b Key Laboratory for Molecular Enzymology and Engineering of Ministry of Education, School of Life Sciences, Jilin University, Changchun 130012, China
Protein glycosylation plays an important role in living organisms, which not only participates in many biological processes such as protein translation regulation, protein degradation, immune protection and signal transduction regulation, but also has an important impact on protein characteristics such as structure, solubility, charge or sensitivity to proteolysis [1-3]. Human body fluids are one of the main sources for the discovery of related disease biomarkers, and often used as clinical biological specimens [4, 5]. Changes in content of glycoproteins and glycopeptides in body fluids usually reflect the physical status of human body [6]. Therefore, the quantitative and qualitative analysis of glycoproteins and glycopeptides in human body fluids are crucial for the screening and discovery of clinical disease biomarkers. Mass spectrometry (MS) analysis is a powerful and high-throughput method that plays a central role in glycomics and glycoproteomics [7, 8]. Nevertheless, systematic and comprehensive analysis of protein glycosylation is still challenging [9, 10]. For one thing, the high abundance of non-glycoproteins and non-glycopeptides seriously hinders the detection of low-abundance glycoproteins and glycopeptides in complex biological samples. For another, the heterogeneity of glycans further reduces the relative content of each glycan form. In addition, the analysis of glycosylation sites and glycan components requires complex bioinformatics software to explain massive mass spectrometry data. Hence, developing an effective pre-concentration method is very important to overcome these obstacles and enables the comprehensive analysis of glycoproteins and glycopeptides with MS.
Up to now, the specific enrichment methods for glycoproteins and glycopeptides mainly include hydrophilic interaction liquid chromatography (HILIC) [11-13], boronate affinity chromatography [14, 15], hydrazine chemical method [16, 17], and lectin affinity chromatography [18]. The enrichment mechanism of HILIC is primarily the hydrophilic distribution of the compound between the organic-rich mobile phase and the water-rich layer formed on the adjacent surface of the HILIC stationary phase [19]. What is more, hydrogen bonding and electrostatic interactions also participate in the retention of HILIC [20]. The mechanism of boronate affinity chromatography can be explained by the reversible formation/dissociation of covalent complexes between boric acid groups and compounds containing cis-diol groups in alkaline/acidic aqueous solutions [21]. In the hydrazide chemistry method, cis-diol groups of glycoproteins and glycopeptides are usually oxidized into aldehydes by periodate, followed by the covalent bonding with hydrazide [22]. Lectin affinity chromatography is a highly specific method for capturing glycoproteins and glycopeptides. Its enrichment mechanism depends on the biological specific recognition between lectins and glycans. Usually, a lectin can only recognize a subset of glycoproteins [23]. In recent years, enrichment materials based on the above enrichment strategies have been widely developed to meet the requirements of low-abundance glycoproteins and glycopeptides enrichment in complex samples.
Magnetic solid phase extraction (MSPE) is widely employed in glycoproteomics because of its simple operation, short adsorption and desorption time, low cost and ecological friendliness [24]. As the core of MSPE technology, magnetic adsorbents possess the ability of rapid separation of target analytes, which benefiting from the excellent magnetic responsiveness of Fe3O4 nanoparticles. However, the practical application of Fe3O4 is restricted due to its high surface activity, easy agglomeration and unstable long-term storage. Therefore, the reasonable functionalization of Fe3O4 is crucial, which can not only improve surface properties and stability, but also broaden the scope of its application [25]. So far, some reviews have been reported for the applications of magnetic materials in the enrichment of glycoproteins and glycopeptides. In 2014, Deng's group [26] reviewed the synthesis of core-shell magnetic microspheres and their applications in protein sample preparation, including the separation and enrichment of phosphoproteins, glycoproteins and other low-abundance proteins/peptides. In 2020, Cao's group [27] described the preparation and functionalization of boronate affinity-based magnetic composites, and summarized the applications of boronate affinity magnetic separation technology in affinity separation, proteomics, detection methods and enzyme immobilization.
In this review, we focus on the application of MSPE technology and magnetic adsorbents for the separation and enrichment of glycoproteins and glycopeptides in human body fluids (Fig. 1). Firstly, the principle and development of MSPE technology are briefly outlined. Secondly, magnetic adsorbents of MSPE are classified based on different functional materials and discussed one by one. Thirdly, the application of MSPE technology for glycoproteins and glycopeptides enrichment in different human body fluids is summarized and commented. Finally, the future trends and application prospects of MSPE technology in glycoproteomics are described.

|
Download:
|
| Fig. 1. Graphical representation of the main points involved in this review. | |
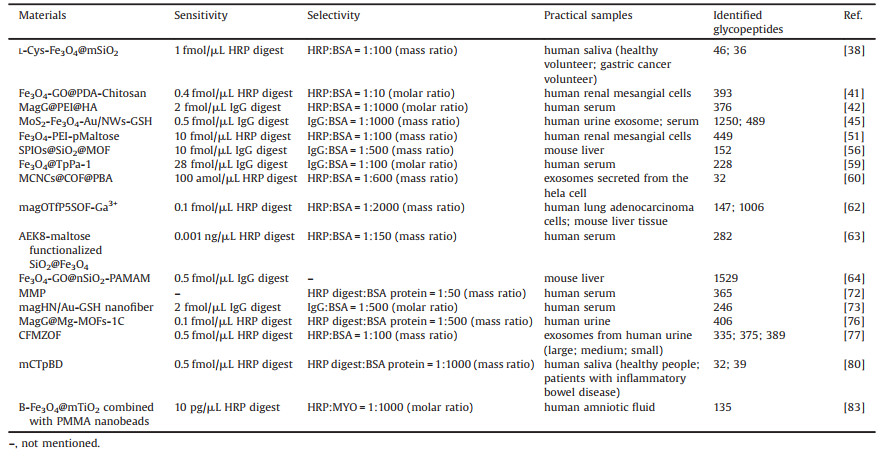
The concept of MSPE was firstly proposed by Šafaříková and Šafařík in 1999 as a new sample pretreatment technology based on solid phase extraction (SPE) [28]. MSPE technology combines magnetic separation and SPE, utilizing magnetic materials as adsorbents to separate and enrich target analytes. It overcomes the defects of traditional SPE such as easy blockage, time consumption and low extraction efficiency, and realizes the rapid separation and enrichment of targets in complex matrix [29]. With the rapid development of MSPE technology, it has been widely used in the fields of environment, food and biology for sample analysis [30]. In a typical MSPE process (Fig. 2), magnetic adsorbents are added to the sample solution, which are fully dispersed with the aid of ultrasound or vibration. The target analytes in the sample solution are selectively adsorbed on the magnetic adsorbents, followed by separating from the sample solution via an external magnet. Then the target analytes are eluted from the magnetic adsorbents for subsequent analysis. The whole process is easy operation and time-saving and can realize the separation of trace analytes from multiple samples.
|
Download:
|
| Fig. 2. Graphical illustration of main steps in MSPE process. | |
Magnetic adsorbents have been considered as the key to realize rapid separation and efficient enrichment. Typically, magnetic adsorbents consist of magnetic particles and their surface-modified functional materials. Magnetic particles realize the rapid separation of the target analytes from the sample solution, while the types of functional materials affect the sensitivity and selectivity of separation and enrichment [31]. Among these magnetic particles such as metals (Fe, Co, Ni), alloys (Fe-Co and Fe-N alloys) and metal oxides (Fe3O4 and γ-Fe2O3), Fe3O4 has become the most commonly used material for MSPE owing to its advantages of easy preparation, low toxicity and low cost [32]. However, bare Fe3O4 has little adsorption capacity for the analytes. In addition, it is prone to agglomerate and oxidize in the air due to its small size and high surface activity. Therefore, researchers apply appropriate physical or chemical methods to functionalize Fe3O4, which not only overcomes its own defects, but also facilitates the introduction of specific groups for enhanced enrichment capacity [33, 34]. As for the separation and enrichment of glycoproteins and glycopeptides in complex samples, the functional materials of magnetic adsorbents in MSPE are mainly divided into the following categories: inorganic nanomaterials, organic small molecules, polymers, porous framework materials and others.Table 1 summarizes some representative magnetic materials and their enrichment performance toward glycopeptides.
|
|
Table 1 Representative list of magnetic adsorbent for glycoproteins and glycopeptides enrichment. |
Inorganic nanomaterials functionalized Fe3O4 nanoparticles reduce the effects of agglomeration and possess certain affinity to glycoproteins and glycopeptides. What is more, its large specific surface area provides sites for subsequent modification [35]. The common inorganic nanomaterials used for functionalizing Fe3O4 include silicon nanomaterials, carbon nanomaterials, metal nanomaterials, etc.
3.1.1. Silicon nanomaterialsSilicon nanomaterials are one of the most important classes of inorganic nanomaterials and have widespread application for the functionalization of magnetic nanoparticles due to their good biocompatibility, little toxicity and high surface-to-volume ratios [36]. Generally, silicon nanomaterials are introduced to the surface of Fe3O4 through the formation of Si-O bonds and accompanied by additional surface modification [37].
To date, researchers have exploited various magnetic adsorbents functionalized by silicon nanomaterials and applied them to the separation and enrichment of glycoproteins and glycopeptides. For instance, Sun and co-workers [38] introduced cysteine onto the aminosilanized Fe3O4 via the formation of Fe-S bond, followed by the deposition of layered mesoporous silica on the surface of core-shell materials. Finally, a zwitterionic magnetic mesoporous silica material designed as l-Cys-Fe3O4@mSiO2 was obtained. The outer mesoporous silica possessed size-exclusion effect, which could selectively enrich glycopeptides and exclude large-size proteins even the mass ratio of horseradish peroxidase (HRP) tryptic digests/bovine serum albumin (BSA) reached to 1:500. Then this material was applied to enrichment glycopeptides from saliva of healthy people and gastric cancer patients, 46 and 36 endogenous glycopeptides were identified, respectively. From the enrichment results of real samples, the enrichment performance of this material towards glycopeptides was not ideal, but it provided new ideas for the design of silicon nanomaterials functionalized magnetic adsorbents for effective separation of endogenous glycopeptides.
3.1.2. Carbon nanomaterialsCarbon nanomaterials represented by graphene and carbon nanotubes have great potential in the enrichment of glycoproteins and glycopeptides due to their large specific surface area, high chemical stability and functional groups like hydroxyl and carboxyl carried on the surface [39]. The combination of carbon nanomaterials with Fe3O4 to prepare novel magnetic adsorbents has displayed advantages in the field of separation and enrichment [40]. Chen et al. [41] used a simple two-step synthesis strategy (dopamine polymerization and Michael addition) to prepare a hydrophilic chitosan functionalized magnetic nanocomposite (Fe3O4-GO@PDA-Chitosan). Thanks to the large specific surface area of graphene oxide, more modification sites were provided for the immobilization of chitosan, which further improved the enrichment efficiency of glycopeptides. Finally, 393 N-glycopeptides were selectively enriched in tryptic digests of human renal mesangial cells (HRMC), corresponding to 195 glycoproteins and 458 glycosylation sites. What is more, Zhao et al. [42] sequentially deposited polyethylenimine (PEI) and hyaluronic acid (HA) on the negatively charged magnetic graphene oxide surface via electrostatic interaction and a hydrophilic magnetic material denoted as MagG@PEI@HA was obtained. The feasibility of the as-prepared magnetic adsorbent toward glycopeptides enrichment was demonstrated by applying MagG@PEI@HA to capture glycopeptides from 2 μL serum sample. As a result, 376 N-glycopeptides originated from 102 glycoproteins were detected. These works provided a feasible strategy to design magnetic affinity materials for glycopeptides enrichment.
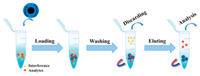
3.1.3. Metal nanomaterialsGold nanoparticles (Au NPs) and silver nanoparticles (Ag NPs) are often used as connecting intermediates to interact with amino groups or sulfhydryl groups, which facilitated the introduction of specific molecules and further improved the enrichment performance of materials [43]. Our group [44] utilized Au NPs as a cross-linker to prepare hydrophilic glutathione (GSH) modified magnetic nanocomposites (Fe3O4@Au-GSH). Massive Au NPs endowed Fe3O4 with enhanced stability and active sites for immobilizing GSH through the formation of Au-S bonds (Fig. 3). The acquired magnetic nanocomposites showed good enrichment performance for glycopeptides based on the superior superparamagnetism of Fe3O4, favorable biocompatibility of Au NPs and good hydrophilicity of GSH. Similarly, Qin et al. [45] introduced an ultra-thin two-dimensional MoS2 nanomaterial with exposed sulfur atoms on its surface for the immobilization of Au nanowire. Integrating the good magnetic response of Fe3O4, unique bonding sites supplied by layered MoS2, enlarged surface area and enhanced biocompatibility supported by Au nanowire and superior hydrophilicity of GSH, the obtained materials (MoS2-Fe3O4-Au/NWs-GSH) exhibited outstanding enrichment performance, 1250 and 489 N-glycopeptides were identified from human urinary exosomes and serum, respectively. These results indicated that metal nanomaterials possessed great potential in the functionalization of magnetic materials.

|
Download:
|
| Fig. 3. (A) Synthetic route of Fe3O4@Au-GSH. (B) Workflow of glycopeptides enrichment by Fe3O4@Au-GSH. Reproduced with permission [44]. Copyright 2019, Elsevier Ltd. | |
Small organic molecules with multiple functional groups are commonly used to modify Fe3O4 nanoparticles. For one thing, organic small molecules could be effectively fixed on the surface of Fe3O4 nanoparticles, for another, organic small molecules are employed as reaction groups in order to introduce functional groups with high affinity towards glycoproteins and glycopeptides. β-Cyclodextrin (β-CD), a cyclic oligosaccharide with abundant polar hydroxyl groups, has aroused great interests that exploiting it for glycoproteins and glycopeptides enrichment [46]. Tian's group [47] developed a CD-based magnetic material via the carbodiimide activation strategy between carboxymethyl-β-cyclodextrin (CMCD) and Fe3O4. The CMCD-bonded Fe3O4 presented good enrichment performance for glycopeptides from the tryptic digests of proteins extracted from 200 μg mouse liver and 666 N-glycosylation sites corresponding to 494 glycoproteins were successfully identified. Besides, Chen's group [48] prepared hydrophilic gluconamide modified magnetic mesoporous silica with high surface area and size-exclusion effect. This affinity material with typical core-shell structure was applied to capture glycopeptides from tryptic digest of HRP or glycans from human serum. The detection limit of this material toward glycopeptides enrichment was determined to be 20 fmol of HRP digest. These magnetic adsorbents usually possessed simple synthesis process and low cost, and its enrichment efficiency of glycoproteins and glycopeptides was better than that of commercial affinity materials.
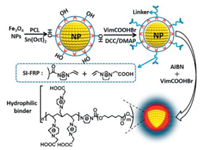
3.3. PolymersTheoretically speaking, polymers have higher affinity for glycoproteins and glycopeptides than small molecular. The main reason is that the bonding of small molecular on magnetic nanoparticles is often single layered while the immobilization of polymer is multiple layered [49]. Taking advantages of tight graft layer, massive active sites and high magnetic susceptibility, Wang et al. [50] described a polymer ionic liquid modified magnetic material (Fe3O4@PCL-PILs) with unique core-corona structure. The scheme of the synthetic procedure for Fe3O4@PCL-PILs was displayed in Fig. 4. The existence of polymer layer could aid the selective enrichment of immunoglobulin G (IgG) since much grafting sites were provided for immobilizing ionic liquid. They demonstrated the adsorption ability of the as-prepared Fe3O4@PCL-PILs by using it to selectively adsorb IgG from human whole blood and high adsorption capacity as high as 1136.4 mg/g was achieved. In addition, Zhang's group [51] employed maltose polymer brushes as functional affinity groups to graft on the PEI coated Fe3O4. This magnetic adsorbent was applied for the selective enrichment of glycopeptides from the tryptic digests of 200 μg HRMC, and identified 449 N-glycopeptides representing 323 glycoproteins and 476 glycosylation sites. Compared with other materials [41], the better enrichment ability of the material was attributed to the grafting of maltose polymer brush and an effective assembly strategy. These results supported the viewpoint that polymer offered more sites for bonding functional groups, resulting in improved enrichment ability to glycoproteins and glycopeptides.
|
Download:
|
| Fig. 4. Scheme of synthetic procedure for Fe3O4@PCL-PILs. Reproduced with permission [50]. Copyright 2018, American Chemical Society. | |
The porous framework materials have high specific surface area, structural diversity, periodic porous structure and variable surface properties [52], which have been reported to be useful for the separation and enrichment of glycoproteins and glycopeptides. The special functional groups (such as amino, carboxyl, and sulfonic acid groups) carried by the porous framework materials can not only serve as binding sites to directly adsorb glycoproteins and glycopeptides, but also serve as carriers for the post-modification process to introduce functional groups, which were beneficial to enhance the enrichment performance of materials towards glycoproteins and glycopeptides.
3.4.1. Metal organic frameworkIn recent years, metal organic framework (MOF) materials composed of metal ions and organic ligands have attracted extensive attention in proteomics research because of their high specific surface area, ordered nano-channels and abundant binding sites [53, 54]. Chen et al. [55] established a dual-functional magnetic MOF (Fe3O4@SiO2@UiO-PBA) for glycopeptides enrichment based on the hydrophilic amino groups of UiO-66-NH2 and high affinity of PBA. The fabrication of this material was adopted a post-modification strategy of MOF materials. PBA was modified on the surface of magnetic MOF via a general crosslinking reaction. Finally, Fe3O4@SiO2@UiO-PBA exhibited high enrichment capacity towards ovalbumin in egg white. Similarly, Wu and co-workers [56] introduced a novel magnetic MOF material (SPIOs@SiO2@MOF) constructed by Zr4+ and terephthalic acid (PTA) and 1, 4-phenylenebisboronic acid (designed as PBA). The utilization of double organic ligands in material synthesis procedure not only provided scaffolds for the construction of MOF materials, but also introduced PBA which could specifically recognize glycoproteins and glycopeptides, avoiding the tedious post-modification process and simplifying the synthesis procedure. Based on boronate affinity strategy, 152 glycopeptides were detected from the tryptic digests of mouse liver. Although this enrichment result wasn't satisfactory, it supplied a novel design platform for multifunctional magnetic MOF used to enrich peptides. It could be concluded that regardless of the presence or absence of the post-modification process of magnetic MOF materials, they all presented potential in the field of glycoproteins and glycopeptides enrichment.
3.4.2. Covalent organic frameworkCovalent organic framework (COF) represents a new type of porous nanomaterials composed of organic architecture units by forming strong covalent bonds. Researchers have employed organic units containing special groups or post-modification to synthesize functional COF materials [57, 58]. So far, various magnetic COF composites have been developed for the efficient separation of glycoproteins and glycopeptides in complex samples. Qian's group [59] developed a sea urchin-type magnetic COF material (Fe3O4@TpPa-1) without post-modification. The obtained composites showed good hydrophilicity, well-ordered pores and fast magnetic response. As a consequence, Fe3O4@TpPa-1 selectively enriched 228 glycopeptides within 114 glycoproteins from the tryptic digests of human serum, which was better than that in commercial HILIC materials. In addition, Lin's group [60] built a boronate-based magnetic COF via a simple click reaction. Briefly, azide-phenylboronic acid (N3-PBA) was grafted onto the magnetic MOF layer containing massive yne groups via azide-alkyne cycloaddition reaction. By employing a simple and efficient click reaction, this fabrication strategy provided a new way for the post-modification of COF. Combining the unique features of Fe3O4, COFs and PBA, the composites successfully applied to enrich glycoproteins and glycopeptides from HeLa cell exosomes digests. At least, 34 N-glycosylation sites corresponding to 32 glycopeptides were identified, which may contribute to the high complexity of the practical samples.
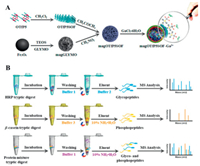
3.4.3. Supramolecular organic frameworkAs a promising member of framework materials, supramolecular organic framework (SOF) materials based on supramolecular macrocycles are usually assembled by non-covalent supramolecular interactions (such as hydrogen bonding, van der Waals force, π-π stacking and dipole interaction) [61]. Up to now, SOF-based enrichment materials for glycoproteins and glycopeptides are still scarce, but its application in the field of glycoproteomics analysis is convincing. In our recent research [62], a pillar[5]arene-based SOF material (magOTfP5SOF-Ga3+) were prepared and applied to construct a MSPE platform for glycopeptides and phosphopeptides enrichment. The whole synthesis and enrichment process were illustrated in Fig. 5. MagOTFP5SOF-Ga3+ featured the large specific surface area, good biocompatibility and superparamagnetism. Combing with the MS method, the bifunctional SOF material was employed to analyze glycopeptides and phosphopeptides from human body fluid, human lung adenocarcinoma cell line and mouse liver tissue. The excellent enrichment property of MagOTFP5SOF-Ga3+ for phosphopeptides is not described here. As for glycopeptides enrichment, the established MSPE platform identified 1006 N-glycopeptides and 665 glycoproteins from tryptic digests of mouse liver. Moreover, a gene onthology analysis illustrated that these glycoproteins detected from tryptic digests of mouse liver showed significantly overrepresented in the biological process of phagocytosis, glycosaminoglycan metabolic process and cell adhesion. These results confirmed the superior enrichment performance of MagOTFP5SOF-Ga3+, which contributed to deepen the understanding of protein glycosylation.
|
Download:
|
| Fig. 5. (A) Synthetic route of magOTfP5SOF-Ga3+. (B) Workflow of glycopeptides and phosphopeptides enrichment by magOTfP5SOF-Ga3+ individually and simultaneously. Reproduced with permission [62]. Copyright 2020, American Chemical Society. | |
Apart from the common magnetic adsorbents mentioned above, several other types of magnetic adsorbents have also been reported. For instance, Dang's group [63] utilized a maltose-modified oligopeptides (AEK8-maltose) to functionalize magnetic nanoparticles. The self-assembled oligopeptides layer on the surface of magnetic nanoparticles gave the magnetic material the ability to remain intact after the repetitive washing process with acidic organic solutions. As a result, the as-fabricated materials identified 282 glycopeptides from human serum digests. To further increase affinity sites for higher enrichment performance, Zhang et al. [64] coated PAMAM dendrimer onto magnetic grapheme-silica hydrophilic substrate, followed by grafting massive maltose on dendrimer via Au served as a cross-linker. Thanks to the large specific surface area, high magnetic response and high density of affinity sites, the acquired Fe3O4-GO@nSiO2-PAMAM nanocomposites identified 1529 N-glycopeptides within 760 glycoproteins from 50 mg tryptic digests of mouse liver, implying the application optional of dendrimer in glycopeptides enrichment. Furthermore, magnetic molecularly imprinted materials have attracted widespread attention due to their high specificity towards glycoproteins. Shi's group [65] described a magnetic molecular imprint polymer material (Fe3O4@pTiO2@MIP) based on boronate affinity, in which the flowerlike structure of TiO2 replaced the smooth SiO2 and more sites provided. It should be mentioned that Ti4+ possessed the strong electron-withdrawing capability decreased the p Ka values of 4-viylphenylboronic acid (VPBA), enabling the identification of glycoproteins from moderate acidic samples. These materials mentioned above enriched the categories of magnetic adsorbents used in glycoproteins and glycopeptides enrichment.
4. Application of MSPE in human body fluidsStudies have shown that 70% of human proteins contain one or more sugar chains, and 1% of human genome participates in the synthesis and modification of sugar chains [66, 67]. The changes in the kinds and concentration of glycoproteins in human body fluids can not only reflect the glycosylation level of proteins, but also make contributions to the search and discovery of disease biomarkers [68]. Therefore, the qualitative and quantitative analysis of glycoproteins and glycopeptides in human body fluids play important roles in the screening and discovery of disease biomarkers with clinical significance [69]. In this section, the application of MSPE technology in separation and enrichment of glycoproteins and glycopeptides will be reviewed from four aspects: blood, urine, saliva and other body fluids. Representative materials and their performance toward glycopeptides enrichment are listed in Table 1.
4.1. BloodHuman blood with complex composition and large difference in component content is one of the most commonly used clinical biological samples and the most complex body fluid used for proteomics analysis. As an easily accessible and minimally invasive clinical sample, the detection of the expression levels of glycoproteins and glycopeptides in the blood is essential for discovering new disease biomarkers and monitoring the progress of diseases [70]. Zhang's group [71] constructed a magnetic boronate affinity material (Fe3O4/PDA/GO/PEI/CBX) with high specific surface area, multivalent binding sites and fine magnetic response. Unlike the common boronate affinity materials, they introduced benzoboroxole (BX) as an improved Wulff-type boronic acid, benefiting from its low p Ka value and good hydrophilicity, Fe3O4/PDA/GO/PEI/CBX presented high affinity towards glycoproteins and glycopeptides in neutral conditions. They used Fe3O4/PDA/GO/PEI/CBX in combination with nano-LC–MS/MS to identify glycoproteins from human plasma. At least, 202 glycoproteins were detected from 20 μL human plasma. It was important to point out that the enrichment ability for labile glycoproteins in alkaline conditions was improved. Moreover, Yang et al. [72] used resorcinol and formaldehyde as precursors, PEI as a cross-linker to prepare a magnetic mesoporous phenolic resin (MMP) with fine hydrophilicity and nitrogen-containing functionality. They employed this material as magnetic adsorbent to enrich glycoproteins and glycopeptides, and 356 N-glycopeptides matching to 119 glycoproteins from 0.35 μL human serum were detected. In virtue of the matrix tolerance, these functionalized magnetic materials showed a promising prospect for the separation and enrichment of glycoproteins and glycopeptides in extremely complex blood samples. Besides, Li and co-workers [73] synthesized magnetic nanofifiber-based affinity materials (magHN/Au-GSH nanofiber) by applying the one-dimensional hydroxyapatite nanofiber as substrates to immobilize Fe3O4 and Au NPs, accompanied by bonding hydrophilic GSH via Au-S and Fe-S. The as-prepared materials were utilized to analyze the N-glycoproteomic profile of human serum. At a consequence, 246 N-glycopeptides from 104 N-glycoproteins were identified from 1 μL human serum. It is worth noting that the enrichment performance of magHN/Au-GSH nanofiber was superior to commercial hydrophilic materials and the as-prepared materials was expected to realize the comprehensive glycoproteomic research including O-glycopeptides.
4.2. UrineUrine produced by kidneys under normal physiological conditions contains a relatively small amount of proteins. The difference of protein content in urine reflects the physiological conditions of local (protein from kidney) and whole body (protein from plasma) from a clinical perspective [74]. Therefore, urine is considered as an ideal source of biomarkers with clinical significance. Furthermore, urine can be obtained repeatedly, in large quantities and non-invasively [75]. In recent years, researches about urine glycoproteomics have attracted wide attention. Zhang and co-workers [76] presented a novel hydrophilic material (MagG@Mg-MOFs-1C) for efficient enrichment glycopeptides from human urine. In this material, the Mg-MOFs layer was assembled to the surface of magnetic graphene via π-π interaction. The acquired materials provided large surface area, massive affinity sites, strong magnetic property and unique porous structure, exhibiting good enrichment performance of glycopeptides from urine. At least, 406 N-glycopeptides within 185 glycoproteins were identified from urine of the bladder cancer patients, in which contained the potential biomarkers for bladder cancer like α-1-antitrypsin, complement C4-B and α-2-macroglobulin. This work paved a way for large-scale screening and discovering glycoproteins as biomarkers. Afterwards, they selected urine exosomes as research object to study the heterogeneity of urine exosomes subpopulations with different size, so as to realize in-deep understanding of its corresponding function as intercellular communication substance [77]. By utilizing size exclusion liquid chromatography (SEC), three distinct-size exosomes (L-Exo, M-Exo and S-Exo) were obtained. Subsequently, a magnetic affinity probe (CFMZOF) combined with LC/MS-MS were introduced to selectively enrich glycoproteins or phosphoproteins from the above exosomes. Finally, 144, 156 and 134 glycoproteins were identified from L-Exo, M-Exo and S-Exo, respectively. These discoveries provided valuable information about illustrating the biological functions of urine exosomes.
4.3. SalivaHuman saliva is a viscoelastic biological fluid and overlaps with blood/plasma by 20%–30% in protein content [78]. Taking advantage of its easy collection, non-invasiveness and cost-effectiveness, saliva is often applied to clinical detection. Researchers have identified many glycoproteins in human saliva as candidate biomarkers for various diseases, demonstrating the great potential of saliva as a clinical biological sample in biomarkers discovery [79]. Deng et al. [80] engineered a magnetic COF material (mCTpBD) without any post-modification. The inherently hydrophilic COF layer was successfully deposited on the amine-group functionalized Fe3O4. Combined with LC/MS-MS technology, the as-fabricated materials were applied to selectively enrich glycopeptides from human saliva. Finally, 28, 32 and 49 glycopeptides were identified from saliva of healthy people and 27, 39 and 40 glycopeptides were identified from saliva of patients with inflammatory bowel disease (IBD) in three independent replicates. In another early example of glycoproteins enrichment in saliva, Hill et al. [81] optimized the enrichment strategy for different salivary glycoproteins based on lectin magnetic bead arrays (saLeMBA). In this method, different lectins were coupled in magnetic beads to construct a lectin magnetic bead arrays (LeMBA) and various parameters of this technology were optimized to enrich salivary glycoproteins. Benefiting from the highly specific recognition between lectins and glycans, glycoproteins with differences in glycan structures were identified from human saliva. However, lectins were usually expensive, which were not conducive to the construction of arrays with multiple lectins.
4.4. OthersAs a novel biological fluid, quantitative and qualitative analysis of amniotic fluid may be helpful to identify pregnancy complications and discover specific biomarkers of fetal disease [82]. Wang et al. [83] described a synergistic strategy for specific enrichment of glycopeptides in amniotic fluid. Briefly, 3-formylbenzeneboronic acid modified magnetic materials (B-Fe3O4@mTiO2) and poly(methyl methacrylate) beads were utilized to selectively capture glycopeptides and non-glycopeptides, respectively. Based on the synergistic enrichment effects, the recovery of N-glycopeptides from standard glycoprotein digests reached to 92.1%, which improved by 20% compared with B-Fe3O4@mTiO2 alone. They tested the applicability of this method by selecting amniotic fluid as biological sample, and finally 126 N-glycopeptides corresponding to 97 glycoproteins were identified. This integration provided an efficient enrichment platform for glycoproteins and glycopeptides.
5. Conclusion and perspectiveIn summary, glycoproteins and glycopeptides are vulnerable to interference and ion inhibition. Therefore, effective separation and enrichment are required prior to MS detection. In the past decade, novel strategies used for the separation and enrichment of glycoproteins and glycopeptides have sprung up. With the aid of these strategies, improved enrichment efficiency, selectivity and sensitivity of glycoproteins and glycopeptides have been realized, which are beneficial to the analysis and characterization of glycoproteins and glycopeptides in human body fluids (such as serum, urine and saliva) and further promote the development of large-scale glycoproteomics analysis. MSPE technology possessed distinctive features such as easy preparation and time-saving has great potential in the separation and enrichment of glycoproteins and glycopeptides from human body fluids. In addition, the development of functionalized magnetic adsorbents greatly reduces the enrichment time, simplifies the enrichment process compared with other enrichment materials, and enhances the enrichment performance for glycoproteins and glycopeptides with low-abundance and various types to a certain extent.
Screening and discovering glycoproteins and glycopeptides from human body fluids as disease biomarkers, especially diagnostic biomarkers with high specificity and sensitivity for early disease detection, are a challenging task. Though effective separation and enrichment of glycoproteins and glycopeptides from human body fluids have been well developed, systematic researches about screening and identifying of glycoproteins and glycopeptides biomarkers is relatively scarce, which leave space for further research on the quantitative analysis of glycoproteins and glycopeptides from human body fluids and the screening of disease biomarkers. Future researches should focus on several aspects: developing novel magnetic adsorbents with higher efficiency and selectivity; enlarging the sample size to make it more representative; in addition, monitoring the concentration changes of potential glycoproteins and glycopeptides biomarkers in body fluids of patients with various diseases so that we could find the relationship between biomarkers and clinical diagnosis, and further verify the possibility of regarding them as diagnostic biomarkers of diseases. Through these systematic analysis and verification, it can be anticipated that the screening of abnormal glycoproteins and glycopeptides in human body fluids will be realized by MSPE technology with higher specificity and sensitivity, which is of vital significance for the screening of biomarkers and early detection of clinical diseases.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThis work was supported by the Natural Science Foundation of Jilin Provincial Science & Technology Department (No. 20190201079JC).
| [1] |
T. Walski, K. De Schutter, E.J.M. Van Damme, G. Smagghe, Insect Biochem. Mol. Biol. 83 (2017) 21-34. DOI:10.1016/j.ibmb.2017.02.005 |
| [2] |
K.T. Schjoldager, Y. Narimatsu, H.J. Joshi, H. Clausen, Nat. Rev. Mol. Cell Bio. 21 (2020) 729-749. DOI:10.1038/s41580-020-00294-x |
| [3] |
Q. Lu, C. Chen, Y. Xiong, G. Li, et al., Anal. Chem. 92 (2020) 6269-6277. DOI:10.1021/acs.analchem.9b02643 |
| [4] |
Y. Liu, X.H. Xun, J.M. Yi, Y. Xiang, J. Hua, Chin. Chem. Lett. 28 (2017) 1093-1098. DOI:10.1016/j.cclet.2016.11.026 |
| [5] |
I.H. Chen, H.A. Aguilar, J.S. Paez Paez, et al., Anal. Chem. 90 (2018) 6307-6313. DOI:10.1021/acs.analchem.8b01090 |
| [6] |
M.M. Wang, J.H. Zhu, D.M. Lubman, C.F. Gao, Clin. Chem. Lab. Med. 57 (2019) 407-416. DOI:10.1515/cclm-2018-0379 |
| [7] |
L.R. Ruhaak, G. Xu, Q. Li, E. Goonatilleke, C.B. Lebrilla, Chem. Rev. 118 (2018) 7886-7930. DOI:10.1021/acs.chemrev.7b00732 |
| [8] |
S. Suttapitugsakul, F. Sun, R. Wu, Anal. Chem. 92 (2020) 267-291. DOI:10.1021/acs.analchem.9b04651 |
| [9] |
T. Zhao, C. Zhang, W. Ma, et al., Anal. Chim. Acta 1058 (2019) 107-116. DOI:10.1016/j.aca.2019.01.044 |
| [10] |
H. Zheng, J. Ma, W. Feng, Q. Jia, J. Chromatogr. A 1512 (2017) 88-97. DOI:10.1016/j.chroma.2017.07.032 |
| [11] |
H. Zheng, X. Li, Q. Jia, ACS Appl. Mater. Interfaces 10 (2018) 5909-5917. DOI:10.1021/acsami.7b18999 |
| [12] |
H. Zheng, X. Li, Q. Jia, ACS Appl. Mater. Interfaces 10 (2018) 19914-19921. DOI:10.1021/acsami.8b01445 |
| [13] |
X.D. Wang, Y.J. Liu, F.J. Li, Z.L. Li, Chin. Chem. Lett. 28 (2017) 1018-1026. DOI:10.1016/j.cclet.2017.02.001 |
| [14] |
H. Xiao, W. Chen, J.M. Smeekens, R. Wu, Nat. Commun. 9 (2018) 1692. DOI:10.1038/s41467-018-04081-3 |
| [15] |
Z. Bie, Y. Chen, H. Li, R. Wu, Z. Liu, Anal. Chim. Acta 834 (2014) 1-8. DOI:10.1016/j.aca.2014.04.035 |
| [16] |
H. Bai, Y. Pan, L. Qi, et al., Talanta 186 (2018) 513-520. DOI:10.1016/j.talanta.2018.04.098 |
| [17] |
J. Huang, H. Wan, Y. Yao, et al., Anal. Chem. 87 (2015) 10199-10204. DOI:10.1021/acs.analchem.5b02669 |
| [18] |
H. Zheng, T. Zhu, X. Li, J. Ma, Q. Jia, Anal. Chim. Acta 983 (2017) 141-148. DOI:10.1016/j.aca.2017.06.034 |
| [19] |
A.J. Alpert, J. Chromatogr. A 499 (1990) 177-196. DOI:10.1016/S0021-9673(00)96972-3 |
| [20] |
G. Qing, J. Yan, X. He, X. Li, X. Liang, TrAC-Trends Anal. Chem. 124 (2020) 115570. DOI:10.1016/j.trac.2019.06.020 |
| [21] |
D. Li, Y. Chen, Z. Liu, Chem. Soc. Rev. 44 (2015) 8097-8123. DOI:10.1039/C5CS00013K |
| [22] |
J. Chen, P. Shah, H. Zhang, Anal. Chem. 85 (2013) 10670-10674. DOI:10.1021/ac401812b |
| [23] |
E. Song, R. Zhu, Z.T. Hammoud, Y. Mechref, J. Proteome Res. 13 (2014) 4808-4820. DOI:10.1021/pr500570m |
| [24] |
Y. Zhang, M. Kuang, L. Zhang, P. Yang, H. Lu, Anal. Chem. 85 (2013) 5535-5541. DOI:10.1021/ac400733y |
| [25] |
X.S. Li, G.T. Zhu, Y.B. Luo, B.F. Yuan, Y.Q. Feng, TrAC-Trends Anal. Chem. 45 (2013) 233-247. DOI:10.1016/j.trac.2012.10.015 |
| [26] |
M. Zhao, Y. Xie, C. Deng, X. Zhang, J. Chromatogr. A 1357 (2014) 182-193. DOI:10.1016/j.chroma.2014.04.078 |
| [27] |
H. Zheng, H. Lin, X. Chen, et al., TrAC-Trends Anal. Chem. 129 (2020) 115952. DOI:10.1016/j.trac.2020.115952 |
| [28] |
Mirka Šafařı'ková, Ivo Šafařı'k, J. Magn, Magn. Mater. 194 (1999) 108-112. DOI:10.1016/S0304-8853(98)00566-6 |
| [29] |
S. Zhu, B. Chen, M. He, T. Huang, B. Hu, Talanta 171 (2017) 213-219. DOI:10.1016/j.talanta.2017.04.068 |
| [30] |
A.L. Capriotti, C. Cavaliere, G. La Barbera, et al., Chromatographia 82 (2019) 1251-1274. DOI:10.1007/s10337-019-03721-0 |
| [31] |
L. Khoshmaram, M. Saadati, F. Sadeghi, Microchem. J. 152 (2020) 104344. DOI:10.1016/j.microc.2019.104344 |
| [32] |
J. Yang, J.Q. Qiao, S.H. Cui, et al., J. Sep. Sci. 38 (2015) 1969-1976. DOI:10.1002/jssc.201500167 |
| [33] |
W. Zhang, Y. Zhang, Q. Jiang, et al., Anal. Chem. 88 (2016) 10523-10532. DOI:10.1021/acs.analchem.6b02583 |
| [34] |
D. Jiang, X. Li, X. Lv, Q. Jia, Talanta 185 (2018) 461-468. DOI:10.1016/j.talanta.2018.04.006 |
| [35] |
C. Wang, H. Zhong, W. Wu, et al., ACS Omega 4 (2019) 1652-1661. DOI:10.1021/acsomega.8b03157 |
| [36] |
F. Peng, Y. Su, Y. Zhong, et al., Accounts Chem. Res. 47 (2014) 612-623. DOI:10.1021/ar400221g |
| [37] |
Y. Jung, Y. Huh, D. Kim, Micropor. Mesopor. Mater. 310 (2021) 110673. DOI:10.1016/j.micromeso.2020.110673 |
| [38] |
H. Chen, Y. Li, H. Wu, N. Sun, C. Deng, ACS Sustain. Chem. Eng. 7 (2019) 2844-2851. DOI:10.1021/acssuschemeng.8b06258 |
| [39] |
N. Li, H.L. Jiang, X. Wang, et al., TrAC-Trends Anal. Chem. 102 (2018) 60-74. DOI:10.1016/j.trac.2018.01.009 |
| [40] |
X.L. Liu, C. Wang, Q.H. Wu, Z. Wang, Chin. Chem. Lett. 25 (2014) 1185-1189. DOI:10.1016/j.cclet.2014.03.030 |
| [41] |
C. Bi, Y. Yuan, Y. Tu, et al., Sci. Rep. 10 (2020) 71. DOI:10.1038/s41598-019-56944-4 |
| [42] |
Q. Zhan, H. Zhao, Y. Hong, et al., Microchim. Acta 186 (2019) 600. DOI:10.1007/s00604-019-3712-2 |
| [43] |
M.C. Daniel, D. Astruc, Chem. Rev. 104 (2004) 293-346. DOI:10.1021/cr030698+ |
| [44] |
H. Qi, Z. Li, H. Zheng, L. Fu, Q. Jia, Chin. Chem. Lett. 30 (2019) 2181-2185. DOI:10.1016/j.cclet.2019.06.046 |
| [45] |
H. Zhang, Y. Lv, J. Du, et al., Anal. Chim. Acta 1098 (2020) 181-189. DOI:10.1016/j.aca.2019.11.012 |
| [46] |
H. Zheng, N. Song, X. Li, Q. Jia, Analyst 142 (2017) 4773-4781. DOI:10.1039/C7AN01436H |
| [47] |
P.P. Song, P.W. Huang, T.J. Huang, et al., Rapid Commun. Mass Spectrom. 30 (2016) 190-195. DOI:10.1002/rcm.7626 |
| [48] |
Y. Liu, W. Ma, Y. He, Z. Chen, Z. Lin, Anal. Lett. (2020) 1-13. |
| [49] |
N. Sun, H. Wu, H. Chen, X. Shen, C. Deng, Chem. Commun. 55 (2019) 10359-10375. DOI:10.1039/C9CC04124A |
| [50] |
Z.Y. Guo, X. Hai, Y.T. Wang, et al., Biomacromolecules 19 (2018) 53-61. DOI:10.1021/acs.biomac.7b01231 |
| [51] |
C.F. Bi, Y.L. Liang, L.J. Shen, et al., ACS Omega 3 (2018) 1572-1580. DOI:10.1021/acsomega.7b01788 |
| [52] |
S.H. Zhang, Q. Yang, C. Wang, X.L, et al., Adv. Sci. 5 (2018) 1801116. DOI:10.1002/advs.201801116 |
| [53] |
L. Hao, X.L. Liu, J.T. Wang, et al., Chin. Chem. Lett. 27 (2016) 783-788. DOI:10.1016/j.cclet.2016.01.021 |
| [54] |
N. Huang, K. Wang, H. Drake, et al., J. Am. Chem. Soc. 140 (2018) 6383-6390. DOI:10.1021/jacs.8b02710 |
| [55] |
S. Li, Y. Qin, G. Zhong, et al., ACS Appl. Mater. Interfaces 10 (2018) 27612-27620. DOI:10.1021/acsami.8b07671 |
| [56] |
B. Luo, Q. Chen, J. He, et al., ACS Sustain. Chem. Eng. 7 (2019) 6043-6052. DOI:10.1021/acssuschemeng.8b06171 |
| [57] |
F. Xiong, L. Jiang, Q. Jia, Anal. Chim. Acta 1099 (2020) 103-110. DOI:10.1016/j.aca.2019.11.058 |
| [58] |
D.D. Jiang, T.T. Hu, H.J. Zheng, G.X. Xu, Q. Jia, Chem. Eur. J. 24 (2018) 10390-10396. DOI:10.1002/chem.201800092 |
| [59] |
H. Wang, F. Jiao, F. Gao, J, et al., J. Mater. Chem. B 5 (2017) 4052-4059. DOI:10.1039/C7TB00700K |
| [60] |
C. Gao, J. Bai, Y. He, et al., ACS Sustain. Chem. Eng. 7 (2019) 18926-18934. DOI:10.1021/acssuschemeng.9b04293 |
| [61] |
L.L. Tan, H. Li, Y. Tao, et al., Adv. Mater. 26 (2014) 7027-7031. DOI:10.1002/adma.201401672 |
| [62] |
H. Zheng, J. Jia, Z. Li, Q. Jia, Anal. Chem. 92 (2020) 2680-2689. DOI:10.1021/acs.analchem.9b04691 |
| [63] |
L. Zhang, X. Yue, N. Li, et al., Anal. Chim. Acta 1088 (2019) 63-71. DOI:10.1016/j.aca.2019.08.040 |
| [64] |
H. Wan, J. Huang, Z. Liu, et al., Chem. Commun. 51 (2015) 9391-9394. DOI:10.1039/C5CC01980J |
| [65] |
X.Y. Sun, R.T. Ma, J. Chen, Y.P. Shi, Microchim. Acta 185 (2018) 565. DOI:10.1007/s00604-018-3092-z |
| [66] |
D.M. Ratner, E.W. Adams, M.D. Disney, P.H. Seeberger, Chembiochem 5 (2004) 1375-1383. DOI:10.1002/cbic.200400106 |
| [67] |
R. Raman, S. Raguram, G. Venkataraman, J.C. Paulson, R. Sasisekharan, Nat. Methods 2 (2005) 817-824. DOI:10.1038/nmeth807 |
| [68] |
L. Dang, L. Jia, Y. Zhi, et al., TrAC-Trends Anal. Chem. 114 (2019) 143-150. DOI:10.1016/j.trac.2019.02.009 |
| [69] |
M.D. Zhao, Y.H. Yang, Z.G. Guo, et al., Proteom. Clin. Appl. 12 (2018) 1800008. DOI:10.1002/prca.201800008 |
| [70] |
Q. Li, M.J. Kailemia, A.A. Merleev, et al., Anal. Chem. 91 (2019) 5433-5445. DOI:10.1021/acs.analchem.9b00776 |
| [71] |
Q. Wu, B. Jiang, Y. Weng, et al., Anal. Chem. 90 (2018) 2671-2677. DOI:10.1021/acs.analchem.7b04451 |
| [72] |
Q. Zhang, Y. Huang, B. Jiang, et al., Anal. Chem. 90 (2018) 7357-7363. DOI:10.1021/acs.analchem.8b00708 |
| [73] |
W. Huan, J. Zhang, H. Qin, et al., Nanoscale 11 (2019) 10952-10960. DOI:10.1039/C9NR01441A |
| [74] |
A. Beasley-Green, Int. Neurourol. J. 20 (2016) 70-75. DOI:10.5213/inj.1612720.360 |
| [75] |
Thongboonkerd, Proteom. Clin. Appl. 1 (2007) 780-791. DOI:10.1002/prca.200700035 |
| [76] |
J. Wang, J. Li, G. Yan, M. Gao, X. Zhang, Nanoscale 11 (2019) 3701-3709. DOI:10.1039/C8NR10074H |
| [77] |
H. Zheng, S. Guan, X. Wang, J, et al., Anal. Chem. 92 (2020) 9239-9246. DOI:10.1021/acs.analchem.0c01572 |
| [78] |
S. Chojnowska, T. Baran, I. Wilinska, et al., Adv. Med. Sci. 63 (2018) 185-191. DOI:10.1016/j.advms.2017.11.002 |
| [79] |
Y. Zhang, X. Wang, D. Cui, J. Zhu, Proteomics 16 (2016) 3173-3182. DOI:10.1002/pmic.201600127 |
| [80] |
Y. Wu, N. Sun, C. Deng, ACS Appl. Mater. Interfaces 12 (2020) 9814-9823. DOI:10.1021/acsami.9b22601 |
| [81] |
M. Caragata, A.K. Shah, B.L. Schulz, M.M. Hill, C. Punyadeera, Anal. Biochem. 497 (2016) 76-82. DOI:10.1016/j.ab.2015.11.024 |
| [82] |
C.K.J. Cho, S.J. Shan, E.J. Winsor, E.P. Diamandis, Mol. Cell Proteomics 6 (2007) 1406-1415. DOI:10.1074/mcp.M700090-MCP200 |
| [83] |
Z. Shi, L. Pu, Y. Guo, et al., Sci. Rep. 7 (2017) 4603. DOI:10.1038/s41598-017-04517-8 |
 2021, Vol. 32
2021, Vol. 32