b Department of Pharmacy, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, China
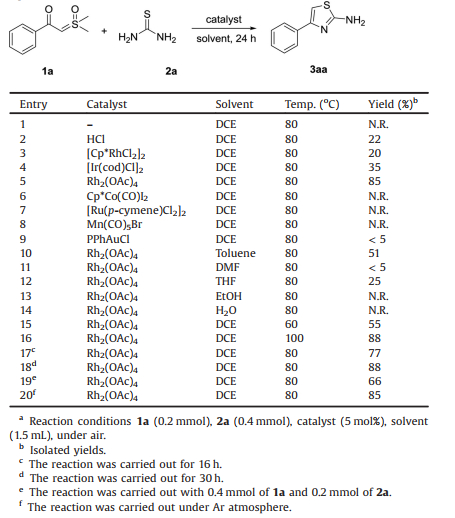
The 2-aminothiazole ring is an important structural motif found in numerous biologically active molecules which exhibits a wide range of activities such as antibacterial [1], anti-inflammatory [2], anticancer [3], anti-hypertension [4] and anti HIV [5]. In addition, 2-aminothiazole ring is frequently found in a variety of market drug such as famotidine [6], cefdinir [7], meloxicam [8] and so on. Besides, in 2019 we reported structure-based design of N-(5-phenylthiazol-2-yl)acrylamides as novel and potent glutathione S-transferase omega 1 (GSTO1-1) inhibitors [9], showing that thiazole derivatives are potential and suitable leading compounds for cancer therapy (Fig. 1). Due to the importance and the broad utility of 2-aminothiazole derivatives, diverse well-established methods have been developed to construct these structural motifs. The Hantzsch synthesis is the most commonly used typical method using α-halocarbonyl compounds and thioureas/thioamides for the synthesis of 2-aminothiazoles (Scheme 1) [10]. Over the years, many improved methods have been reported involving the use of catalysts such as ammonium 12-molybdophosphate [11], β-cyclodextrin [12], iodine [13] and silica chloride [14], new starting materials from α-nitroepoxides and thiocyanate [15]-18], green solvent such as ionic liquids [19], PEG-400 [20] and water [21], and with the aid of microwaves [22, 23]. And some valuable methods have also been developed for constructing fused polycyclic thiazole rings [24, 25]. Despite the progress, some limitations still remain. For example, mostly using α-halocarbonyl compounds as the starting materials is inevitable, which lead to the generation of much chemical waste. Faced with these thorny problems, developing more efficient and sustainable synthetic strategies remains highly desirable.
|
Download:
|
| Fig. 1. Selected examples of bioactive molecules with 2-aminothiazole ring. | |
|
Download:
|
| Scheme 1. Routes to 2-aminothiazole derivatives. | |
Metal-catalyzed X-H (X = N, O, S, etc.) insertion reactions into diazo compounds have been recognized as a powerful method to the construction of diverse carbon-heteroatom bonds [26]. In 2008, Yadav and coworkers reported Cu(Ⅱ)-catalyzed coupling of α-diazoketones with thiourea to generate a variety of 2-aminothiazoles (Scheme 1b) [27]. Following this pioneer work, there were also other works to make amino-thiazoles, starting from diazocompounds catalyzed by PEG400 or BF3 (Scheme 1b, ii and iii) [28, 29]. Nevertheless, diazo compounds suffer from instability, synthetic inconvenience, and potential danger of rapid exothermic reaction. So exploring alternative carbene precursors and their applications in organic synthesis will make a great contribution to green carbene chemistry.
In recent years, sulfoxonium ylides has been widely used as new surrogates of carbene precursors due to their high stability and synthetic convenience compared to diazo compounds. Many studies on carbene insertions and C-H functionalization have been reported to construct versatile carbon-carbon bonds and carbon-heteroatom bonds [30-47]. It was noted that in 2006 sheppeck and co-workers first utilized sulfoxonium ylides and thiourea to synthesize 2-aminothiazole ring with added acid [48]. Probably due to the enough acidity which protonate sulfoxonium ylide to furnish reactive sulfoxonium. However, only three examples were performed and the yields of the obtained products were very low according to this method. Besides, this method requires large amount of hydrochloride as the catalyst which led to the inconvenience of post process and generation of chemical waste. In 2016, a patent described the use of sulfonium salts and thiourea to prepare the 2-amino-4-aryl-5-methylthiothiazole compounds [49]. Still, the synthesis of α-halocarbonyl compounds was inevitable. Our group has been long contributed to the study on diverse reactions of sulfoxonium ylides, and we have developed precedent water-mediated S-H insertion reaction of sulfoxonium ylides with mercaptan [50]. Considering we are in much need of efficient method for preparation of 2-aminothiazoles based on our previously study of GSTO1−1 inhibitors, we envisaged that 2-aminothiazole ring could be obtained through carbene insertion from sulfoxonium ylides and thiourea followed by subsequent cyclocondensation. Herein, we report an efficient strategy for the synthesis of 2-aminothiazoles by means of Rh2(OAc)4-catalyzed coupling of sulfoxonium ylides with thiourea under mild condition. This halogen-free method avoids some safety issues of previous strategies, making it a useful and attractive strategy for the preparation of relevant 2-aminothiazole derivatives.
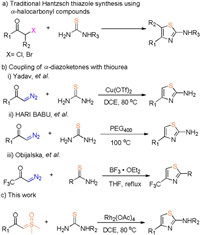
In order to explore the best reaction conditions, the investigation was commenced by using α-benzoyl sulfoxonium ylide 1a (0.2 mmol) and thiourea 2a (0.4 mmol) with different catalytic systems in DCE at 80 ℃ for 24 h under air atmosphere. Of these catalysts, Rh2(OAc)4 was found to be the most effective catalyst in terms of conversion and cleanliness while others like HCl, Cp*Co(CO)I2, [Cp*RhCl2]2, [Ir(cod)Cl]2 or [Ru(p-cymene)Cl2]2 failed to work or gave lower yields, (Table 1, entries 2–9). With regard to solvents, the increase in yield was not observed when solvents were changed (Table 1, entries 10–14). Although higher temperature and longer reaction time could slightly increase the reaction conversion, 80 ℃ and 24 h were chosen as the final reaction conditions in consideration of efficiency and energy conservation (Table 1, entries 16 and 18). Subsequently, further study on the adjustment of the two substrates' ratio indicated that only excess equivalents of thiourea could keep the high yield of the desired product (Table 1, entry 19). The reaction did not proceed at all without any catalyst (Table 1, entry 1).
|
|
Table 1 Optimization of reaction conditions.a |
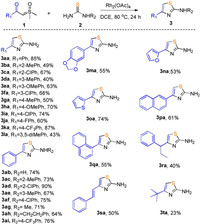
Having determined the optimal conditions, the generality of Rh-catalyzed insertion and annulations reaction was then evaluated by using the diversely substituted sulfoxonium ylides with thiourea. As shown in Scheme 2, a range of sulfoxonium ylides bearing various electron-donating, -withdrawing and halogen substituents at the different positions of the phenyl ring all coupled smoothly with 2a, affording the target products in moderate to excellent yields (3ba-3la). In general, it was indicated that the electron-withdrawing groups attached to ylides were more suitable than electron-donating substituents such as methyl, methoxyl and dimethyl, probably because that inductive effect of electron-withdrawing groups make the carbonyl more susceptible to nucleophilic attack by the amino group in the cyclocondensation step. Notably, other factors including the positions of substitution, size of groups and steric effect had little effect on the reaction. To our delight, the substrates containing 1, 3-benzodioxole group, furan and thiophene rings or naphthalene could also be tolerated well to generate the respective products in 53%–74% yields (3ma-3qa). When the phenyl group was replaced by alkyl groups, the yields of products were decreased to 23%–40% (3ra and 3ta). It was worth mentioning that the reaction using alkenyl substituted ylide also formed corresponding product 3sa in more thermodynamically stable E-configuration. Among these examples, no N-H insertion products were observed in terms of chemoselectivity. We then tried reaction with urea, still no desired product was observed.
|
Download:
|
| Scheme 2. The synthesis of 2-aminothiazoles derivatives. Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), Rh2(OAc)4 (5 mol%), DCE (1.5 mL), desired product 3 was isolated by column chromatography on silica gel (eluent: PE/EA=10/1). | |
Furthermore, the scope of thiourea derivatives was studied under the standard procedure. A series of 1-phenylthiourea derivatives with methyl, trifluoromethyl or chloride group substituted at different positions of phenyl ring all reacted smoothly affording the corresponding products in high yields (3ab-3af, 3ai). Also, 1-methylthiourea exhibited good reactivity giving the product 3ag in 71% yield. Especially, this method could be successfully applied for the synthesis of anti-inflammatory agent Fanetizole 3ah from α-benzoyl sulfoxonium ylide and 2-phenylethylthiourea in 64% yield. It was noted that using these asymmetrical thiourea derivatives did not form any isomers indicating that the cyclization reaction preferred the unsubstituted side, probably due to the less steric hindrance and the stronger nucleophilicity of primary amines.
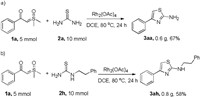
To show the usefulness and practical synthesis of this method, we carried out a large-scale reaction of α-benzoyl sulfoxonium ylide 1a (5 mmol) with thiourea 2a (10 mmol) under standard condition. It turned out that this reaction proceeded smoothly to afford 3aa in a yield of 67% (Scheme 3a). We have also performed a large scale of reaction for synthesizing the anti-inflammatory agent Fanetizole (3ah) directly from α-benzoyl sulfoxonium ylide (5 mmol) and 2-phenylethylthiourea (10 mmol), affording the expected product in 58% yield (Scheme 3b), which justifying the synthetic applications of the current method.
|
Download:
|
| Scheme 3. Large-scale synthesis of 3aa and 3ah. | |
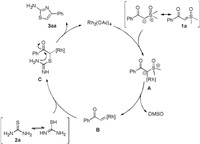
According to the experimental results and relevant literatures [27, 51], we proposed a plausible mechanistic hypothesis shown in Scheme 4. Initially, sulfoxonium ylides were activated in the presence of Rh2(OAc)4 to generate rhodium intermediate A. The rhodium carbene complexes B was obtained from intermediate A by the elimination of DMSO, which underwent insertion into S-H bond of tautomeric thiourea to form the intermediate C, and subsequent nucleophilic attack between the amino group and carbonyl group afforded the product 3aa and released the rhodium catalyst.
|
Download:
|
| Scheme 4. Proposed mechanism for the formation of 3aa. | |
In summary, we presented a simple and efficient Rh-catalyzed carbene insertion and annulation reaction to access a variety of 2-aminothiazoles. This strategy features mild condition, high selectivity, simple operation and employment of sulfoxonium ylides instead of the diazo compounds or α-haloketones, making it more attractive and practical. Moreover, this process exhibites good functional group compatibility and chemoselectivity. We believe that this useful strategy may have promising and significant applications in metal carbene chemistry and preparation of biologically potent 2-aminothiazoles.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsWe are grateful for the support from the National Natural Science Foundation of China (Nos. 81373259 and 81573286) and Sichuan Science and Technology Program (Nos. 2020YJ0221 and 2018JY0537).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2021.02.052.
| [1] |
K.K. Roy, S. Singh, S.K. Sharma, et al., Bioorg. Med. Chem. Lett. 21 (2011) 5589-5593. DOI:10.1016/j.bmcl.2011.06.076 |
| [2] |
O. Kouatly, A. Geronikaki, C. Kamoutsis, et al., Eur. J. Med. Chem. 44 (2009) 1198-1204. DOI:10.1016/j.ejmech.2008.05.029 |
| [3] |
M.D. Charles, J.L. Brookfield, T.C. Ekwuru, et al., J. Med. Chem. 58 (2015) 8309-8313. DOI:10.1021/acs.jmedchem.5b01242 |
| [4] |
B.F. Abdel-Wahab, S.F. Mohamed, G.E. A.E.-Amr, et al., Monatshefte für Chemie-Chemical Monthly 139 (2008) 1083-1090. DOI:10.1007/s00706-008-0896-2 |
| [5] |
F.W. Bell, A.S. Cantrell, M. Hogberg, et al., J. Med. Chem. 38 (1995) 4929-4936. DOI:10.1021/jm00025a010 |
| [6] |
L. Laine, A.J. Kivitz, A.E. Bello, et al., Am. J. Gastroenterol. 107 (2012) 379-386. DOI:10.1038/ajg.2011.443 |
| [7] |
D.R.P. Guay, Clin. Ther. 24 (2002) 473-489. DOI:10.1016/S0149-2918(02)85125-6 |
| [8] |
G. Engelhardt, D. Homma, K. Schlegel, et al., Inflamm. Res. 44 (1995) 423-433. DOI:10.1007/BF01757699 |
| [9] |
W. Dai, S. Samanta, D. Xue, et al., J. Med. Chem. 62 (2019) 3068-3087. DOI:10.1021/acs.jmedchem.8b01960 |
| [10] |
A. Hantzsch, J.H. Weber, Chem. Ber. 20 (1887) 3118-3132. DOI:10.1002/cber.188702002200 |
| [11] |
B. Das, V. Saidi Reddy, R. Ramu, J. Mol. Catal. A: Chem. 252 (2006) 235-237. DOI:10.1016/j.molcata.2006.02.065 |
| [12] |
M. Narender, M.S. Reddy, R. Sridhar, et al., Tetrahedron Lett. 46 (2005) 5953-5955. DOI:10.1016/j.tetlet.2005.06.130 |
| [13] |
H.L. Siddiqui, A. Iqbal, S. Ahmad, et al., Molecules 11 (2006) 206-211. DOI:10.3390/11020206 |
| [14] |
H. Karade, M. Sathe, M.P. Kaushik, Catal. Commun. 8 (2007) 741-746. DOI:10.1016/j.catcom.2006.09.005 |
| [15] |
B. Chen, S. Guo, X. Guo, et al., Org. Lett. 17 (2015) 4698-4701. DOI:10.1021/acs.orglett.5b02152 |
| [16] |
D. Zhao, S. Guo, X. Guo, et al., Tetrahedron 72 (2016) 5285-5289. DOI:10.1016/j.tet.2016.05.010 |
| [17] |
Y. Zhu, W. Chen, D. Zhao, et al., Synthesis 51 (2019) 2023-2029. DOI:10.1055/s-0037-1612101 |
| [18] |
Y. Zhu, Q. Wang, H. Luo, et al., Tetrahedron 74 (2018) 7143-7147. DOI:10.1016/j.tet.2018.09.005 |
| [19] |
T.M. Potewar, S.A. Ingale, K.V. Srinivasan, Tetrahedron 63 (2007) 11066-11069. DOI:10.1016/j.tet.2007.08.036 |
| [20] |
D.V. Jawale, D.L. Lingampalle, U.R. Pratap, et al., Chin. Chem. Lett. 21 (2010) 412-416. DOI:10.1016/j.cclet.2009.11.035 |
| [21] |
T.M. Potewar, S.A. Ingale, K.V. Srinivasan, Tetrahedron 64 (2008) 5019-5022. DOI:10.1016/j.tet.2008.03.082 |
| [22] |
G.W. Kabalka, A.R. Mereddy, Tetrahedron Lett. 47 (2006) 5171-5172. DOI:10.1016/j.tetlet.2006.05.053 |
| [23] |
M. Botta, D. Castagnolo, M. Pagano, et al., Synlett 2009 (2009) 2093-2096. DOI:10.1055/s-0029-1217700 |
| [24] |
A. Dey, A. Hajra, Org. Lett. 21 (2019) 1686-1689. DOI:10.1021/acs.orglett.9b00245 |
| [25] |
S. Jana, A. Chakraborty, V.Z. Shirinian, et al., Adv. Synth. Catal. 360 (2018) 2402-2408. DOI:10.1002/adsc.201800393 |
| [26] |
D. Gillingham, N. Fei, Chem. Soc. Rev. 42 (2013) 4918-4931. DOI:10.1039/c3cs35496b |
| [27] |
J.S. Yadav, B.V.S. Reddy, Y.G. Rao, et al., Tetrahedron Lett. 49 (2008) 2381-2383. DOI:10.1016/j.tetlet.2008.02.068 |
| [28] |
B.H. Babu, K. Vijay, K.B. Murali Krishna, et al., J. Chem. Sci. 128 (2016) 1475-1478. DOI:10.1007/s12039-016-1130-0 |
| [29] |
E. Obijalska, M. Błaszczyk, M.K. Kowalski, et al., J. Fluorine Chem. 220 (2019) 35-40. DOI:10.1016/j.jfluchem.2019.02.003 |
| [30] |
M. Barday, C. Janot, N.R. Halcovitch, et al., Angew. Chem. Int. Ed. 56 (2017) 13117-13121. DOI:10.1002/anie.201706804 |
| [31] |
S. Ji, K. Yan, B. Li, et al., Org. Lett. 20 (2018) 5981-5984. DOI:10.1021/acs.orglett.8b02796 |
| [32] |
H. Jiang, H. Zhang, W. Xiong, et al., Org. Lett. 21 (2019) 1125-1129. DOI:10.1021/acs.orglett.9b00072 |
| [33] |
R. Lai, X. Wu, S. Lv, et al., Chem. Commun. 55 (2019) 4039-4042. DOI:10.1039/C9CC01146C |
| [34] |
X. Wu, H. Xiong, S. Sun, et al., Org. Lett. 20 (2018) 1396-1399. DOI:10.1021/acs.orglett.8b00119 |
| [35] |
Y. Xu, G. Zheng, X. Yang, et al., Chem. Commun. 54 (2018) 670-673. DOI:10.1039/C7CC07753J |
| [36] |
G. Zheng, M. Tian, Y. Xu, et al., Org. Chem. Front. 5 (2018) 998-1002. DOI:10.1039/C7QO01033H |
| [37] |
Y.Z. Liu, Y. Hu, G.H. Lv, et al., ACS Sustain. Chem. Eng. 7 (2019) 13425-13429. DOI:10.1021/acssuschemeng.9b02803 |
| [38] |
J. Li, H. He, M. Huang, et al., Org. Lett. 21 (2019) 9005-9008. DOI:10.1021/acs.orglett.9b03410 |
| [39] |
J. Vaitla, A. Bayer, K.H. Hopmann, Angew. Chem. Int. Ed. 56 (2017) 4277-4281. DOI:10.1002/anie.201610520 |
| [40] |
R.M. Dias, A.C. Burtoloso, Org. Lett. 18 (2016) 3034-3037. DOI:10.1021/acs.orglett.6b01470 |
| [41] |
R. Nie, R. Lai, S. Lv, et al., Chem. Commun. 55 (2019) 11418-11421. DOI:10.1039/C9CC05804D |
| [42] |
Z. Shen, C. Pi, X. Cui, et al., Chin. Chem. Lett. 30 (2019) 1374-1378. DOI:10.1016/j.cclet.2019.01.033 |
| [43] |
X. Wu, Y. Xiao, S. Sun, et al., Org. Lett. 21 (2019) 6653-6657. DOI:10.1021/acs.orglett.9b02249 |
| [44] |
W. Xie, X. Chen, J. Shi, et al., Org. Chem. Front. 6 (2019) 2662-2666. DOI:10.1039/C9QO00524B |
| [45] |
Y. Xu, X. Yang, X. Zhou, et al., Org. Lett. 19 (2017) 4307-4310. DOI:10.1021/acs.orglett.7b01974 |
| [46] |
Y. Xu, X. Zhou, G. Zheng, et al., Org. Lett. 19 (2017) 5256-5259. DOI:10.1021/acs.orglett.7b02531 |
| [47] |
Y. Yuan, X.F. Wu, Org. Lett. 21 (2019) 5310-5314. DOI:10.1021/acs.orglett.9b01926 |
| [48] |
J. Sheppeck, T.G.M. Dhar, L. Doweyko, et al., Patent, US20060154973, 2006.
|
| [49] |
Zhiling Cao, Qiao Wu, Jing Chen, et al., Patent, CN106565626A, 2016.
|
| [50] |
Y. Xu, X. Huang, G. Lv, et al., Eur. J. Org. Chem. 2020 (2020) 4635-4638. DOI:10.1002/ejoc.202000716 |
| [51] |
I.K. Mangion, I.K. Nwamba, M. Shevlin, et al., Org. Lett. 11 (2009) 3566-3569. DOI:10.1021/ol901298p |
 2021, Vol. 32
2021, Vol. 32