b Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education, Hefei 230601, China;
c Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, School of Science, Westlake University, Hangzhou 310024, China
2, 3-Dihydrobenzofuran is one of the most important oxygen-containing heterocycles, which can be frequently found in various natural isolates and synthetic bioactive molecules [1]. As some selected examples shown in Fig. 1, Abiesinol E was isolated from the bark of Abies sachalinensis with remarkable anti-tumor activities [2a]. Other 2, 3-dihydrobenzofuran containing structures, such as (+)-Obtusafuran exhibits anticarcinogenic and antifeedant activities [2b], and Pterocarpan is found to show impressive antiproliferative activities [2c]. Due to their broad spectra of pharmaceutical applications, a great deal of efforts has been devoted to the development of efficient methods towards the synthesis of 2, 3-dihydrobenzofurans over the past several years [3]. Among the reactions reported, [4 + 1] cycloaddition of para-quinone methides (p-QMs) [4] with a suitable C1 building block has been witness as a reliable method for the construction of 2, 3-dihydrobenzofurans. In 2018, Yuan et al. disclosed a catalyst-free synthesis of 2, 3-dihydrobenzofuran derivatives via [4 + 1] cycloaddition of p-QMs with sulfur ylides [5]. Shortly after this discovery, other commonly used C1 synthons, such as sulfonium salts [6], ammonium bromides [7] and α-halogenated carbonyl compounds [8] were sequentially investigated to conduct such valuable cycloaddition reactions. In addition, Waser group demonstrated a [4 + 1] cycloaddition of para-quinone methides with allenoates as C1 cycloaddition partner by using commercially available chiral phosphine as catalyst [9]. Despite these elegant progresses, further exploring of other C1 units for the synthesis of biologically important 2, 3-dihydrobenzofurans is still appealing and highly desirable.

|
Download:
|
| Fig. 1. Bioactive compounds containing 2, 3-dihydrobenzofuran moiety. | |
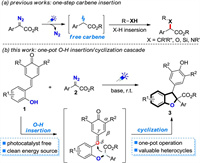
Visible light-promoted chemical transformation has attracted much attentions in the past decade not only because it utilizes visible-light or direct sun-light as green and sustainable energy source, but also due to the fact that it can create unique reaction manifolds in synthetic organic chemistry which are generally difficult or unavailable to realize under traditional thermal conditions [10]. Pioneering works disclosed by the group of Jurberg and Davies [11], He and Zhou [12] revealed that aryl diazoacetates could efficiently undergo photolysis to generate free carbene species under blue LED irradiation. Compared with classical transition metal-catalyzed carbene formation by using α-diazocarbonyl compounds as carbene precursors [13], these photochemical processes could be achieved without the need of exogenous metal catalysts under very mild reaction conditions [14]. The formed free carbene species has been successfully applied to the synthesis of various useful products through X-H insertion [15], cyclopropanation [16], rearrangement reaction [17] and others [18]. Regarding to X-H insertion reactions [15], to the best of our knowledge, the reported photochemical process mainly limited to one-step carbene insertion to construct single C–N, C–O or C–Si bond (Scheme 1a).
|
Download:
|
| Scheme 1. 2, 3-Dihydrobenzofuran synthesis via one-pot visible light-promoted O-H insertion/cyclization cascade. | |
Within the frame of our ongoing research interests in visible-light-induced chemical transformations [19] and [4 + m] cycloaddition reaction of p-QMs [4l, 4m], we envisage that ortho-hydroxyphenyl-substituted p-QMs 1 might be suitable carbene trapping reagents because of the suitable pKA of phenol and easy formation of hydrogen bond between phenols and aryl diazoacetates [15d], [15f]. As shown in Scheme 1b, upon visible light irradiation, A would be formed through insertion reaction of photogenerated carbene species into O-H bonds of 1. Then, an intramolecular cyclization of A might occur with the assistance of an appropriate base. This expected one-pot two step annulation process could provide a green and efficient method to access 2, 3-dihydrobenzofurans 3. Herein, we would like to describe our preliminary investigations on this study.
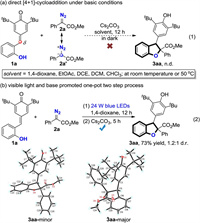
To test the feasibility of our design, we started our investigation with the reaction of ortho-hydroxyphenyl-substituted p-QM 1a and methyl 2-diazo-2-phenylacetate 2a as model substrates (Scheme 2). It is well known that diazo compounds can serve as 1, 1′-dipolar C1 synthons in various cycloaddition processes. However, the desired 2, 3-dihydrobenzofuran 3aa could not be obtained by direct reacting of 1a and 2a in the presence of Cs2CO3 as base at room temperature or 50 ℃ which might due to the steric effect (Scheme 2a). In contrast, treatment of 1a and 2a in 1, 4-dioxane under the irradiation of 24 W blue LEDs for 12 h, followed by addition of Cs2CO3 lead to the formation of 3aa in 73% yield over two steps with 1.2:1 diastereoselectivity (Scheme 2b). Note that, the two diastereomers of 3aa were unambiguously confirmed by X-ray diffraction analysis (CCDC: 2039981 and CCDC: 2039982). For detailed condition optimizations, please see Supporting information.
|
Download:
|
| Scheme 2. Initial investigation. | |
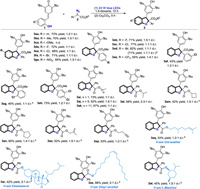
Next, we turned our attention to investigate the substates scope of this visible light and base promoted 2, 3-dihydrobenzofuran formation process (Scheme 3). Regarding to ortho-hydroxyphenyl-substituted p-QMs components, both electron-donating groups (e.g., -Me) and electron-withdrawing groups (e.g., -F, -Cl, -Br, -NO2) can be successfully introduced to aryl ring of 1, affording the corresponding heterocycles 3aa-ga in good yields with moderate diastereoselectivities. Only one-step O-H insertion product was isolated in 10% yield when methoxy substituted p-QM 1c was involved. It was reported that hydrogen-bonding interaction between the proton of phenols and carbonyl group of aryl diazoacetates played an important role for O-H insertion reaction [15d, 15f]. The pKa value of para-methoxy phenol (10.21) showed that this type of substrate had weaker acidic activity and low reaction efficiency for O-H insertion. Moreover, a wide range of substations on the different positions of phenyl ring of the diazo compounds could be well tolerated, yielding the dihydrobenzofurans 3ab−3ag in synthetically useful to high yields with moderate dr. To our delight, 3ad still could be isolated in 71% yield when expand the reaction to 0.5 mmol scale. It was noteworthy that disubstituted aryl diazoacetate 2h was also amenable substrate to give product 3ah in 73% yield with 1.2:1 dr. Then, substituent modification of ester group in diazo compounds was investigated. Replacement of methyl group in 2a with other primary alkyl groups (3ai-ak, 3ao, 3ap), secondary alkyl group (3al), cyclic alkyl groups (3am and 3an) were all proved to be successful, providing the corresponding heterocycles in acceptable results (42%–73% yields, 1:1−2.3:1 dr). It should be pointed out that carbene cyclopropanation or cyclopropenation products were not observed in the cases of substrates containing unsaturated double or triple bonds. Some commonly used simple diazo compounds, such as ethyl diazoacetate and 3-diazopentane-2, 4-dione are not suitable for this transformation. More significantly, the synthetic potential of the method could be further highlighted by successfully introducing some useful molecules, such citronellol, cholesterol, oleyl alcohol and L-menthol, into the final heterocycles (3aq-3at). It was found that tert-Bu group in p-QMs 1 had significant effect on the reaction efficiency. Only one-step O-H insertion product can be obtained when the two tert-Bu groups were replaced by i-Pr groups.
|
Download:
|
| Scheme 3. Reaction scopes. Reaction condition: 1 (0.4 mmol), 2 (0.1 mmol), Cs2CO3 (0.4 mmol), in 1, 4-dioxane (2.0 mL), irradiation by 24 W blue LEDs at room temperature. Isolated yield. a Reaction was conducted with 0.5 mmol scale. b Sodium bis(trimethylsilyl)amide (0.4 mmol) was used as base. | |
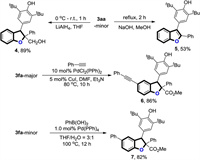
To further demonstrate the synthetic utility of this reaction, some follow-up chemistry was performed (Scheme 4). Treatment of one single isomer of 3aa with LiAlH4 in THF provided the reductive product 5 in 89% yield. In addition, a decarboxylative aromatization of 3aa in NaOH/MeOH under reflux conditions afforded 2, 3-diaryl substituted benzofuran 6 in 53% isolated yield. Note that, further functionalization of C-Br bond in final product 3fa was succeed by using employing palladium catalyzed Suzuki cross-coupling and Sonogashira cross-coupling as examples, affording the corresponding products 6 and 7 with 86% and 82% yield, respectively.
|
Download:
|
| Scheme 4. Synthetic transformations. | |
To better understanding of the reaction mechanism, some control experiments were subsequently conducted. As shown in Scheme 5, treatment of 1a and 2a under standard reaction conditions for 12 h gave the one-step O-H insertion product 8 in 81% isolated yield. Note that, visible light irradiation was crucial for the formation of 8 (Scheme 5a). 8 could transfer to final 2, 3-dihydrobenzofuran 3aa in 87% yield by treating them with Cs2CO3 in 1, 4-dioxane (Scheme 5b).

|
Download:
|
| Scheme 5. Mechanism studies. | |
Based on above experiment results and literature reports, a plausible reaction mechanism was proposed in Scheme 5c. Initially, upon irradiation of aryl diazoacetate with blue LED generated carbene species with the release of molecule nitrogen [14]. Then, insertion of carbene species into O-H bond of 1a afforded product 8 [15]. Under basic reaction conditions, deprotonation of 8 gave carbon anion intermediate 9, which subsequently cyclized to produce 10. Finally, protonation of 10 delivered final 2, 3-dihydrobenzofuran 3aa.
In summary, we have developed a visible light and base promoted O-H insertion/cyclization of para-quinone methides with aryl diazoacetates. By using the strategy, a wide range of 2, 3-dihydrobenzofuran derivatives can be obtained in good yields and moderate diastereoselectivities. More significantly, the successful introduction of some useful complex structures into the 2, 3-dihydrobenzofuran heterocycles and synthetic transformation of the final products further renders this approach attractive and valuable. The further discovery of new visible light-promoted carbene transfer reactions is currently underway in our laboratory.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsWe are grateful to the National Natural Science Foundation of China (Nos. 21971001, 21702001), and the Start-up Grant from Anhui University for financial support of this work.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.010.
| [1] |
(a) R.S. Ward, Chem. Soc. Rev. 11 (1982) 75–125; (b) D.C. Chauret, C.B. Bernard, J.T. Arnason, et al., J. Nat. Prod. 59 (1996) 152–155; (c) R.S. Ward, Nat. Prod. Rep. 16 (1999) 75–96; (d) N. Beldjoudi, L. Mambu, M. Labaïed, et al., J. Nat. Prod. 66 (2003) 1447-1450; (e) T. She, X.N. Wang, H.X. Lou, Nat. Prod. Rep. 26 (2009) 916–935; (f) A. Radadiya, A. Shah, Eur. J. Med. Chem. 97 (2015) 356–376; (g) R.J. Nevagi, S.N. Dighe, S.N. Dighe, Eur. J. Med. Chem. 97 (2015) 561–581. |
| [2] |
(a) A. Goel, A. Kumar, A. Raghuvanshi, Chem. Rev. 113 (2013) 1614–1640; (b) H.Q. Yin, B.W. Lee, Y.C. Kim, D.H. Sohn, B.H. Lee, Arch. Pharm. Res. 27 (2004) 919–922. |
| [3] |
(a) T.D. Sheppard, J. Chem. Res. 35 (2011) 377–385; (b) C. Zhu, Y. Ding, L.W. Ye, Org. Biomol. Chem. 13 (2015) 2530–2536; (c) J.R. Chen, X.Q. Hu, L.Q. Lu, W.J. Xiao, Chem. Rev. 115 (2015) 5301–5365; (d) B.M. Trost, O.R. Thiel, H.C. Tsui, J. Am. Chem. Soc. 125 (2013) 13155–13164; (e) E.D. Coy B, L. Jovanovic, M. Sefkow, Org. Lett. 12 (2010) 1976–1979; (f) J. Meng, T. Jiang, H.A. Bhatti, et al., Org. Biomol. Chem. 8 (2010) 107–113; (g) D.H. Wang, J.Q. Yu, J. Am. Chem. Soc. 133 (2011) 5767–5769; (h) X.X. Sun, H.H. Zhang, G.H. Li, L. Meng, F. Shi, Chem. Commun. 52 (2016) 2968–2971; (i) Y. Cheng, Z. Gang, W. Li, P. Li, Org. Chem. Front. 5 (2018) 2728–2733; (j) T. Zhou, T. Xia, Z. Liu, L. Liu, J. Zhang, Adv. Synth. Catal. 360 (2018) 4475–4479. |
| [4] |
(a) J.Y. Wang, W.J. Hao, S.J. Tu, B. Jiang, Org. Chem. Front. 7 (2020) 1743–1778; (b) C.G.S. Lima, F.P. Pauli, D.C.S. Costa, et al., Eur. J. Org. Chem. 2020 (2020) 2650–2692; (c) L. Caruana, M. Fochi, L. Bernardi, Molecules 20 (2015) 11733–11764; (d) K. Zhao Y. Zhi, T. Shu, et al., Angew. Chem. Int. Ed. 55 (2016) 12104–12108; (e) A. Parra, M. Tortosa, ChemCatChem 7 (2015) 1524–1526; (f) P. Chauhan, U. Kaya, D. Enders, Adv. Synth. Catal. 359 (2017) 888–912; (g) W. Li, X. Xu, P. Zhang, P. Li, Chem. Asian J. 13 (2018) 2350–2359; (h) Y.J. Xiong, S.Q. Shi, W.J. Hao, S.J. Tu, B. Jiang, Org. Chem. Front. 5 (2018) 3483–3487; (i) S. Liu, X.C. Lan, K. Chen, et al., Org. Lett. 19 (2017) 3831–3834; (j) W. Li, H. Yuan, Z. Liu, et al., Adv. Synth. Catal. 360 (2018) 2460–2464; (k) Q. Liu, S. Li, X.Y. Chen, K. Rissanen, D. Enders, Org. Lett. 20 (2018) 3622-3626; (l) F. Jiang, F.R. Yuan, L.W. Jin, G.J. Mei, F. Shi, ACS Catal. 8 (2018) 10234–10240; (m) X.L. Jiang, S.F. Wu, J.R. Wang, G.J. Mei, F. Shi, Adv. Synth. Catal. 360 (2018) 4225–4235; (n) J.R. Wang, X.L. Jiang, Q.Q. Hang, et al., J. Org. Chem. 84 (2019) 7829–7839; (o) Y.C. Cheng, C.S. Wang, T.Z. Li, et al., Org. Biomol. Chem. 17 (2019) 6662-6679; (p) M. Sun, C. Ma, S.J. Zhou, et al., Angew. Chem. Int. Ed. 58 (2019) 8703–8708; (q) S. Zhang, X. Yu, J. Pan, et al., Org. Chem. Front. 6 (2019) 3799–3803; (r) Z. Li, W. Wang, H. Jian, et al., Chin. Chem. Lett. 30 (2019) 386–388; (s) X. Cheng, S.J. Zhou, G.Y. Xu, et al., Adv. Synth. Catal. 362 (2020) 523–527; (t) S.J. Zhou, X. Cheng, C.X. Hu, et al., Sci. China Chem. 64 (2021) 61–65; (u) J.P. Tan, H. Zhang, Z. Jiang, et al., Adv. Synth. Catal. 362 (2020) 1058–1063; (v) S.F. Wu, M.S. Tu, Q.Q. Hang, et al., Org. Biomol. Chem. 18 (2020) 5388–5399. |
| [5] |
X.M. Chen, K.X. Xie, D.F. Yue, et al., Tetrahedron 74 (2018) 600-605. DOI:10.1016/j.tet.2017.12.038 |
| [6] |
Y. Zhi, K. Zhao, C.V. Essen, K. Rissanen, D. Enders, Org. Chem. Front. 5 (2018) 1348-1351. DOI:10.1039/C8QO00008E |
| [7] |
L. Liu, Z. Yuan, R. Pan, et al., Org. Chem. Front. 5 (2018) 623-628. |
| [8] |
(a) J. Zhou, G. Liang, X. Hu, L. Zhou, H. Zhou, Tetrahedron 74 (2018) 1492–1496; (b) J.P. Tan, P. Yu, J.H. Wu, et al., Org. Lett. 21 (2019) 7298–7302; (c) G. Singh, S. Kumar, A. Chowdhury, R.V. Anand, J. Org. Chem. 84 (2019) 15978–15989; (d) H. Lu, H.X. Zhang, C.Y. Tan, J. Org. Chem. 84 (2019) 10292–10305. |
| [9] |
K. Zielke, O. Kováč, M. Winter, J. Pospíšil, M. Waser, Chem. Eur. J. 25 (2019) 8163-8168. DOI:10.1002/chem.201901784 |
| [10] |
(a) J.M.R. Narayanam, C.R.J. Stephenson, Chem. Soc. Rev. 4 (2011) 102–113; (b) Y. Xi, H. Yi, A. Lei, Org. Biomol. Chem. 11 (2013) 2387–2403; (c) C.K. Prier, D.A. Rankic, D.W.C. MacMillan, Chem. Rev. 113 (2013) 5322–5363; (d) M.N. Hopkinson, B. Sahoo, J.L. Li, F. Glorius, Chem. Eur. J. 20 (2014) 3874–3886; (e) N.A. Romero, D.A. Nicewicz, Chem. Rev. 116 (2016) 10075–10166; (f) L. Marzo, S. Pagire, O. Reiser, B. König, Angew. Chem. Int. Ed. 57 (2018) 10034–10072; (g) Y.Z. Cheng, X. Zhang, S.L. You, Sci. Bull. 63 (2018) 809–811; (h) L. Lu, J.L. Zhang, Chin. J. Org. Chem. 39 (2019) 3308–3309; (i) B.G. Cai, J. Xuan, W.J. Xiao, Sci. Bull. 64 (2019) 337–350; (j) Y. Chen, L.Q. Lu, D.G. Yu, C.J. Zhu, W.J. Xiao, Sci. China Chem. 62 (2019) 24–57; (k) T.Y. Shang, L.H. Lu, Z. Cao, et al., Chem. Commun. 55 (2019) 5408–5419; (l) X.Y. Yu, Q.Q. Zhao, J. Chen, W.J. Xiao, J.R. Chen, Acc. Chem. Res. 53 (2020) 1066–1083; (m) J. Xuan, X.K. He, W.J. Xiao, Chem. Soc. Rev. 49 (2020) 2546–2556; (n) W. Xiao, J. Wu, Chin. Chem. Lett. 31 (2020) 3083–3094; (o) H.G. Zhang, H. Chen, C. Zhu, S. Yu, Sci. China Chem. 63 (2020) 637–647; (p) X. Lv, H. Xu, Y. Yin, X. Zhao, Z. Jiang, Chin. J. Chem. 38 (2020) 1480–1488; (q) K. Sun, Q.Y. Lv, X.L. Chen, et al., Green Chem. 23 (2021) 232–248. |
| [11] |
I. Jurberg, H.M.L. Davies, Chem. Sci. 9 (2018) 5112-5118. DOI:10.1039/C8SC01165F |
| [12] |
T. Xiao, M. Mei, Y. He, L. Zhou, Chem. Commun. 54 (2018) 8865-8868. DOI:10.1039/C8CC04609C |
| [13] |
(a) H.M.L. Davies, J.R. Manning, Nature 451 (2008) 417–424; (b) M.P. Doyle, R. Duffy, M. Ratnikov, Z. Zhou, Chem. Rev. 110 (2010) 704–724; (c) X. Guo, W. Hu, Acc. Chem. Res. 46 (2013) 2427–2440; (d) S.F. Zhu, Q.L. Zhou, Sci. Rev. 1 (2014) 580–603; (e) H.J. Knöker, I. Bauer, Chem. Rev. 115 (2015) 3170–3387; (f) L. Mertens, R.M. Koenigs, Org. Biomol. Chem. 14 (2016) 10547–10556; (g) Y. Xia, D. Qiu, J. Wang, Chem. Rev. 117 (2017) 13810–13889. |
| [14] |
(a) L.W. Ciszewski, K. Rybicka-Jasinska, D. Gryko, Org. Biomol. Chem. 17 (2019) 432–448; (b) T.B. Hua, Q.Q. Yang, Y.Q. Zou, Molecules 24 (2019) 3191; (c) Z. Yang, M.L. Stivanin, I.D. Jurberg, R.M. Koenigs, Chem. Soc. Rev. 49 (2020) 6833–6847; (d) S. Jana, Y. Guo, R.M. Koenigs, Chem. Eur. J. 27 (2021) 1270–1281. |
| [15] |
(a) C. Empel, F.W. Patureau, R.M. Koenigs, J. Org. Chem. 84 (2019) 11316–11322; (b) M.L. Stivanin, A.A.G. Fernandes, A.F. da Silva, C.Y. Okada Jr, I.D. Jurberg, Adv. Synth. Catal. 362 (2020) 1106–1111; (c) S. Jana, Z. Yang, F. Li, et al., Angew. Chem. Int. Ed. 59 (2020) 5562–5566; (d) F. He, F. Li, R.M. Koenigs, J. Org. Chem. 85 (2020) 1240–1246; (e) C. Empel, S.J. Orcid, C. Pei, T.V. Nguyen, R.M. Koenigs, Org. Lett. 22 (2020) 7225–7229; (f) J. Chen, S. Liu, X. Lv, et al., J. Org. Chem. 85 (2020) 13920–13928; (g) J. Yang, G. Wang, S. Chen, et al., Org. Biomol. Chem. 18 (2020) 9494–9498. |
| [16] |
(a) Y. Guo, T.V. Nguyen, R.M. Koenigs, Org. Lett. 21 (2019) 8814–8818; (b) F. He, R.M. Koenigs, Chem. Commun. 55 (2019) 4881–4884; (c) X. Zhang, C. Du, H. Zhang, et al., Synthesis 51 (2019) 889–898; (d) R. Hommelsheim, Y. Guo, Z. Yang, C. Empel, R.M. Koenigs, Angew. Chem. Int. Ed. 58 (2019) 1203–1207. |
| [17] |
(a) J. Yang, J. Wang, H. Huang, et al., Org. Lett. 21 (2019) 2654–2657; (b) Z. Yang, Y. Guo, R.M. Koenigs, Chem. Eur. J. 25 (2019) 6703–6706; (c) K. Orłowska, K. Rybicka-Jasin'ska, P. Krajewski, D. Gryko, Org. Lett. 22 (2020) 1018–1021. |
| [18] |
(a) Y. Wei, S. Liu, M.M. Li, et al., J. Am. Chem. Soc. 141 (2019) 133–137; (b) S. Jana, Z. Yang, C. Pei, X. Xu, R.M. Koenigs, Chem. Sci. 10 (2019) 10129-10134; (c) Q.L. Zhang, Q. Xiong, et al., Angew. Chem. Int. Ed. 59 (2020) 14096–14100; (d) A.F. da Silva, M.A.S. Afonso, R.A. Cormanich, I.D. Jurberg, Chem. Eur. J. 26 (2020) 5648–5653; (e) R. Cheng, C. Qi, L. Wang, et al., Green Chem. 22 (2020) 4890–4895; (f) M.A. Ansari, D. Yadav, M.S. Singh, Chem. Eur. J. 26 (2020) 8083–8089. |
| [19] |
(a) J. Xuan, A. Studer, Chem. Soc. Rev. 46 (2017) 4329–4346; (b) B.G. Cai, Z.L. Chen, G.Y. Xu, J. Xuan, W.J. Xiao, Org. Lett. 21 (2019) 4234-4238; (c) Q.Q. Ge, J.S. Qian, J. Xuan, J. Org. Chem. 84 (2019) 8691–8701; (d) X.K. He, B.G. Cai, Q.Q. Yang, L. Wang, J. Xuan, Chem. Asian J. 14 (2019) 3269-3273; (e) J. Lu, X.K. He, X. Cheng, et al., Adv. Synth. Catal. 362 (2020) 2178–2182; (f) X.K. He, J. Lu, A.J. Zhang, et al., Org. Lett. 22 (2020) 5984–5989; (g) B.G. Cai, S.S. Luo, L. Li, et al., CCS Chem. 2 (2020) 2764–2771; (h) C. Ye, B.G. Cai, J. Lu, et al., J. Org. Chem. 86 (2021) 1012–1022. |
 2021, Vol. 32
2021, Vol. 32 

