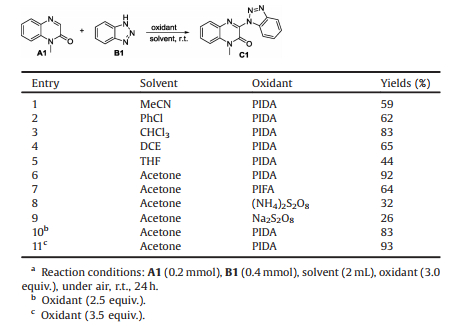
Quinoxalinones are highly valuable structural scaffolds in various natural products and biologically active compounds [1]. As shown in Fig. 1, a wide range of 3-aminoquinoxalinone derivatives such as aldose reductase inhibitor [2], chloroquinoxaline sulfonamide (CQS) [3] and XL147 [4] have been explored because of their therapeutic applications. According, the pursuit of practical methods for the preparation of quinoxalinones has become a hot topic in organic synthesis. At present various of functionalized quinoxalinones with different substituents on C3 position have been reported [5]. For example, phosphonation [6], arylation [7], acylation [8], cyanoalkylation [9], alkylation [10], sulfenylation [11] and amination [12] of quinoxalinones have all been realized efficiently. The iodine-catalyzed cross-dehydrogenative coupling between quinoxalinones and amines was developed by Jain and coworkers (Scheme 1a) [12a]. Wei group found that the amination of quinoxalinones can also be realized in the presence of visible light (Scheme 1b) [12b]. In 2016, the copper catalyzed oxidative amination of quinoxalinones with aliphatic amines was described by Cui group (Scheme 1c) [12c]. For these reported amination reactions, the substrates amine are all aliphatic amines. Amination of quinoxalinones using aromatic amines still remains unexplored.
|
Download:
|
| Fig. 1. Pharmacoactive 3-aminoquinoxalinones. | |

|
Download:
|
| Scheme 1. C-H amination of quinoxalinones. | |
Organic chemical reactions mediated by iodine, especially by hypervalent iodine(Ⅲ) has gained considerable interest in recent years [13]. Various radicals could be produced in the presence of PhI(OAc)2, then the radicals could be used to react with other substrates via different ways for the synthesis of various compounds. Morpholine radical which was derived from the reaction of morpholine and (diacetoxy)iodobenzene (PIDA) was described by Hajra group [14]. Georg group found that CF3 radical can be obtained from TMSCF3 in the presence of PIDA [15]. Kuhakarn group realized the formation of sulfonyl radical via the reaction of sodium sulfinate and PIDA [16]. As our continuing interest in iodine mediated reactions and functionalization of heterocyclic compounds [17], herein, we describe a PIDA mediated direct C–H amination of quinoxalinones with heteroaromatic amines under metal free conditions.
In order to optimize the reaction conditions, the reaction of 1-methylquinoxalin-2(1H)-one (A1) with 1H-benzo[d][1, 2, 3]triazole (B1) was chosen as a model reaction. Screening of the reaction solvents revealed that acetone was the most effective solvent for this reaction (Table 1, entry 6). The product can be isolated with 83% yield when the reaction was performed in chloroform (Table 1, entry 3). The use of other solvents such as MeCN, PhCl, DCE and THF led to moderate yields (Table 1, entries 1, 2, 4, 5). The oxidant also palyed an important role in this reaction. Moderate yield of the product was observed when PIFA was used as oxidant (Table 1, entry 7). To our disappointment, (NH4)2S2O8 and Na2S2O8 delivered the products in poor yields (Table 1, entries 8 and 9). The effect of oxidant amount for this reaction was also investigated. Decreasing of amount of PIDA to 2.5 equiv. led to declined yields of 83% (entry 10). However, no significant change was observed on the yield when the amount of PIDA was increased to 3.5 equiv. (entry 11).
|
|
Table 1 Optimization of the reaction conditions.a |
After completion of the search for the optimized reaction conditions, the generality and scope of quinoxalinones and heteroaromatic amines were investigated. The results are shown in Scheme 2. Quinoxalinones bearing either electron-withdrawing groups or electron-donating groups could react with 1H-benzo[ d]-[1, 2, 3]triazole smoothly to deliver the desired products in moderate to high yields (C1- C7). Subsequently, various N-substituted quinoxalinones were involved to examine the generality of this method. Propyl, allyl, propargyl, benzyl groups and so on were all suitable for this transformation (C8- C14). The corresponding products can be obtained with moderate yields. Notably, the useful ester group could be well tolerated in the system (C13). Moreover, 1H-benzo[ d][1, 2, 3]triazole with different groups were investigated (C15- C20). Furthermore, when the benzene ring of triazole was monosubstituted, the reaction would take place either on N1 or N3 position, delivering the products with a mixture of two isomers (C21 and C22). To our delight, when 1H-benzo[ d]imidazole and 1H-1, 2, 4-triazole were used as substrates, the desired products could be detected in 84%–94% yields (C23 and C24).

|
Download:
|
| Scheme 2. Reaction scope. Reaction conditions: A (0.2 mmol), B (0.4 mmol), acetone (2 mL), PIDA (3.0 equiv.). under air, r.t., 24 h. | |
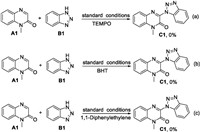
To gain some insight into the reaction mechanism, several control experiments were performed. When the reaction was performed in the presence of TEMPO, BHT or 1, 1-diphenylethylene, no desired products were isolated (Scheme 3). These results indicated that radical mechanism maybe involved in the present transformation.
|
Download:
|
| Scheme 3. Control experiments. | |
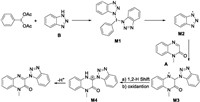
Based on the above-mentioned observations and related references [7a, 18], a probable reaction mechanism is depicted in Scheme 4. Firstly, the reaction of PIDA with 1H-benzo[d][1,2,3]triazole B afforded intermediate M1. Decomposition of M1 gives radical M2, which reacted with 1-methylquinoxalin-2(1H)-one A to give radical M3. Then intermediate M4 was produced via 1, 2-hydrogen shift of M3 and oxidation. Finally, the corresponding product was obtained from the deprotonation of the intermediate M4.
|
Download:
|
| Scheme 4. Tentative mechanism. | |
In summary, an efficient and facile method for C–H amination of quinoxalinones with heteroaromatic amines under metal-free conditions has been described. In the presence of hypervalent PIDA reagent, the desired products with various groups were obtained with moderate to high yields. Further studies on the reaction mechanism and the application of this transformation to more complicated compounds are underway in our lab.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21772107), Shandong Province Key Research and Development Plan (No. 2019GSF108017). We also thank Cai-Zhen Ding, Yan-Li Wang and Hong-Di Yang for their useful help.
| [1] |
(a) J.A. Pereira, A.M. Pessoa, M.N.D.S. Cordeiro, et al., Eur. J. Med. Chem. 97 (2015) 664-672; (b) R. Liu, Z. Huang, M.G. Murray, X. Guo, G. Liu, J. Med. Chem. 54 (2011) 5747-5768; (c) C.H. Chen, Q.Y. Wu, C. Wei, et al., Green Chem. 20 (2018) 2722-2729; (d) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30 (2019) 2287-2290; (e) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30 (2019) 2132-2138. |
| [2] |
(a) J.A. Pereira, A.M. Pessoa, M.N.D.S. Cordeiro, et al., Eur. J. Med. Chem. 97 (2015) 664-672; (b) R. Liu, Z. Huang, M.G. Murray, X. Guo, G. Liu, J. Med. Chem. 54 (2011) 5747-5768; (c) C.H. Chen, Q.Y. Wu, C. Wei, et al., Green Chem. 20 (2018) 2722-2729; (d) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30 (2019) 2287-2290; (e) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30 (2019) 2132-2138. |
| [3] |
T.S. Bekaii-Saab, A. Mortazavi, L.G. Hicks, et al., Invest. New Drugs 24 (2006) 343-346. DOI:10.1007/s10637-005-4827-3 |
| [4] |
C. Yang, X. Zhang, Y. Wang, et al., ACS Med. Chem. Lett. 8 (2017) 875-880. DOI:10.1021/acsmedchemlett.7b00222 |
| [5] |
Q. Ke, G. Yan, J. Yu, X. Wu, Org. Biomol. Chem. 17 (2019) 5863-5881. |
| [6] |
M. Gao, Y. Li, L. Xie, R. Chauvin, X. Cui, Chem. Commun. 52 (2016) 2846-2849. DOI:10.1039/C5CC08049E |
| [7] |
(a) L. Wang, P. Bao, W. Liu, et al., Chin. J. Org. Chem. 38 (2018) 61-68; (b) S. Paul, J.H. Ha, G.E. Park, Y.R. Lee, Adv. Synth. Catal. 359 (2017) 1515-1521; (c) M. Tian, S. Liu, X. Bu, J. Yu, X. Yang, Chem. Eur. J. 26 (2020) 369-373; (d) L.Y. Xie, S. Peng, L.H. Yang, et al., Green Chem. 23 (2021) 374-378. |
| [8] |
(a) J. Lu, X.K. He, X. Cheng, et al., Adv. Synth. Catal. 362 (2020) 2178-2182; (b) L.Y. Xie, Y.S. Bai, X.Q. Xu, et al., Green Chem. 22 (2020) 1720-1725. |
| [9] |
L. Yang, P. Gao, X.H. Duan, Y.R. Gu, L.N. Guo, Org. Lett. 20 (2018) 1034-1037. DOI:10.1021/acs.orglett.7b03984 |
| [10] |
(a) J. Fu, J. Yuan, Y. Zhang, et al., Org. Chem. Front. 5 (2018) 3382-3390; (b) S. Mao, K. Luo, L. Wang, et al., Org. Lett. 21 (2019) 2365-2368; (c) N. Meng, Y. Lv, Q. Liu, et al., Chin. Chem. Lett. 32 (2021) 258-262; (d) L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. Chin. Chem. 62 (2019) 460-464. |
| [11] |
(a) Q.H. Teng, Y. Yao, W.X. Wei, et al., Green Chem. 21 (2019) 6241-6245; (b) L.Y. Xie, Y.L. Chen, L. Qin, et al., Org. Chem. Front. 6 (2019) 3950-3955. |
| [12] |
(a) A. Gupta, M.S. Deshmukh, N. Jain, J. Org. Chem. 82 (2017) 4784-4792; (b) W. Wei, L. Wang, P. Bao, et al., Org. Lett. 20 (2018) 7125-7130; (c) Y. Li, M. Gao, L. Wang, X. Cui, Org. Biomol. Chem. 14 (2016) 8428-8432; (d) L.Y. Xie, J.L. Hu, Y.X. Song, et al., ACS Sustainable Chem. Eng. 7 (2019) 19993-19999. |
| [13] |
(a) Z. Zheng, D. Zhang-Negrerie, Y. Du, K. Zhao, Sci. Chin. Chem. 57 (2013) 189-214; (b) X. Wang, A. Studer, Acc. Chem. Res. 50 (2017) 1712-1724; (c) T. Luo, J.P. Wan, Y. Liu, Org. Chem. Front. 7 (2020) 1107-1112; (d) Q. Deng, W. Xia, M.I. Hussain, et al., J. Org. Chem. 85 (2020) 3125-3133; (e) D.Q. Dong, W.J. Chen, D.M. Chen, et al., Chin. J. Org. Chem. 39 (2019) 3190-3198; (f) D.Q. Dong, W.J. Chen, Y. Yang, X. Gao, Z.L. Wang, ChemistrySelect 4 (2019) 2480-2483; (g) X. Zhang, Y. Cong, G. Lin, et al., Chin. J. Org. Chem. 36 (2016) 2513-2529; (h) T. Lan, H. Qin, W. Chen, W. Liu, C. Chen, Chin. Chem. Lett. 31 (2020) 357-360; (j) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30 (2019) 2259-2262; (k) Q.W. Gui, F. Teng, S. -N. Ying, et al., Chin. Chem. Lett. 31 (2020) 3241-3244. |
| [14] |
(a) L. Wang, P. Bao, W. Liu, et al., Chin. J. Org. Chem. 38 (2018) 61-68; (b) S. Paul, J.H. Ha, G.E. Park, Y.R. Lee, Adv. Synth. Catal. 359 (2017) 1515-1521; (c) M. Tian, S. Liu, X. Bu, J. Yu, X. Yang, Chem. Eur. J. 26 (2020) 369-373; (d) L.Y. Xie, S. Peng, L.H. Yang, et al., Green Chem. 23 (2021) 374-378. |
| [15] |
(a) J. Lu, X.K. He, X. Cheng, et al., Adv. Synth. Catal. 362 (2020) 2178-2182; (b) L.Y. Xie, Y.S. Bai, X.Q. Xu, et al., Green Chem. 22 (2020) 1720-1725. |
| [16] |
P. Katrun, S. Hlekhlai, J. Meesin, et al., Org. Biomol. Chem. 13 (2015) 4785-4794. DOI:10.1039/c5ob00417a |
| [17] |
(a) G.H. Li, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31 (2020) 3255-3258; (b) Q.Q. Han, G.H. Li, Y.Y. Sun, et al., Tetrahedron Lett. 61 (2020) 151704; (c) D.Q. Dong, H. Yang, J.L. Shi, et al., Org. Chem. Front. 7 (2020) 2538-2575; (d) D.Q. Dong, Y.Y. Sun, G.H. Li, et al., Chin. J. Org. Chem. 40 (2020) 4071-4086; (e) D. Dong, G. Li, D. Chen, et al., Chin. J. Org. Chem. 40 (2020) 1766-1771; (f) D. Chen, Y. Sun, D. Dong, Q. Han, Z. Wang, Chin. J. Org. Chem. 40 (2020) 4267-4273; (g) D.Q. Dong, L.X. Li, G.H. Li, et al., Chin. J. Catal. 40 (2019) 1494-1498; (h) G.H. Li, D.Q. Dong, Y. Yang, X.Y. Yu, Z.L. Wang, Adv. Synth. Catal. 361 (2019) 832-835; (i) G.H. Li, D.Q. Dong, X.Y. Yu, Z.L. Wang, New J. Chem. 43 (2019) 1667-1670; (j) G.H. Li, D.Q. Dong, Q. Deng, S.Q. Yan, Z.L. Wang, Synthesis51 (2019)3313-3319. |
| [18] |
(a) L.Y. Xie, J. Qu, S. Peng, et al., Green Chem. 20 (2018) 760-764; (b) L.Y. Xie, Y.S. Liu, H.R. Ding, et al., Chin. J. Catal. 41 (2020) 1168-1173; (c) Y.F. Si, X.L. Chen, X.Y. Fu, et al., ACS Sustainable Chem. Eng. 8 (2020) 10740-10746; (d) N. Meng, L. Wang, Q. Liu, et al., J. Org. Chem. 85 (2020) 6888-6896; (e) X.K. He, J. Lu, A.J. Zhang, et al., Org. Lett. 22 (2020) 5984-5989; (f) J. Yuan, S. Liu, Y. Xiao, et al., Org. Biomol. Chem. 17 (2019) 876-884; (g) X.F. Xia, Z. Gu, W. Liu, et al., J. Org. Chem. 80 (2015) 290-295; (h) D.M. Chen, Y.Y. Sun, Q.Q. Han, Z.L. Wang, Tetrahedron Lett. 61 (2020) 152482; (i) Q. Yang, X. Han, J. Zhao, H.Y. Zhang, Y. Zhang, J. Org. Chem. 84 (2019) 11417-11424; (j) L. Wang, Y. Zhang, F. Li, et al., Adv. Synth. Catal. 360 (2018) 3969-3977; (k) W.L. Chen, S.Y. Wu, X.L. Mo, et al., Org. Lett. 20 (2018) 3527-3530; (l) J.Y. Chen, C.T. Zhong, Q.W. Gui, et al., Chin. Chem. Lett. 32 (2021) 475-479. |
 2021, Vol. 32
2021, Vol. 32