b College of Chemistry and Pharmaceutical Engineering, Nanyang Normal University, Nanyang 473061, China;
c College of Chemistry, Zhengzhou University, Zhengzhou 450052, China
Compounds containing a sulfamate moiety are important synthetic targets because they have a broad spectrum of biological activities and serve as preeminent synthetic reagents or intermediates in organic synthesis [1]. For example, readily accessible and stable cyclic sulfamate aldimines have been widely employed in organic synthesis. Among these transformations on the elaboration of cyclic sulfamate aldimines, nucleophilic addition reactions (Scheme 1a) [2], [3-7], annulation reactions (Scheme 1b) [8], ring-expansion reactions (Scheme 1c) [9] and others have been intensively investigated [10-12]. Recently, we have disclosed a silver-catalyzed direct C-H functionalization of cyclic sulfamate aldimines with tertiary cycloalkanols via radical-mediated C-C bond cleavage process (Scheme 1d) [13]. Although a vast array of prominent transformations of cyclic sulfamate aldimines have been excavated, direct decarboxylative C-H functionalization of cyclic aldimines with aliphatic carboxylic acids through transition-metal catalyzed radical process has not been reported so far.
|
Download:
|
| Scheme 1. Catalytic reaction types of cyclic sulfamate aldimines. | |
Silver-catalyzed decarboxylative radical functionalization of heterocycles is well-known as Minisci reaction [14]. In the past decades, the flourishing Ag-catalyzed decarboxylative strategies have been employed in multitudinous substrates. Among these splendid transformations, aliphatic carboxylic acids are the most frequently used alkyl precursors owing to the advantages of readily available starting materials, low cost, effectiveness, and ease of use in organic synthesis [15]. Since the pioneering report on silver-catalyzed decarboxylative chlorination of aliphatic carboxylic acids developed by Li's group in 2012 [16], a vast array of radical transformations of aliphatic carboxylic acids has been successfully achieved [17, 18]. Despite these impressive advances, the development of more robust and readily available methods for the direct C-H functionalization through the decarboxylative cross-coupling of aliphatic carboxylic acids to construct valuable motifs with CO2 as the only byproduct still remains desirable. Herein, we disclose the first silver-catalyzed decarboxylative C-H functionalization of cyclic aldimines for the assembly of cyclic ketimines via a radical pathway (Scheme 1e).
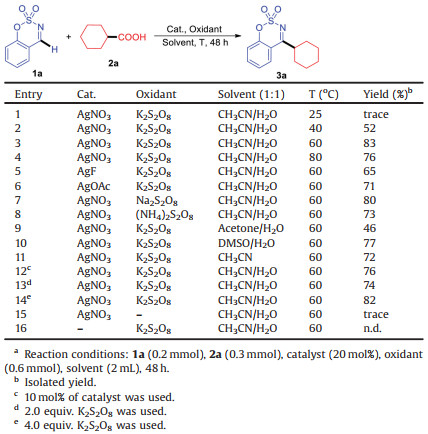
At the outset, cyclic aldimines (1a) and cyclohexanecarboxylic acid (2a) were selected as model substrates to identify the optimal conditions (Table 1). Pleasingly, the desired product 3a was obtained in 52% yield with AgNO3 (20 mol%) as catalyst and K2S2O8 (3 equiv.) as oxidant in CH3CN/H2O (1:1) at 40 ℃ (entry 2). Further investigation of reaction temperature (entries 2-4) revealed that 60 ℃ was the optimal choice (entry 3). Other Ag salts such as AgF and AgOAc could also promote the reaction albeit in slightly lower yields (entries 5 and 6). Subsequently, other oxidants were screened and the results showed that K2S2O8 was the optimal oxidant (entries 7 and 8). Next, solvent examination indicated that the reaction worked unfavorably in mixed solvents such as acetone/H2O and DMSO/H2O (entries 9 and 10). Using CH3CN as reaction medium, a decreased yield of 3a was obtained (entry 11). Then, the amount of catalyst and oxidant were explored. The results showed that changing the amount of catalyst and oxidant led to negative results (entries 12-14). Control experiments indicated that the reaction did not work in the absence of catalyst or oxidant (entries 15 and 16). Thus, based on the above experiments, the optimized conditions were determined to be 1a (0.2 mmol), 2a (0.3 mmol), AgNO3 (20 mol%), K2S2O8 (0.6 mmol) in CH3CN/H2O (1:1) at 60 ℃ for 48 h.
|
|
Table 1 Optimization of reaction conditions.a |
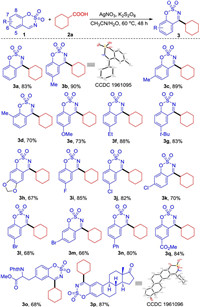
With the optimized reaction conditions in hand, we set out to explore the substrate scope of this reaction. First, different cyclic aldimines were investigated. As shown in Scheme 2, better results were achieved when substituent was 6-Me or 7-Me (3b, 3c) and the structure of 3b was confirmed through X-ray crystallographic data. 8-Me-substituted cyclic aldimine gave a relatively lower yield of 3d, owing to the electronic effect. Substrates with electron-donating substituents such as OMe, Et, tBu, 6, 7−OCH2O displayed good reactivity to afford the products 3e-3h in decent yields. In addition, cyclic aldimines with halogen substituents were well compatible in this transformation, delivering the products in moderate to good yields (3i-3m). Cyclic aldimine with 6-Ph was also a good candidate, giving the desired product 3n in 80% yield. In addition, the cyclic aldimine originated from tyrosine was suitable for this transformation, delivering the expected product 3o in 68% yield. It is noteworthy that estrone-derived cyclic aldimine could also undertook the radical process to give the desired product 3p in 87% yield without affecting the ketone group. The structure of 3p was confirmed through X-ray crystallographic data. The product 3o and 3p arguably highlight the current protocol could be applied for late-stage modification of bioactive molecules. To our delight, ester-substituted imine was amenable to this transformation, providing 3q in 84% yield.
|
Download:
|
| Scheme 2. Scope of cyclic aldimines. Reaction conditions: 1 (0.2 mmol), 2a (0.3 mmol), AgNO3 (20 mol%), K2S2O8 (0.6 mmol), CH3CN/H2O (1 mL/1 mL), 48 h, isolated yields. | |
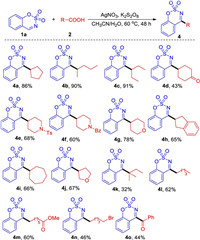
Next, we started to investigate the scope of aliphatic carboxylic acids. As depicted in Scheme 3, secondary cyclic or noncyclic aliphatic carboxylic acids were proved to be suitable alkyl donors, giving the corresponding products 4a-4j in moderate to excellent yields. However, tertiary aliphatic carboxylic acid exhibited low reactivity probably owing to the steric hindrance (4k). Moreover, primary aliphatic carboxylic acids and 2-oxo-2-phenylacetic acid could be engaged in this transformation to give the desired products in moderate yields (4l-4o).
|
Download:
|
| Scheme 3. Scope of aliphatic carboxylic acids. Reaction conditions: 1a (0.2 mmol), 2 (0.3 mmol), AgNO3 (20 mol%), K2S2O8 (0.6 mmol), CH3CN/H2O (1 mL/1 mL), 48 h, isolated yields. | |
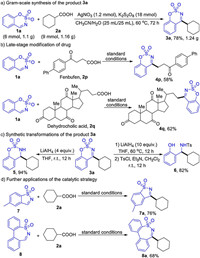
To evaluate the synthetic potential of this methodology, the gram-scale reaction, late-stage modification of drug, transformations of the product, and further application of the catalytic strategy were performed. The product 3a was readily obtained in 78% yield (1.24 g) on a gram-scale reaction by prolonged reaction time (Scheme 4a). Under the standard conditions, fenbufen-a nonsteroidal antiinflammatory drug, and dehydrocholic acid-a choleretic drug, could be modified to corresponding cyclic ketimine derivatives4p, 4q (58%, 62%) without affecting the ketone group (Scheme 4b). To further demonstrate the synthetic utility of this protocol, synthetic transformations of the product 3a were conducted (Scheme 4c). Reduction of 3a with 4 equiv. of LiAlH4 gave cyclic sulfamate 5 in 94% yield. When LiAlH4 was increased to 10 equiv., the ring of sulfonylimine was opened and β-aminophenol 6 was obtained in 82% yield after trapping with TsCl. Subsequently, we extended this strategy to other cyclic imines (Scheme 4d). To our delight, the reaction of five- and seven-membered cyclic aldimines efficiently proceeded under the standard conditions, and the corresponding product 7a and 8a were obtained in 76% and 68% yields, respectively [19].
|
Download:
|
| Scheme 4. Synthetic applications. | |
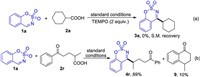
To confirm that radical intermediates might be involved in the reaction, radical-trapping experiments were performed (Scheme 5). When radical scavenger TEMPO was added to mixture of 1a and 2a under the standard conditions, the reaction was totally suppressed and the substrates were recovered (Scheme 5a). With aliphatic carboxylic acid 2r as substrate, along with normal product 4r, the cyclization by-product 9 was obtained in 10% yield (Scheme 5b). All these experiments suggest that a radical pathway might be involved in the reaction.
|
Download:
|
| Scheme 5. Mechanistic studies. | |
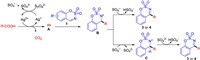
According to these mechanistic studies and other previous investigations, a plausible mechanism for the reaction is proposed in Scheme 6. First, Ag2+ intermediate is generated by oxidizing Ag+ specie with K2S2O8, which undergoes the single-electron transfer (SET) with the aliphatic carboxylic acid to generate radical intermediate A via a decarboxylation. Then, radical A attacks cyclic aldimines to afford N-radical intermediate B, along with the formation of a new C-C bond. Finally, N-radical B loses a H atom to give the target product. In addition, sulfate radical anion might oxidize the intermediate B nitrogen centered radical via SET to generate nitrogen cation intermediate C, and then the deprotonation of the intermediate C occurs to give the desired product.
|
Download:
|
| Scheme 6. Proposed mechanism of decarboxylative radical C-H functionalization. | |
In conclusion, we have developed a novel, practical and straightforward silver-catalyzed decarboxylative radical reaction of aliphatic carboxylic acids with cyclic aldimines under mild reaction conditions. The desired products were obtained in moderate to good yields. This transformation features easy accessibility of aliphatic carboxylic acids and broad substrate scopes. In addition, late-stage modification of bioactive molecules, gram-scale synthesis and further synthetic transformations of the product indicate the synthetic potential of this approach. Moreover, five- and seven-membered cyclic aldimines were also applicable for this transformation. Mechanistic studies showed that the reaction might undergo a radical pathway. Further exploration of the synthetic utility of this transformation is ongoing in our laboratory.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis research was financially supported by the National Natural Science Foundation of China (Nos. 21402116, 21502111, 21572126), the Science and Technology Innovation Talents of Henan Province (No. 2018JQ0011) and the Key Science Research of Education Committee in Henan Province (No. 21A150044). The author also thanks Dr. Yong-Gui Zhou (Dalian Institute of Chemical Physics, Chinese Academy of Sciences) for his encouragement and generous support.
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.011.
| [1] |
(a) K.C. Majumdar, S. Mondal, Chem. Rev. 111 (2011) 7749-7773; (b) S.J. Williams, Expert Opin. Ther. Pat. 23 (2013) 79-98; (c) S. Debnath, S. Mondal, Eur. J. Org. Chem. 2018 (2018) 933-956. |
| [2] |
(a) Y. Luo, H.B. Hepburn, N. Chotsaeng, H.W. Lam, Angew. Chem. Int. Ed. 51 (2012) 8309-8313; (b) J.I. Martinez, J.J. Smith, H.B. Hepburn, H.W. Lam, Angew. Chem. Int. Ed. 55 (2016) 1108-1112; (c) J. Park, S. Choi, Y. Lee, S.H. Cho, Org. Lett. 19 (2017) 4054-4057. |
| [3] |
(a) H. Wang, T. Jiang, M.H. Xu, J. Am. Chem. Soc. 135 (2013) 971-974; (b) M. Quan, L. Tang, J. Shen, G. Yang, W. Zhang, Chem. Commun. 53 (2017) 609-612; (c) B. Zhou, K. Li, C. Jiang, Y. Lu, T. Hayashi, Adv. Synth. Catal. 359 (2017) 1969-1975; (d) W. Sun, H. Gu, X. Lin, J. Org. Chem. 83 (2018) 4034-4043. |
| [4] |
(a) L.D. Munck, A. Monleón, C. Vila, J.R. Pedro, Adv. Synth. Catal. 359 (2017) 1582-1587; (b) Y.L. Li, J.X. Liu, X.P. Chen, et al., Adv. Synth. Catal. 362 (2020) 3202-3207. |
| [5] |
(a) Y. Luo, A.J. Carnell, H.W. Lam, Angew. Chem. Int. Ed. 51 (2012) 6762-6766; (b) S.S. Zhang, T.J. Hu, M.Y. Li, et al., Angew. Chem. Int. Ed. 58 (2019) 3387-3391. |
| [6] |
(a) H.X. Zhang, J. Nie, H. Cai, J.A. Ma, Org. Lett. 16 (2014) 2542-2545; (b) C.M. Jia, H.X. Zhang, J. Nie, J.A. Ma, J. Org. Chem. 81 (2016) 8561-8569; (c) X.Y. Cui, H.X. Duan, Y. Zhang, Y.Q. Wang, Chem. Asian J. 11 (2016) 3118-3125; (d) J. Zhao, Y. Li, L.Y. Chen, X. Ren, J. Org. Chem. 84 (2019) 5099-5108. |
| [7] |
(a) S.G. Lee, S.G. Kim, RSC Adv. 7 (2017) 34283-34286; (b) C. Vila, A. Tortosa, G. Blay, et al., New J. Chem. 43 (2019) 130-134. |
| [8] |
(a) Y. Liu, T.R. Kang, Q.Z. Liu, et al., Org. Lett. 15 (2013) 6090-6093; (b) J. Wang, F. Li, Q. Shen, et al., Synthesis 48 (2016) 441-447; (c) B. Mao, W. Shi, J. Liao, et al., Org. Lett. 19 (2017) 6340-6343; (d) K. Spielmann, A. Lee, et al., Org. Lett. 20 (2018) 1444-1447; (e) J. Zhou, H. Zhang, X.L. Chen, et al., J. Org. Chem. 84 (2019) 9179-9187; (f) J.U. Park, H.I. Ahn, H.J. Cho, et al., Adv. Synth. Catal. 362 (2020) 1836-1840; (g) C.C. Wang, Z.W. Ma, Y.L. Qu, et al., Chem. Asian J. 15 (2020) 560-563; (h) J.A. Xiao, X.L. Cheng, H. Peng, et al., Sci. China Chem. 63 (2020) 785-791; (i) H.I. Ahn, J.U. Park, Z. Xuan, J.H. Kim, Org. Biomol. Chem. 18 (2020) 9826-9830. |
| [9] |
(a) Z. Yang, H. Yu, L. Zhang, et al., Chem. Asian J. 9 (2014) 313-318; (b) A.J. Xia, T.R. Kang, L. He, et al., Angew. Chem. Int. Ed. 55 (2016) 1441-1444. |
| [10] |
(a) X. Qian, J. Han, L. Wang, RSC Adv. 6 (2016) 89234-89237; (b) F. Li, J. Wang, W. Pei, et al., Tetrahedron Lett. 59 (2018) 3010-3014. |
| [11] |
K. Parthasarathy, A.R. Azcargorta, Y. Cheng, C. Bolm, Org. Lett. 16 (2014) 2538-2541. DOI:10.1021/ol500918t |
| [12] |
(a) B.N. Lai, J.F. Qiu, H.X. Zhang, J. Nie, J.A. Ma, Org. Lett. 18 (2016) 520-523; (b) L.D. Munck, C. Vila, M.C. Muñoz, J.R. Pedro, Chem. Eur. J. 22 (2016) 17590-17594; (c) J. Liu, X. Tong, M. Chen, J. Org. Chem. 85 (2020) 5193-5202; (d) K.P. Jethava, J. Fine, Y. Chen, A. Hossain, G. Chopra, Org. Lett. 22 (2020) 8480-8486; (e) Y. Zhao, X.Q. Wang, Y.J. Yu, Y.G. Zhou, J. Org. Chem. 86 (2021) 1262-1272. |
| [13] |
J. Wang, X. Liu, Z. Wu, et al., Chem. Commun. 57 (2021) 1506-1509. DOI:10.1039/D0CC07181A |
| [14] |
F. Minisci, R. Bernardi, F. Bertini, R. Galli, M. Perchinummo, Tetrahedron 27 (1971) 3575-3579. DOI:10.1016/S0040-4020(01)97768-3 |
| [15] |
(a) C.J. Li, X. Bi, Silver Catalysis in Organic Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2018, pp. 183-269; (b) Q.Z. Zheng, N. Jiao, Chem. Soc. Rev. 45 (2016) 4590-4627; (c) M. Yan, J.C. Lo, J.T. Edwards, P.S. Baran, J. Am. Chem. Soc. 138 (2016) 12692-12714; (d) Q. Feng, Y.W. Zhao, Q.L. Song, Chin. Chem. Lett. 27 (2016) 571-574; (e) Y. Wei, P. Hu, M. Zhang, W. Su, Chem. Rev. 117 (2017) 8864-8907; (f) G. Fang, X. Cong, G. Zanoni, Q. Liu, X. Bi, Adv. Synth. Catal. 359 (2017) 1422-1502; (g) J. Schwarz, B. König, Green Chem. 20 (2018) 323-361; (h) X. Yin, W. Li, B. Zhao, C. Kai, Chin. J. Org. Chem. 38 (2018) 2879-2887; (i) R.S. J. Proctor, R.J. Phipps, Angew. Chem. Int. Ed. 58 (2019) 13666-13699; (j) S. Wang, Z. Fu, Z. Huang, Y. Jiang, S. Guo, H. Cai, Chin. Chem. Lett. 30 (2019) 1173-1177. |
| [16] |
Z. Wang, L. Zhu, F. Yin, et al., J. Am. Chem. Soc. 134 (2012) 4258-4263. DOI:10.1021/ja210361z |
| [17] |
(a) F. Yin, Z. Wang, Z. Li, C. Li, J. Am. Chem. Soc. 134 (2012) 10401-10404; (b) X. Liu, Z. Wang, X. Cheng, C. Li, J. Am. Chem. Soc. 134 (2012) 14330-14333; (c) W.P. Mai, J.T. Wang, L.R. Yang, et al., Org. Lett. 16 (2014) 204-207; (d) P.F. Wang, X.Q. Wang, J.J. Dai, Y.S. Feng, H.J. Xu, Org. Lett. 16 (2014) 4586-4589; (e) F. Hu, X. Shao, D. Zhu, L. Lu, Q. Shen, Angew. Chem. Int. Ed. 53 (2014) 6105-6109; (f) C. Liu, X. Wang, Z. Li, L. Cui, C. Li, J. Am. Chem. Soc. 137 (2015) 9820-9823; (g) Y. Zhu, X. Li, X. Wang, et al., Org. Lett. 17 (2015) 4702-4705; (h) P. Natarajan, R. Chaudhary, P.J. Venugopalan, J. Org. Chem. 80 (2015) 10498-10504; (i) G. Shi, C. Shao, S. Pan, et al., Org. Lett. 17 (2015) 38-41; (j) X.F. Xia, S.L. Zhu, C. Chen, H. Wang, Y.M. Liang, J. Org. Chem. 81 (2016) 1277-1284; (k) D. Zhu, X. Shao, X. Hong, L. Lu, Q. Shen, Angew. Chem. Int. Ed. 55 (2016) 15807-15811; (l) L. Cui, H. Chen, C. Liu, C. Li, Org. Lett. 18 (2016) 2188-2191; (m) Q.W. Zhang, A.T. Brusoe, V. Mascitti, et al., Angew. Chem. Int. Ed. 55 (2016) 9758-9762; (n) Y. Zhu, X. Wen, S. Song, N. Jiao, ACS Catal. 6 (2016) 6465-6472; (o) L. Lv, S. Lu, Y. Chen, Z. Li, Org. Chem. Front. 4 (2017) 2147-2152; (p) X. Tan, Z. Liu, H. Shen, et al., J. Am. Chem. Soc. 139 (2017) 12430-12433; (q) X. Tan, T. Song, Z. Wang, et al., Org. Lett. 19 (2017) 1634-1637; (r) Y. Dong, Z. Wang, C. Li, Nat. Commun. 8 (2017) 277; (s) W. Shu, A. Lorente, E. Gómez-Bengoa, C. Nevado, Nat. Commun. 8 (2017)13832; (t) R.G. Xing, Y.N. Li, B.W. Zhang, Chin. Chem. Lett. 28 (2017) 407-411; (u) Y. Guo, M.W. Huang, X.L. Fu, et al., Chin. Chem. Lett. 28 (2017) 719-728; (v) H. Hu, X. Chen, K. Su, et al., Org. Chem. Front. 5 (2018) 2925-2929; (w) K. Sun, S.J. Li, X.L. Chen, et al., Chem. Commun. 55 (2019) 2861-2864; (x) Z. Wang, C.Y. Guo, C. Yang, J.P. Chen, J. Am. Chem. Soc. 141 (2019) 5617-5622; (y) C.G. Li, Q. Xie, X.L. Xu, et al., Org. Lett. 21 (2019) 8496-8500; (z) R. Matsubara, H. Kim, T. Sakaguchi, et al., Org. Lett. 22 (2020) 1182-1187. |
| [18] |
(a) R. Xia, M.S. Xie, H.Y. Niu, G.R. Qu, H.M. Guo, Org. Lett. 16 (2014) 444-447; (b) W.M. Zhao, X.L. Chen, J.W. Yuan, et al., Chem. Commun. 50 (2014) 2018-2020; (c) W.P. Mai, B. Sun, L.Q. You, et al., Org. Biomol. Chem. 13 (2015) 2750-2755; (d) P.S. Mahajan, S.B. Mhaske, Org. Lett. 20 (2018) 2092-2095; (e) J.Y. Guo, T. Guan, J.Y. Tao, K. Zhao, T.P. Loh, Org. Lett. 21 (2019) 8395-8399. |
| [19] |
(a) Z. Yan, B. Wu, X. Gao, M.W. Chen, Y.G. Zhou, Org. Lett. 18 (2016) 692-695; (b) Z.B. Zhao, L. Shi, Y. Li, F.J. Meng, Y.G. Zhou, Org. Biomol. Chem. 17 (2019) 6364-6368; (c) Z.B. Zhao, L. Shi, F.J. Meng, Y. Li, Y.G. Zhou, Org. Chem. Front. 6 (2019) 1572-1576. |
 2021, Vol. 32
2021, Vol. 32