b Engineering Research Center for Clean Production of Textile Dyeing and Printing, Ministry of Education, Wuhan 430073, China
Recently, antibiotic contamination in water has become one of the most serious problems for human beings. As an important type of antibiotics, tetracycline is widely used for human antivirus and animal growth [1, 2]. Due to the limited availability of tetracycline, such pollutants accumulate in the aquatic environment, thus cause the spread of antibiotic resistance genes and eventually endanger human health [3, 4]. Meanwhile, tetracycline degrades very slowly in natural water bodies and will produce toxic intermediates that resistant to the biodegradation process. Until now, tetracycline has attracted a great deal of concern, and it is urgent to pay attention to the treatment of antibiotic-containing wastewater [5, 6].
In the past, various advanced oxidation processes (AOPs) had been proposed for tetracycline removal [7, 8]. Among them, photocatalysis has developed rapidly in recent years due to its mild reaction conditions and strong oxidation ability [9]. Photocatalysis has a good degradation effect on tetracycline, but the toxic intermediates of tetracycline are difficult to degrade [10]. Apart from photocatalytic strategies, biological treatment of pollutants has the advantages of thorough treatment and no secondary pollution accumulation [11]. Although it is almost impossible to treat an antibiotic by itself, the biological method has a good degradation performance for the intermediate products produced by tetracycline [12-14]. Therefore, the combination of chemical oxidation and biodegradation is expected to remove tetracycline in water.
Recently, the intimate coupling of photocatalysis and biodegradation (ICPB) has been developed to overcome the difficulties of photocatalysis or biodegradation alone [15]. Particularly, ICPB has attracted great attention due to the good degradation efficiency of pollutants and convenient operation process [16, 17]. For example, Ding and co-workers used inexpensive and highly efficient belt-shaped one-dimensional oxygen-rich Bi12O17Cl2 to degrade oxytetracycline. They found that the degradation rate of oxytetracycline and total organic carbon (TOC) in ICPB system could reach 96.16% and 88.03% in 4 h, which were increased by 3.8% and 14.3% than photocatalytic alone [18]. Xiong et al. used Ag-TiO2 to degrade tetracycline (TC), and the results indicated the degradation efficiency of ICPB was increased by 10% than photocatalytic alone [5]. Dong et al. developed composite-cubes by using self-assembly to coat a thin layer of visible-light-responsive photocatalyst (Er3+: YAlO3/TiO2) on sponge-type carriers. The removal efficiencies of phenol and dissolved organic carbon (DOC) in visible-light photocatalysis coupled with biodegradation (VPCB) system were 99.8% and 65.2% within 16 h, while only 71.6% and 50.0% were achieved in visible-light photocatalysis (VPC) system [16].
To date, most published articles have focused on the preparation of novel photocatalysts and the exploration of better loading methods [19]. However, the selection of carriers is also an important issue for the application of ICPB [20]. This requires the materials to have excellent properties, such as low-cost, easy to use, and good hydrophilicity [21]. As a promising biomass material, non-woven cotton fabrics with good biodegradability and high stability have been favored by researchers [15]. This kinds of material with sufficient surface area have been used in preparing functional materials adopted in many fields, such as pharmaceutical, chemical production, and wastewater treatment [22-25]. Until now, no researches had been conducted for the application of non-woven cotton fabrics as the carrier in the chemical-biological coupled system. It is expected that the photocatalytic material can be loaded on the outer surface of the non-woven cotton fabric, while the microorganisms are attached to the inside of non-woven cotton fabric, which induces the reactions to take place in different areas [18]. Moreover, with the aid of the microporous carrier, it is convenient for intermediate processing and subsequent recovery and recycling [16]. Theoretically, the non-woven cotton fabric can be adopted in ICPB system, though its application in this area has been rarely reported.
Based on the above discussion, the objective of this study is to validate the performance of photocatalytic materials with non-woven cotton fabric as a carrier in ICPB system. Three typical photocatalysts, TiO2, N-TiO2 and Ag-TiO2 were loaded onto the non-woven cotton fabric, and the degradation performances were compared. Cycling experiments were performed to explore the recyclability of various systems and the stability of composite materials. The coexisting inorganic, organic matter and authentic water were added to simulate the effects of water matrix in the natural environment. The degradation mechanisms of pollutants were discussed in detail.
The chemicals, synthesis and characterization of materials were displayed in Texts S1-S5 (Supporting information). The morphology of photocatalytic materials and biofilms attach to non-woven cotton fabric were observed by scanning electron microscopy (SEM, Zeiss, Gemini 300, Germany). The crystal structures of materials were detected by X-ray diffraction (XRD, Miniflex600, Rigaku Corporation, Japan) with Ni-filtered Cu Kα radiation in the range of 2θ from 10° to 80°. The chemical states of different elements in samples were characterized by X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha, U. S. A.). Electron paramagnetic resonance (EPR) spectrometry was employed to detect the active radicals by using 5, 5-dimethyl-1-pyrroline-N-oxide (DMPO) as the spin trapping agents.
SEM image of the non-woven cotton fabric was shown in Fig. 1a. This material exhibited a loose and porous structure, which provided good conditions for the loading of photocatalytic materials and the attachment of microorganisms [26, 27]. The SEM images of fabrics loaded with photocatalysts were displayed in Figs. 1b–d, respectively. The photocatalysts could be observed on the outer surface of the carrier. Compared with the original fabric, the materials exhibited more expansion and loosely, which was beneficial for the attachment and growth of microbes. Moreover, as displayed in Fig. 1e, the inner pores of the non-woven cotton fabric were loaded with microbial membranes [10]. After a cycle of ICPB degradation experiment, there were still a lot of microbes preserved inside the non-woven cotton fabric (Fig. 1f). The partial shedding of microbial membranes might be caused by the shearing force of the liquid. Meanwhile, ultraviolet light irradiation and tetracycline could also cause microbial growth inhibition and death on the outside of the non-woven cotton fabric [16, 28, 29].

|
Download:
|
| Fig. 1. SEM images of (a) non-woven cotton fabric, (b) non-woven cotton fabric loaded with TiO2, (c) non-woven cotton fabric loaded with N-TiO2, (d) non-woven cotton fabric loaded with Ag-TiO2, (e) non-woven cotton fabric loaded with Ag-TiO2 and microbes, (f) material after reactions, (g) XRD pattern of different materials. | |
As displayed in Fig. 1g, anatase phase (at 2θ values of 25.2°, 37.8°, 47.9°, 53.9° and 55.0°) was detected in the three materials, which was consistent with the results of the literature [14, 30]. Moreover, the metallic Ag phase (at 2θ values of 32.2°, 46.2°) was observed in Ag-TiO2 [31]. From energy dispersive spectrometer (EDS) in Fig. S1 (Supporting information), N and Ag were found in N-TiO2 and Ag-TiO2, indicating these elements had been successfully doped on the surface of TiO2 [22, 23]. XPS analysis were conducted to determine the surface composition and chemical states of as-prepared materials. As displayed in Figs. S2–S4 (Supporting information), the materials were mainly composed of Ti and O, while N and Ag were detected in XPS spectra of N-TiO2 and Ag-TiO2, which was consistent with EDS results. The two peaks of the binding energies of 458.7 eV and 464.3 eV were corresponded to anatase type TiO2 [7, 23, 25]. The main peak of O 1s at the binding energy of 529.6 eV was corresponded to Ti-O bond. Two peaks of Ag 3d5/2 and 3d3/2 were located at the binding energy of 373.6 eV and 367.5 eV respectively, proving the existence of metallic Ag [32-34]. All results proved that the preparation and loading of TiO2, N-TiO2 and Ag-TiO2 to non-woven cotton fabric were successfully.
In order to reflect degradation performance of TC, three synthetic materials were adopted for comparison. As shown in Fig. 2a, the adsorption (AD) of TC by raw fabric was limited (~10%). While in ICPB systems, the degradation rates of TiO2, N-TiO2 and Ag-TiO2 after 5 h were 70.9%, 79% and 94.7%, respectively. The results indicated Ag-TiO2 had better degradation efficiency than TiO2 and N-TiO2, as the modified material had wider light reaction band than the original photocatalytic material [35]. The enhancement of TC degradation in the existence of Ag was mainly ascribed to two reasons. Firstly, the e− and h+ recombination was depressed due to the Schottky barrier brought by Ag particles, then the utilization of excitons was largely promoted. Secondly, the adsorption affinity of catalyst toward dissolved oxygen was promoted in the presence of Ag, thus leading to enhanced production of ROS and TC degradation [36-38]. Moreover, TC degradation by photocatalysis alone was also investigated. According to Fig. 2b, the degradation curves of photocatalytic systems composed of TiO2, N-TiO2 and Ag-TiO2 were always smooth, and the final degradation rates were 65.5%, 72.6% and 78.2%, respectively. About 10% higher degradation rate of tetracycline was observed in ICPB system than that by photocatalytic system, which was attributed to the function of microorganisms [39]. As shown in Fig. 2c, the chemical oxygen demand (COD) removal rates of ICPB with TiO2, N-TiO2 and Ag-TiO2 were 46%, 58% and 66%, respectively. The maximum COD removal rates could be achieved with the aid of Ag-TiO2, and this trend was also consistent with that for TC. In single photocatalytic systems, the mineralization rates were 36%, 46% and 52% for TiO2, N-TiO2 and Ag-TiO2, respectively (Fig. 2d). The COD removal rates were about 10% lower for system without microorganisms, indicating the microorganisms could strengthen pollutants eliminating in photocatalytic system [17].

|
Download:
|
| Fig. 2. Tetracycline removal by (a) ICPB system, (b) photocatalytic. COD removal by (c) ICPB system, (d) photocatalytic. Cycling degradation of TC by different ICPB system: (e) TiO2, (f) N-TiO2. (g) Ag-TiO2 ([TC]0=20 mg/L, pH0 6.0). | |
Cycling experiments were conducted to investigate the feasibility of ICPB system for tetracycline degradation. As shown in Figs. 2e–g, ICPB system exhibited stable pollutant degradation rate for three kinds of photocatalysts. After 5 cycles, the removal rate of tetracycline by Ag-TiO2 was 82.9%, which was 11.8% decrease compared with the first cycle (Fig. 2e). However, the removal efficiencies of the ICPB reaction system composed of TiO2 and N-TiO2 photocatalytic materials were relatively poor. The degradation rates of the two systems after 5 cycles were 46% and 53%, respectively, which were 26% and 25% lower than the initial degradation rates (Figs. 2f and g). Such results further proved that Ag-TiO2 had a good removal ability toward tetracycline. After five cycles of experiments, the residual rates of TiO2, N-TiO2 and Ag-TiO2 were 89.4%, 93.1% and 86.7%, respectively (Fig. S5 in Supporting information). The results indicated liquid shear force had a small effect on the material [11, 20]. In summary, Ag-TiO2 coupled microorganisms have good and cause less secondary pollution to the environment, so the material has a good application prospect. Considering the removal efficiency of pollutants as well as recycling characteristics, Ag-TiO2 was selected as the photocatalytic material for further studies.
The effect of photocatalyst loads on TC removal was displayed in Fig. S6a (Supporting information). Within 2 h, the better degradation effect could be achieved when more photocatalytic materials were loaded. This may be attributed to more photoelectrons generated for removing pollutants [40]. The degradation rates of photocatalytic materials with loads of 20% and 25% were almost the same. Further increase of photocatalysts load will lead to microbial shedding, therefore, the photocatalytic material with 20% load was selected as the optimum condition. Furthermore, the effect of initial pHs on degradation of TC were investigated. As shown in Fig. S6b (Supporting information), the degradation rate of TC at pH 3.0 was 84.1% within 5 h, while the highest removal efficiency (94.7%) was obtained at pH 6.0. According to the literature, different forms of TC (e.g., TCH3+, TCH20, TCH−, TC2‒) were found at varied pH range, and the reactivity toward ·OH were in the order: TCH- > TC 2‒ > TCH 20 > TCH 3+ [41, 42]. At pH 3.0, TC molecular became less reactive toward UV light due to its protonated as TCH3+. With the increased of pH, TCH20, TCH− and TC2‒ species were found, which result in the enhancement of TC removal. Therefore, the optimum pH was set at 5.0–7.0.
In order to evaluate the effects of water matrix on the catalytic performance of composite fabric, four types of inorganic anions (Cl−, SO42‒, PO43‒ and CO32‒), one organic (citric acid) and two authentic background (Tangxun Lake water and Yangtze River water in Wuhan) were selected. As shown in Figs. S7a–S7d (Supporting information), both Cl− and SO42‒ had limited influence on TC degradation, while only 73% removal rate was obtained in the presence of 10 mmol/L CO32‒. According to previous studies, CO32‒ could consume ·OH to form carbonate radical with low reactivity [42, 43]. Meanwhile, the degradation efficiency of TC was only 77% when 10 mmol/L PO43‒ was added to the solution. This phenomenon may be ascribed to ·OH scavenge brought by PO43‒ [39]. The effect of citric acid on removal of TC was displayed in Fig. S7e (Supporting information). The degradation rate decreased after adding organic matter, which was ascribed to the competition of free radicals. Although coexisting organic matter could provide nutrients for microorganisms growing, the promotion was still limited [29, 39, 44]. The degradation performances of TC in real water were displayed in Fig. S7f (Supporting information). Compared with deionized water system, the removal efficiencies of TC in Tangxun Lake water and Yangtze River water were decreased to 74.9% and 85.8%, respectively. The possible reason for this phenomenon was that the free radicals were consumed by organic matter existed in natural water and thus affected the removal of TC.
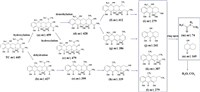
The intermediates of TC in ICPB system were analyzed by UPLC-MS (Fig. S8 in Supporting information). As shown in Scheme 1, a total of 14 intermediates (a–n) were identified, and the possible degradation pathways of TC were proposed. After the initial photodegradation of TC, it was oxidized, hydroxylated and dehydrated to produce molecules with m/z values of 479, 459, 427. Subsequently, molecules with m/z value of 459 was demethylated and hydroxylated to produce substances with m/z values of 428 and 479. Substances with an m/z value of 427 produced molecules with m/z values of 399 and 339 by demethylation, deamination through the breaking of the C-N bond. In fact, cyclic molecules are very stable and difficult to be destroyed [10, 28]. After further degradation of intermediate products by microorganisms, molecules withm/z values of 279, 241, 307 were produced through ring-opening, demethylation, deamination and dehydration. Finally, with the further development of the reaction, some small molecules with m/z values of 169, 74 were eventually degraded to CO2 and H2O [5, 45, 46].
|
Download:
|
| Scheme 1. The proposed transformation pathways of TC degradation. | |
The main reactive oxygen species responsible for TC degradation was detected by EPR analysis. As can be seen in Fig. 3a, the EPR spectra showed signal peak of height ratio of 1:2:2:1, corresponding to DMPO-·OH formation [47, 48]. In the dark, no free radicals were detected, but the signal peaks obviously appeared after the UV light irradiation, which may be ascribed to TiO2 being excited by UV light [49]. Moreover, as shown in Fig. 3b, the signal peaks of DMPO-O2·− appeared after the UV light, indicating that Ag accelerated the generation and migration of e‒ and realized the process of e‒ reduction to dissolve O2. These results suggested that the main reactive oxygen species for degradation of TC in ICPB system were ·OH and O2·−.

|
Download:
|
| Fig. 3. (a, b) EPR spectra of DMPO-radical adducts in the reaction systems. (c) Proposed mechanisms of TC degradation by ICPB system. | |
Based on the discussion mentioned above, the plausible mechanisms of ICPB system for removing tetracycline in water were proposed. As displayed in Fig. 3c, ·OH and O2·− responsible for TC degradation were generated on the surface of photocatalysts with the assistance of UV light. The by-products were produced by active radicals during photocatalysis process, and further transformed to CO2 and H2O by microbes loaded on the non-woven fabric. Through the above processes, the effective degradation of tetracycline was finally achieved.
In this study, three types of photocatalysts (i.e., TiO2, N-TiO2 and Ag-TiO2) were loaded on non-woven cotton fabric for constructing ICPB reaction systems. Ag-TiO2 based ICPB system showed good removal performance for tetracycline, which was much better than those with TiO2 and N-TiO2. Compared with single photocatalysis process, the removal efficiencies of tetracycline and COD in Ag-TiO2-ICPB system were enhanced by approximately 16.5% and 14%. After five cycles, the degradation rate of tetracycline could be maintained at ~82.9%, indicating the photocatalysts and biofilms kept good stability. Tetracycline could be transferred to CO2 and H2O through ring-opening, demethylation, deamination and dehydration reactions. This study provided a novel ICPB system for eliminating antibiotics in water. Further studies related to other pollutants removal by this hybrid system are also need to be carried out.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThis work was financially supported by the Central Government Guidance for Local Science and Technology Development Projects for Hubei province, China (No. 2019ZYYD068).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.02.028.
| [1] |
F. Zhao, L. Chen, H. Yen, et al., Environ. Int. 134 (2020) 105327. DOI:10.1016/j.envint.2019.105327 |
| [2] |
Z. Li, C. Shen, Y. Liu, et al., Appl. Catal. B 260 (2020) 118204. DOI:10.1016/j.apcatb.2019.118204 |
| [3] |
F. Liu, Y. Liu, Q. Yao, et al., Environ. Sci. Technol. 54 (2020) 5913-5921. DOI:10.1021/acs.est.0c00427 |
| [4] |
S. Gai, J. Zhang, R. Fan, et al., ACS Appl. Mater. Inter. 12 (2020) 8650-8662. DOI:10.1021/acsami.9b19583 |
| [5] |
H. Xiong, S. Dong, J. Zhang, et al., Water Res. 136 (2018) 75-83. DOI:10.1016/j.watres.2018.02.061 |
| [6] |
Y. Wang, C. Chen, D. Zhou, et al., Chemosphere 237 (2019) 124491. DOI:10.1016/j.chemosphere.2019.124491 |
| [7] |
J. Hu, J. Li, J. Cui, et al., J. Hazard. Mater. 384 (2020) 121399. DOI:10.1016/j.jhazmat.2019.121399 |
| [8] |
Y. Liu, G. Gao, C.D. Vecitis, Acc. Chem. Res. 53 (2020) 2892-2902. DOI:10.1021/acs.accounts.0c00544 |
| [9] |
X. Lei, M. You, F. Pan, et al., Chin. Chem. Lett. 30 (2019) 2216-2220. DOI:10.1016/j.cclet.2019.05.039 |
| [10] |
H. Cai, L. Sun, Y. Wang, et al., Chem. Eng. J. 369 (2019) 1078-1092. DOI:10.1016/j.cej.2019.03.143 |
| [11] |
D.C. Botía, M.S. Rodríguez, V.M. Sarria, Chemosphere 89 (2012) 732-736. DOI:10.1016/j.chemosphere.2012.06.046 |
| [12] |
C.Y. Chan, H.S. Chan, P.K. Wong, Chemosphere 222 (2019) 371-380. DOI:10.1016/j.chemosphere.2019.01.127 |
| [13] |
K. Stylianou, E. Hapeshi, M.I. Vasquez, D.F. Kassinos, I. Vyrides, J. Environ. Chem. Eng. 6 (2018) 3242-3248. DOI:10.1016/j.jece.2018.04.052 |
| [14] |
D. Zhou, S. Dong, J. Shi, et al., Chem. Eng. J. 317 (2017) 882-889. DOI:10.1016/j.cej.2017.02.128 |
| [15] |
Z. Qin, Z. Zhao, W. Jiao, et al., Environ. Res. 183 (2020) 109135. DOI:10.1016/j.envres.2020.109135 |
| [16] |
S. Dong, S. Dong, X. Tian, et al., J. Hazard. Mater. 302 (2016) 386-394. DOI:10.1016/j.jhazmat.2015.10.007 |
| [17] |
D. Ma, D. Zou, D. Zhou, et al., Int. Biodeter. Biodegr. 104 (2015) 178-185. DOI:10.1016/j.ibiod.2015.06.004 |
| [18] |
R. Ding, W. Yan, Y. Wu, et al., Water Res. 143 (2018) 589-598. DOI:10.1016/j.watres.2018.06.068 |
| [19] |
G. Li, S. Park, D.W. Kang, R.K. Brown, B.E. Rittmann, Environ. Sci. Technol. 45 (2011) 8359-8367. DOI:10.1021/es2016523 |
| [20] |
G. Li, S. Park, B.E. Rittmann, Water Res. 46 (2012) 6489-6496. DOI:10.1016/j.watres.2012.09.029 |
| [21] |
D. Caschera, F. Federici, T. de Caro, et al., Appl. Surf. Sci. 427 (2018) 81-91. DOI:10.1016/j.apsusc.2017.08.015 |
| [22] |
M.S. Stan, M.A. Badea, G.G. Pircalabioru, et al., Mater. Sci. Eng. C 94 (2019) 318-332. DOI:10.1016/j.msec.2018.09.046 |
| [23] |
P. Bhattacharya, D. Mukherjee, S. Dey, S. Ghosh, S. Banerjee, Mater. Chem. Phys. 229 (2019) 106-116. DOI:10.1016/j.matchemphys.2019.02.094 |
| [24] |
S. L Jr., J. Carneiro, O.S.G.P. Soares, et al., J. Mater. Res. Technol. 8 (2019) 1933-1943. DOI:10.1016/j.jmrt.2018.06.025 |
| [25] |
S. Noothongkaew, H.K. Jung, O. Thumtan, K.S. An, Mater. Lett. 233 (2018) 153-157. DOI:10.1016/j.matlet.2018.08.116 |
| [26] |
P. Xiao, Q. Meng, L. Zhao, et al., Mater. Des. 129 (2017) 164-172. DOI:10.1016/j.matdes.2017.05.035 |
| [27] |
B. Xu, J. Ding, L. Feng, et al., Surf. Coat. Technol. 262 (2015) 70-76. DOI:10.1016/j.surfcoat.2014.12.017 |
| [28] |
F. Li, X. Lan, L. Wang, et al., Chem. Eng. J. 383 (2020) 123092. DOI:10.1016/j.cej.2019.123092 |
| [29] |
Y. Ma, H. Xiong, Z. Zhao, et al., Chem. Eng. J. 351 (2018) 967-975. DOI:10.1016/j.cej.2018.06.167 |
| [30] |
S.J. Jang, M.S. Kim, B.W. Kim, Water Res. 39 (2005) 2178-2188. DOI:10.1016/j.watres.2005.03.028 |
| [31] |
M.P. Reddy, A. Venugopal, M. Subrahmanyam, Water Res. 41 (2007) 379-386. DOI:10.1016/j.watres.2006.09.018 |
| [32] |
Z. Zafar, I. Ali, S. Park, J.O. Kim, Ceram. Int. 46 (2020) 3353-3366. DOI:10.1016/j.ceramint.2019.10.045 |
| [33] |
M. Anjum, R. Kumar, M.A. Barakat, J. Environ. Manage. 212 (2018) 65-76. DOI:10.1016/j.jenvman.2018.02.006 |
| [34] |
S. Boufi, M. Abid, S. Bouattour, et al., Int. J. Biol. Macromol. 128 (2019) 902-910. DOI:10.1016/j.ijbiomac.2019.01.218 |
| [35] |
H. Lei, H. Zhang, Y. Zou, et al., J. Alloys Compd. 809 (2019) 151840. DOI:10.1016/j.jallcom.2019.151840 |
| [36] |
S. Zhao, J. Chen, Y. Liu, et al., Chem. Eng. J. 367 (2019) 249-259. DOI:10.1016/j.cej.2019.02.123 |
| [37] |
Z. Xiong, J. Ma, W.J. Ng, T.D. Waite, X.S. Zhao, Water Res. 45 (2011) 2095-2103. DOI:10.1016/j.watres.2010.12.019 |
| [38] |
L. Liu, Z. Liu, H. Bai, D.D. Sun, Water Res. 46 (2012) 1101-1112. DOI:10.1016/j.watres.2011.12.009 |
| [39] |
H. Xiong, D. Zou, D. Zhou, Chem. Eng. J. 316 (2017) 7-14. DOI:10.1016/j.cej.2017.01.083 |
| [40] |
F. Shi, H. Shan, D. Li, et al., J. Colloid Interface Sci. 538 (2019) 620-629. DOI:10.1016/j.jcis.2018.12.028 |
| [41] |
L. Ge, Q. Dong, C. Halsall, et al., Environ. Sci. Pollut. Res. 25 (2018) 15726-15732. DOI:10.1007/s11356-018-1765-0 |
| [42] |
C.H. Han, H.D. Park, S.B. Kim, et al., Water Res. 172 (2020) 115514. DOI:10.1016/j.watres.2020.115514 |
| [43] |
H. Li, J. Qian, B. Pan, Chem. Eng. J. 403 (2021) 126395. DOI:10.1016/j.cej.2020.126395 |
| [44] |
X. Luo, H. Liang, F. Qu, et al., Chemosphere 200 (2018) 237-247. DOI:10.1016/j.chemosphere.2018.02.113 |
| [45] |
M. Bilal, M. Adeel, T. Rasheed, Y. Zhao, H.M.N. Iqbal, Environ. Int. 124 (2019) 336-353. DOI:10.1016/j.envint.2019.01.011 |
| [46] |
C. Wang, Y. Li, H. Tan, Chem. Eng. J. 359 (2019) 1065-1074. DOI:10.1016/j.cej.2018.11.077 |
| [47] |
Y. Ye, Z. Jiang, Z. Xu, et al., Water Res. 126 (2017) 172-178. DOI:10.1016/j.watres.2017.09.021 |
| [48] |
Y. Ye, C. Shan, X. Zhang, et al., Environ. Sci. Technol. 52 (2018) 10657-10664. DOI:10.1021/acs.est.8b01693 |
| [49] |
X. Lei, X. Li, Z. Ruan, et al., J. Mol. Liq. 266 (2018) 122-131. DOI:10.1016/j.molliq.2018.06.053 |
 2021, Vol. 32
2021, Vol. 32 

