Chromone (CMO, Scheme 1), also known as benzo-γ-pyranone, is the parent of natural chromone compounds. Natural chromone compounds are a natural oxygen-containing heterocyclic compounds widely found in plants, which exhibit a variety of biological activities such as anti-inflammatory, antibacterial, anti-platelet, antiviral and anticancer [1-4] activities. CMO and its derivatives can also be used as colorimetric and fluorescent probes used for the effective detection of metal ions in an aqueous solution due to their special structures [5].
|
Download:
|
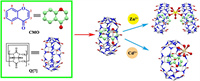
| Scheme 1. The chemical structures of Q[7] and CMO and a schematic representation of the recognition of Zn2+ and Cd2+ in an neutral aqueous solution by CMO@Q[7]. | |
Cucurbit[n]urils, (Q[n]s, Scheme 1) are a new type of highly symmetrical barrel-shaped macrocyclic molecule with a large hydrophobic cavity and hydrophilic portals that can form host-guest complexes with a variety of metal cations or organic small molecules via hydrophobic interactions, hydrogen bonding and ion dipole non-bonding interactions, which exhibit unique host-guest chemistry [6-15] and coordination chemistry [16-19].
Zinc is the second largest transition metal ion after iron found in the human body, which plays an important role in many cell functions [20]. Many nervous system diseases, including Alzheimer's disease, cerebral ischemia and epilepsy, are associated with disorders in Zn2+ metabolism [21-23]. Cadmium is a heavy metal element with the highest biological toxicity, which has high chemical activity, high mobility and long-lasting toxicity in the environment. It is easy to endanger human health through its enrichment in the food chain [24]. The rapid, efficient and accurate detection of Zn2+ and Cd2+ in the human body and living environment has important application value for the protection of the environment and human health. At present, electrochemical methods, atomic absorption spectrometry and spectrophotometry are commonly used for the detection of Zn2+ and Cd2+ [25]. When compared with these traditional techniques, fluorescence spectroscopy has attracted a lot of attention due to its high selectivity and sensitivity, simple operation, real-time detection and no damage to the substrate [26-28]. Therefore, fluorescence detection has developed rapidly in recent years and has become a common analysis method in practical investigation [29-31].
In this work, CMO interacted with Q[7] in a neutral aqueous solution to produce a 1:1 inclusion complex (CMO@Q[7]). The inclusion complex had high selectivity and sensitivity for zinc and cadmium ion recognition in water. When zinc or cadmium ions were added, the fluorescence intensity at 364 nm increased, which was used as a fluorescent probe to quantitatively detect and monitor zinc and cadmium ions in the environment and life systems.
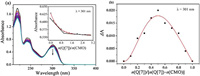
The interaction between Q[7] and CMO was first evaluated using UV–vis spectroscopy. The UV absorption spectrum of the interaction between CMO and Q[7] is shown in Fig. 1. CMO had strong absorption at 301 nm and the absorption intensity of CMO decreased upon increasing the concentration of Q[7]. The decrease in absorption was attributed to the host-guest interaction formed between CMO and Q[7]. In the molar ratio method, when n(Q[7])/n(CMO) approached 1.0 equiv., the UV absorption spectrum became steady and then there was almost no significant change. In the Job's method, when n(Q[7])/[n(Q[7])+n(CMO)]=0.5, the absorption value change reached its maximum, indicating that the ratio of CMO to Q[7] was 1:1.
|
Download:
|
| Fig. 1. UV–vis spectra of CMO and Q[7] in an aqueous solution: (a) Molar ratio method and (b) Job's method. | |
Isothermal titration calorimetry (ITC) and mass spectrometry (MS) can also provide some evidence in regards to the interaction between CMO and Q[7] to support the determination of the binding constant (K). The ITC titration spectra and thermodynamic parameters obtained upon adding an aqueous solution of Q[7] (2.0 × 10–3 mol/L) to an aqueous solution of CMO (1.0 × 10–4 mol/L) at 25 ℃, indicated that the inclusion process of CMO and Q[7] was mainly driven by enthalpy at a molar ratio of 1:1 and K = 3.54 × 105 L/mol (Fig. S1, Table S1 in Supporting information). MS showed that the parent ion peaks of the CMO@Q[7] complex was located at m/z 1309.3863 [M+H]+ (calcd. 1309.3876 [M+H]+) (Fig. S2 in Supporting information), supporting the formation of a 1:1 inclusion complex between CMO and Q[7].
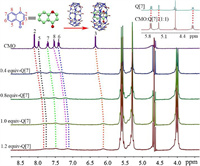
The interaction between Q[7] and CMO was further investigated using 1H NMR spectroscopy. Fig. 2 and Table S2 (Supporting information) show the changes in the 1H NMR spectra of CMO, in which all the proton resonance peaks of CMO underwent an upfield shift after the gradual addition of Q[7] in a deuterated aqueous solution. At the same time, after the host-guest interaction occurred, the resonance peak of Q[7] gradually shifted to a downfield (Fig. 2 upper right illustration). This indicated that CMO and Q[7] had a host-guest inclusion, and the whole CMO molecule entered the cavity of Q[7]. On the other hand, the addition of Q[7] caused the peaks observed for all the protons in CMO to broaden, which was due to the self-assembly and self-dissociation of the host-guest interactions occurring at the same time and the weak transition between them. The fluorescence titration results showed that the fluorescence intensity had no obvious change in the range of n(Q[7])/n(CMO) from 0 to 3.0 (Fig. S3 in Supporting information).
|
Download:
|
| Fig. 2. 1H NMR spectra (400 MHz) of CMO (5.0 × 10–4 mol/L) in the different amount of Q[7] in D2O. | |
First of all, we needed to confirm whether metal ions had an effect on the fluorescence of the guest. Fig. 3a shows that CMO itself did not exhibit an obvious fluorescence change to the abovementioned metal cations. This result indicated that metal cations and free CMO were difficult to form complexes in aqueous solutions. In addition, the addition of the Q[7] to the CMO solution also did not cause significant fluorescence spectra changes (Fig. 3b). However, when various metal cations, such as Li+, Na+, K+, Rb+, Cs+, Mg2+, Ca2+, Sr2+, Ba2+, Al3+, Hg2+, Fe2+, Fe3+, Cr3+, Co2+, Pb2+, Zn2+, Cd2+, Ni2+, Cu2+, and Mn2+, were added to an aqueous solution of CMO@Q[7] (1:1), the results showed that the fluorescence intensity of CMO@Q[7] (1:1) was almost unaffected by the addition of other ions except Zn2+ or Cd2+ (Fig. 3b). Simultaneously, an increase in the absolute fluorescence quantum yield (QY) was observed for the CMO@Q[7]@Zn2+ and CMO@Q[7]@Cd2+ complexes (Table S3 in Supporting information). It was found that the QY of CMO@Q[7]@Zn2+ and CMO@Q[7]@Cd2+ were 2.25 and 2.67-fold stronger than CMO, respectively.

|
Download:
|
| Fig. 3. Fluorescence response of ions to CMO (a) and CMO@Q[7] (b) in an aqueous solution. | |
Consequently, CMO@Q[7] was applied toward the detection of Zn2+ and Cd2+ in aqueous solution. Upon the gradual addition of increasing concentrations of Zn2+ or Cd2+ to an aqueous solution of CMO@Q[7] (Fig. 4), the fluorescence titration spectra showed a linear increase trend within the Zn2+ or Cd2+ concentration range of 0–3.0 × 10–3 mol/L at the maximum emission wavelength of 364 nm. The respective linear regression equations and correlation coefficients were ΔI=74047.678c + 10.691 and R2=0.9905 (Zn2+, Fig. 4c); ΔI=79447c + 7.6572 and R2=0.9922 (Cd2+, Fig. 4f). In addition, the limit of detection (LOD=3σ/slope) for Zn2+ and Cd2+ was 2.03 × 10–6 and 1.89 × 10–6 mol/L, respectively, based on a standard deviation of 0.05. In Figs. 4a and d, when n(Zn2+ or Cd2+): n(CMO@Q[7]) > 40, the increasing trend in the fluorescence intensity decreased and the fluorescence intensity gradually became stable. The Job's method was used to obtain the ratio of Zn2+ or Cd2+ to CMO@Q[7]. When n(Zn2+)/[n(Zn2+)+n(CMO@Q[7])]=0.33, the fluorescence intensity changed the most (Fig. 4b), indicating that the ratio of Zn2+ and CMO@Q[7] was 1:2, when n(Cd2+)/[n(Cd2+)+n(CMO@Q[7])]=0.5, the fluorescence intensity changed (Fig. 4e), indicating that the ratio of Cd2+ and CMO@Q[7] was 1:1.

|
Download:
|
| Fig. 4. (a, d) Fluorescence spectra recorded for CMO@Q[7] (molar ratio=1:1) in the presence of increasing concentrations of Zn2+ and Cd2+ in an aqueous solution; (b, e) Job's pot and (c, f) linear relationship of Zn2+ and Cd2+ ions. | |
In order to investigate the interference of other metal ions on the CMO@Q[7] probe during the detection of Zn2+ and Cd2+, aqueous solutions containing different metal ions with the same molar concentration were added to a solution of CMO@Q[7]@Zn2+ or CMO@Q[7]@Cd2+, and the fluorescence intensity measured (Fig. S4 in Supporting information). The results showed that the fluorescence intensity of the system was almost unaffected by the other ions except Cd2+ and Zn2+ when the CMO@Q[7]@Zn2+ or CMO@Q[7]@Cd2+ complex were mixed with the other metal ion. Therefore, metal ions did not interfere with the detection results.
The mode of interaction between CMO@Q[7] and Zn2+ or Cd2+ was inferred using a 1H NMR titration experiment. Fig. 5 shows the changes observed in the 1H NMR spectra recorded for CMO@Q[7] after the addition of Zn2+ or Cd2+ in D2O. Upon increasing the concentration of Zn2+ or Cd2+, the chemical shifts observed for the proton resonant peaks in CMO were continuously shifted downfield, especially the chemical shift corresponding to 3-H, which changed significantly, indicating that CMO was pulled to the portal of Q[7] from the Q[7] cavity by Zn2+ or Cd2+. At the same time, the proton resonance peaks in Q[7] also shifted upon the addition of Zn2+ or Cd2+ (Fig. S5 in Supporting information). It can be inferred that Zn2+ or Cd2+ combined with CMO@Q[7] to form stable host-guest-metal ion complexes. The MS results (Fig. S6 in Supporting information) showed the CMO@Q[7]@Zn2+ parent ion peak was located at m/z 1341.3410 M2+ (calcd. 1341.3452 M2+) and the CMO@Q[7]@Cd2+ parent ion peak was located at m/z 711.1404 M2+ (calcd. 711.1420 M2+), which indicated CMO@Q[7]@Zn2+ was a 2:2:1 complex and CMO@Q[7]@Cd2+ was a 1:1:1 complex.

|
Download:
|
| Fig. 5. 1H NMR titration spectra recorded for CMO@Q[7] upon the addition of different molar equivalents of Zn2+ (a) and Cd2+ (b). | |
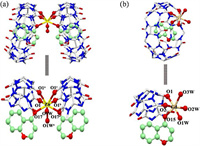
The coordination mode of CMO@Q[7] on Zn2+ or Cd2+ could be further explained by their crystal structures. Fig. 6 shows the crystal structure of the CMO@Q[7] probe with Zn2+ and Cd2+, and the crystal data and refined parameters are shown in Table S4 (Supporting information). Fig. 6a shows that in an asymmetric unit of crystal structure, the Zn2+ ion bridged two CMO@Q[7] to form a 1:2 complex [{Zn(H2O)2(CMO@Q[7])2}2+{2Q[7], 8ClO4−}]. An asymmetric unit contained one Zn2+, two CMO@Q[7] molecules, two uncoordinated Q[7] molecules and eight ClO4− anions. Zn2+ coordinates with eight oxygen atoms, four of which were derived from the carbonyl oxygen atoms (O1, O1a, O1b, O1c) at the ports of Q[7] in two adjacent CMO@Q[7], two were derived from the carbonyl oxygen atoms (O17, O17a) on the CMO molecules in the two adjacent Q[7] cavities and two were derived from two coordinated water molecules (O1W, O1Wa). Under the guidance of Zn2+, the carbonyl oxygen atom of CMO and the Q[7] port in CMO@Q[7] were directly coordinated with Zn2+ and the 3-H in the CMO molecule in the cavity of Q[7] was also moved to the port of Q[7] due to the addition of Zn2+.
|
Download:
|
| Fig. 6. (a) Crystal structure of CMO@Q[7]@Zn2+ system; symmetry code a: 1-x, y, z, b: 1-x, y, 0.5-z, c: x, y, 0.5-z. (b) Crystal structure of CMO@Q[7]@Cd2+ system. | |
Fig. 6b shows that the crystal structures of CMO@Q[7]@Cd2+ and CMO@Q[7]@Zn2+ were quite different. In the asymmetric unit of the crystal structure of CMO@Q[7]@Cd2+, Cd2+ and CMO@Q[7] formed a 1:1 complex [{Cd(H2O)3(CMO@Q[7])}2+{2ClO4−}], that is, an asymmetric unit contained one Cd2+ and one CMO@Q[7] molecule, three coordination water molecules and two ClO4− anions. Cd2+ coordinates with six oxygen atoms, two of which were derived from the carbonyl oxygen atoms (O1, O2) in the port of Q[7] in CMO@Q[7] and one was derived from the CMO in the cavity of Q[7] due to the carbonyl oxygen atom (O15) in the molecule, and three derived from three coordinated water molecules (O1W, O2W, O3W). Under the guidance of Cd2+, the carbonyl oxygen atom of CMO and the Q[7] port in CMO@Q[7] were directly coordinated to Cd2+ and the 3-H on the CMO in the cavity of Q[7] was also moved to the port of Q[7] due to the addition of Cd2+. In the CMO@Q[7]@Zn2+ or Cd2+ systems, the counter anions (ClO4–) surrounded the Q[7] molecules via the outer surface interaction of Q[n]s (OSIQ) and created an electronegative environment, which is favorable for the coordination of Zn2+ or Cd2+ with the portal carbonyl oxygen atoms of Q[7] and the interaction distance was 3.042–3.610 Å (Fig. S7 in Supporting information).
The results showed that the fluorescence response of CMO@Q[7] toward Zn2+ or Cd2+ in neutral aqueous solution was due to their mutual coordination to form clathrates with an interaction ratio of 2:1 or 1:1. Because the carbonyl oxygen atom on the CMO contained lone pair of electrons and there was a PET process, which led to fluorescence quenching. When CMO and Q[7] formed the CMO@Q[7] complex, after adding Zn2+ or Cd2+, the carbonyl oxygen atom on the CMO and Q[7] were complexed with the Zn2+ or Cd2+, and the PET process of CMO was blocked, so that CMO emitted fluorescence. This process belonged to chelation fluorescence enhancement.
In summary, the CMO and Q[7] formed a 1:1 inclusion complex, which selectively recognized Zn2+ and Cd2+ in an aqueous solution. The crystal structure of CMO@Q[7]@Zn2+ showed a 2:2:1 complex and that of CMO@Q[7]@Cd2+ a 1:1:1 complex. In addition, the fluorescence emission of CMO@Q[7] was enhanced by metal ions was the result of a CHEF mechanism of Zn2+ and Cd2+ coordinate with the carbonyl oxygen of CMO, which was encapsulated in the cavity of Q[7]. As a result, the present work indicated that cucurbit[n]urils-assisted fluorophores can be used as fluorescent probe for cations in an aqueous solution via a CHEF mechanism.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThe authors acknowledge the support from the Science and Technology Support Plan of Guizhou Province [Guizhou Science and Technology Cooperation Support (2020) 4Y218].
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.03.071.
| [1] |
A. Gaspar, M.J. Matos, J. Garrido, E. Uriarte, F. Borges, Chem. Rev. 114 (2014) 4960-4992. DOI:10.1021/cr400265z |
| [2] |
J. Reis, A. Gaspar, N. Milhazes, F. Borges, J. Med. Chem. 60 (2017) 7941-7957. DOI:10.1021/acs.jmedchem.6b01720 |
| [3] |
A. Farukh, A. Zeenat, R. Thierry, RSC Adv. 8 (2018) 37375-37390. DOI:10.1039/C8RA06722H |
| [4] |
L. Yoonji, B. Shaherin, C. Sun, J. Med. Chem. 61 (2018) 1-46. DOI:10.1021/acs.jmedchem.6b01453 |
| [5] |
A. Mohammadi, B. Khalili, A.S. Haghayegh, Spectrochim. Acta A 222 (2019) 117193. DOI:10.1016/j.saa.2019.117193 |
| [6] |
J. Lagona, P. Mukhopadhyay, S. Chakrabarti, L. Isaacs, Angew. Chem. Int. Ed. 44 (2005) 4844-4870. DOI:10.1002/anie.200460675 |
| [7] |
W. Wu, S. Song, X.W. Cui, et al., Chin. Chem. Lett. 29 (2018) 95-98. DOI:10.1016/j.cclet.2017.08.049 |
| [8] |
S.K. Li, Y. Gao, Y.F. Ding, A.N. Xu, H.P. Tan, Chin. Chem. Lett. 32 (2021) 313-318. DOI:10.1016/j.cclet.2020.04.049 |
| [9] |
T. Zhang, Y.H. Liu, B.W. Hu, et al., Chin. Chem. Lett. 30 (2019) 949-952. DOI:10.1016/j.cclet.2018.12.029 |
| [10] |
L. Isaacs, Acc. Chem. Res. 47 (2014) 2052-2062. DOI:10.1021/ar500075g |
| [11] |
K.I. Assaf, W.M. Nau, Chem. Soc. Rev. 44 (2014) 394-418. |
| [12] |
X.L. Ni, S.Y. Chen, Y.P. Yang, Z. Tao, J. Am. Chem. Soc. 138 (2016) 6177-6183. DOI:10.1021/jacs.6b01223 |
| [13] |
K.D. Zhang, J. Tian, D. Hanifi, et al., J. Am. Chem. Soc. 135 (2013) 17913-17918. DOI:10.1021/ja4086935 |
| [14] |
J. Liu, Y. Lan, Z. Yu, et al., Acc. Chem. Res. 50 (2017) 208-217. DOI:10.1021/acs.accounts.6b00429 |
| [15] |
W. Liu, S.K. Samanta, B.D. Smith, L. Isaacs, Chem. Soc. Rev. 46 (2017) 2391-2403. DOI:10.1039/C7CS00011A |
| [16] |
M.N. Sokolov, D.N. Dybtsev, V.P. Fedin, Russ. Chem. B 52 (2003) 1041-1060. DOI:10.1023/A:1024771902420 |
| [17] |
J. Lü, J.X. Lin, M.N. Cao, R. Cao, Coord. Chem. Rev. 257 (2013) 1334-1356. DOI:10.1016/j.ccr.2012.12.014 |
| [18] |
X.L. Ni, X. Xiao, H. Cong, et al., Chem. Soc. Rev. 42 (2013) 9480-9508. DOI:10.1039/c3cs60261c |
| [19] |
X.L. Ni, S.F. Xue, Z. Tao, et al., Coord. Chem. Rev. 287 (2015) 89-113. DOI:10.1016/j.ccr.2014.12.018 |
| [20] |
B.L. Vallee, K.H. Falchuk, Physiol. Rev. 73 (1993) 79-118. DOI:10.1152/physrev.1993.73.1.79 |
| [21] |
A.I. Bush, W.H. Pettingell, G. Multhaup, et al., Science 265 (1994) 1464-1467. DOI:10.1126/science.8073293 |
| [22] |
J.Y. Koh, S.W. Suh, B.J. Gwag, et al., Science 272 (1996) 1013-1016. DOI:10.1126/science.272.5264.1013 |
| [23] |
C.F. Walker, R.E. Black, Annu. Rev. Nutr. 24 (2004) 255-275. DOI:10.1146/annurev.nutr.23.011702.073054 |
| [24] |
L. Noël, H.D. Céline, T. Guérin, et al., Biometals 19 (2006) 473-481. DOI:10.1007/s10534-005-5147-y |
| [25] |
H. Xu, L.P. Zeng, D.K. Huang, Y.Z. Xian, L.T. Jin, Food Chem. 109 (2008) 834-839. DOI:10.1016/j.foodchem.2007.12.065 |
| [26] |
Š. Milda, Š. Edmundas, B. Dalė, A. Dalia, Med. Chem. 51 (2015) 100-106. |
| [27] |
L.C. Weng, N.J. Lee, W.T. Yeh, L.T. Ho, W.H. Pan, J. Formos, Med. Assoc. 111 (2012) 651-659. DOI:10.1016/j.jfma.2012.07.038 |
| [28] |
Z. Liu, C.N. Peng, C.X. Guo, et al., Tetrahedron 71 (2015) 2736-2742. DOI:10.1016/j.tet.2015.03.029 |
| [29] |
F. Mauro, F. Vieri, G. Luca, M. Mauro, Coord. Chem. Rev. 256 (2012) 170-192. DOI:10.1016/j.ccr.2011.09.010 |
| [30] |
H.N. Kim, M.H. Lee, H.J. Kim, J.S. Kim, J. Yoon, Chem. Soc. Rev. 37 (2008) 1465-1472. DOI:10.1039/b802497a |
| [31] |
K.G. Vinod, K.S. Ashok, M. Naveen, Electrochim. Acta 117 (2014) 405-412. DOI:10.1016/j.electacta.2013.11.143 |
 2021, Vol. 32
2021, Vol. 32 

