jhazmat.2017.03.010 |
| [34] |
S. Guo, H.J. Wang, W. Yang, et al., Appl. Catal. B:Environ 262 (2020) 118250. DOI:10.1016/j.apcatb.2019.118250 |
| [35] |
N. Tian, X.K. Tian, Y.L. Nie, et al., Chem. Eng. J. 355 (2019) 448-456. DOI:10.1016/j.cej.2018.08.183 |
| [36] |
Y.B. Wang, D. Cao, X. Zhao, Chem. Eng. J. 328 (2017) 1112-1121. DOI:10.1016/j.cej.2017.07.042 |
| [37] |
R. Luo, M. Li, C. Wang, et al., Water Res. 148 (2019) 416-424. DOI:10.1016/j.watres.2018.10.087 |
| [38] |
W. Ren, P. Zhou, G. Nie, et al., Water Res 186 (2020) 116361. DOI:10.1016/j.watres.2020.116361 |
| [39] |
Y. Feng, D.L. Wu, Y. Deng, T. Zhang, K. Shih, Environ. Sci. Technol. 50 (2016) 3119-3127. DOI:10.1021/acs.est.5b05974 |
| [40] |
Y. Zhao, H.Z. An, J. Feng, Y.M. Ren, J. Ma, Environ. Sci. Technol. 53 (2019) 4500-4510. DOI:10.1021/acs.est.9b00658 |
 2021, Vol. 32
2021, Vol. 32