b The Key Laboratory of Water and Air Pollution Control of Guangdong Province, South China Institute of Environmental Sciences, The Ministry of Ecology and Environment of PRC, Guangzhou 510655, China;
c Guangdong Province Engineering Laboratory for Air Pollution Control, South China Institute of Environmental Sciences, The Ministry of Ecology and Environment of PRC, Guangzhou 510655, China;
d Zhuhai Jinwan Liangang Foundation Investment Co., Ltd., Zhuhai 519090, China;
e Hunan Construction Engineering Group, Changsha 410000, China
Selective catalytic reduction (SCR) with ammonia is an efficient way to control NOx emission. Manganese oxide-based catalysts are gaining much attention due to the fact that they exhibit excellent low-temperature activity than V2O5-WO3(MoO3)/TiO2 catalysts at the actual flue gas temperature (< 200 ℃) [1-3]. However, one of the largest drawbacks of the pure MnOx is its relatively narrow temperature range, which limits its practical use in the NH3-SCR process [4]. Ce could be introduced into MnOx, and Mn-Ce oxide possesses as a superior catalyst owes a good deal to the synergistic effects between MnOx and CeO2 [1, 2, 5].
Recent years, a novel hydrolysis driving redox method has been successfully applied to synthesize higher low-temperature activity Mn-Ce catalysts [6, 7]. In this process, KMnO4 reacts with metal ionic salts (i.e., Ce3+) in the presence of H2O2 solution (i.e., 6MnO4− (aq) + 2Ce3+ (aq) + 9H2O2 (l)=6MnO2 (s) + 2Ce(OH)3 (s) + 6H2O (l) + 9O2 (g)). And a MnOx-CeO2 catalyst with a stoichiometric ratio of Mn/Ce=3 is subsequently synthesized. A large average pore size and a high SBET which are beneficial to the catalytic performance are obtained due to a large amount of O2 emitted during the procedure. The as-prepared 3Mn1Ce catalysts were reported to display strong reducibility and favorable performances in the removal of BTEX and chlorobenzene [6, 7]. However, these superior MnOx-CeO2 catalysts have not been tested in the NH3-SCR deNOx process. Moreover, it should be noted that cerium(Ⅲ) nitrate hexahydrate (Ce(NO3)3·6H2O) employed in the preparation process was corrosive and very toxic to aquatic life with long-lasting effects. Meanwhile, some researches showed that catalysts prepared by nitrate (N) precursor exhibited lower activity than those prepared by acetate (Ac) precursor [8, 9]. Thus, the present study chooses Ac precursor to prepare 3Mn1Ce catalysts by hydrolysis driving redox method. Also, the N precursor is applied for comparison purpose.
As one of the representatives of oxygenated VOCs (OVOCs), gaseous acetone usually coexists with NOx in the flue gases from the incomplete combustion of fossil fuels, biomass, and solid wastes, etc. [10-13]. However, in contrast to many works focus on the effect of Cl-contained VOCs (Cl-VOCs, e.g., chlorobenzene) and benzene series (e.g., toluene) on the NH3-SCR process, few researchers play attention to the impact of OVOCs (e.g., acetone) [14, 15]. Previous studies have been confirmed that chlorobenzene could improve the N2 selectivity and widen the active temperature of SCR reaction, while toluene significantly inhibits the deNOx activity and results in even poorer N2 selectivity. Thus, it suggested that different VOCs will make different impacts on the NH3-SCR reaction, and how gaseous acetone influence the NH3-SCR process needs attention. To date, to the best of the authors' knowledge, there is only a very limited report studying the effect of gaseous acetone on the NH3-SCR process.
In this work, 3Mn1Ce catalysts were synthesized using two methods: hydrolysis driving redox method (3Mn1Ce-Ac, and 3Mn1Ce-N) and NH3·H2O co-precipitation method (Cop-3Mn1Ce). In brief, Ce(CH3COO)3·xH2O was the precursor for the 3Mn1Ce-Ac synthesis, while Ce(NO3)3·6H2O for the 3Mn1Ce-N. N2 adsorption-desorption isotherms, X-ray powder diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), H 2 temperature-programmed reduction (H2-TPR), and NH3 temperature-programmed desorption (NH3-TPD), were applied to characterize the physicochemical properties of different catalysts. The activity of the catalyst was then tested by on-line FTIR (GASMET DX-4000). The possible impacts of gaseous acetone on SCR performance and SCR side reactions were finally investigated. Detail catalysts synthesis, experimental setup, and definition of the SCR side reactions were presented in Supporting information.
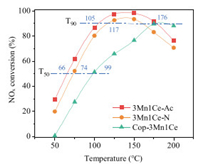
Fig. 1 shows the SCR performance of three 3Mn1Ce catalysts in the low temperature range of 50−200 ℃. The light-off curves for NOx conversion are typical convex curves due to the competition between NO reduction and NH3 oxidation when the temperature increases. The 3Mn1Ce catalysts synthesized by hydrolysis driving redox method (3Mn1Ce-Ac and 3Mn1Ce-N) are more active than that synthesized by NH3·H2O co-precipitation method (Cop-3Mn1Ce). The temperatures T50 and T90 corresponding to NOx conversions of 50% and 90%, respectively, are summarized to better show the catalytic activities. The T50 and T90 of 3Mn1Ce-Ac (66 and 105 ℃) were lower than that of 3Mn1Ce-N (74 and 117 ℃), It is obvious that the acetate precursor was superior for the preparation of higher low-temperature activity MnOx-CeO2 catalysts in hydrolysis driving redox method.
|
Download:
|
| Fig. 1. NOx conversion over 3Mn1Ce catalysts. Reaction conditions: [NH3] = [NO]=500 ppm, O2=10 vol%, GHSV=60, 000 mL g−1h−1. | |
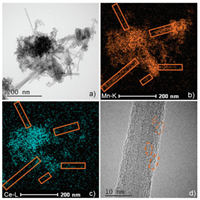
To better explain the catalytic performance of the prepared catalysts, detailed physicochemical characterizations are carried out. The XRD results of the 3Mn1Ce catalysts are depicted in Fig. S1 (Supporting information). It could be seen that all the diffraction peaks are broad, indicating the low crystallinity of the 3Mn1Ce catalysts. Some small peaks corresponding to MnO2 (JCPDS PDF# 72–1982) are found in the 3Mn1Ce-Ac catalyst. According to the high-resolution transmission electron microscopy (HRTEM) images of the 3Mn1Ce-Ac catalyst, some nanorod structures are presented (Fig. 2a). These structures are not observed in 3Mn1Ce-N and Cop-3Mn1Ce catalysts (Fig. S2 in Supporting information). The element distribution mappings of Mn and Ce demonstrate that the MnO2 nanorods are formed, and Ce cations are introduced into the nanorods structure (Figs. 2b and c) [16]. Besides, some lattice defects can be identified in the image (Fig. 2d). The introduction of Ce ions into the lattice matrix of MnO2 nanorods is favorable for the occurrence of lattice defects [7], which promotes the formation of more oxygen vacancies and subsequently enhances the catalytic performance of the 3Mn1Ce-Ac.
|
Download:
|
| Fig. 2. Studies of the 3Mn1Ce-Ac catalyst by TEM (a) TEM image, (b) Mn-element mapping, (c) Ce-element mapping, (d) HRTEM image of the nanorod. | |
The as-prepared 3Mn1Ce catalysts are mesoporous structures according to the N2 isotherms [17] (Fig. S3a in Supporting information). However, the pore size distributions of these catalysts are different (Fig. S3b in Supporting information). Compared with the Cop-3Mn1Ce catalyst, two 3Mn1Ce catalysts synthesized by hydrolysis driving redox method are abundant in mesopores (2−50 nm) due to the inhibition of small particles agglomeration by the O2 produced during the preparation procedure [7, 18]. Moreover, the pore size of the 3Mn1Ce-Ac catalyst is mainly distributed in the range of 7−30 nm, while the majority of the pore size of the 3Mn1Ce-N catalyst is less than 5 nm. Surface morphologies by SEM well confirm the above phenomena (Figs. S4a, S4b and S4d in Supporting information). The BET surface area of the catalyst decreases in the order: 3Mn1Ce-Ac > Cop-3Mn1Ce > 3Mn1Ce-N (Table S1 in Supporting information). 3Mn1Ce-Ac catalyst has the largest BET surface area (145.36 m2/g), pore volume (0.82 cm3/g), and pore diameter (23.87 nm), which are beneficial for the contact of reactants and the occurrence of reactions due to more available active sites provided on the catalyst surface.
Redox properties and surface acidity of the catalyst play important roles in the NOx reduction. Redox properties determined by H2-TPR are displayed in Fig. S5a and Table S2 (Supporting information). Two obvious reduction peaks in the range of 200−400 ℃ are observed over three 3Mn1Ce catalysts as reported in previous studies [7, 19], associating with two stepwise reductions of Mn4+ to Mn3+ and Mn3+ to Mn2+, overlapping the reduction of surface Ce4+. Compared with the reduction peaks of CeO2 (> 450 ℃) and MnOx (~400 ℃) (Fig. S6 in Supporting information), the values of 3Mn1Ce catalysts are significantly lower. This result is related to the formation of a solid solution with Mn-O-Ce structures, which improves the mobility of oxygen species (O2−, O22− and O−) greatly and makes catalysts surface more reducible [20, 21]. The reduction peak of the 3Mn1Ce-Ac starts at 89 ℃, which is lower than that of 3Mn1Ce-N (118 ℃) and Cop-3Mn1Ce (97 ℃). This result indicates that oxygen vacancy can be formed at such a low temperature, which provides the possibility for a low-temperature operation to remove NOx. To further investigate the reducibility of three 3Mn1Ce catalysts, the amount of H2 consumption with AgO as standard material is calculated. H2-TPR curves are fitted by Gaussian function with a fitting quality R2 higher than 0.99. The total H2 consumption is ranked in the order: 3Mn1Ce-Ac > 3Mn1Ce-N > Cop-3Mn1Ce. The H 2 consumption of the 3Mn1Ce-Ac catalyst reaches 7.85 mmol/g, suggesting that a large amount of most active oxygen is involved in the reaction [20, 22]. Moreover, the H2 consumption rate (Fig. S5b, detailed calculation see Supporting information) of 3Mn1Ce-Ac is the highest with the largest temperature range among three catalysts, inferring that the 3Mn1Ce-Ac catalyst poses outstanding oxygen migration ability within a wider temperature range, which is consistent with the deNOx performance.
NH3-TPD is applied to quantify the surface acid amount of 3Mn1Ce catalysts, and the results are presented in Fig. S7 (Supporting information) and Table S2. It is obvious that 3Mn1Ce catalysts synthesized by different methods all present multi-desorption peaks. As previous literature reported, physisorbed NH3 desorption associated with weak acid sites always happens below 400 ℃ and probably relates to Brønsted acid sites, while chemisorbed NH3 desorption associated with strong acid sites would occur above 400 ℃ and assigns to Lewis acid sites [23-26]. Since the area of desorption peaks can clearly show the surface acid amount, quantitative analysis by integrating the NH3-TPD curves is performed. 3Mn1Ce-Ac catalyst has the largest surface acid amount (1.76 mmol/g) for NH3 adsorption among the three 3Mn1Ce catalysts. In the meantime, the contribution of strong acid sites follows the order of 3Mn1Ce-Ac > 3Mn1Ce-N > Cop-3Mn1Ce, which is consistent with the catalytic performance in NH 3-SCR reaction, indicating that the strong acid sites assigned to Lewis acid are highly correlated with catalytic activity.
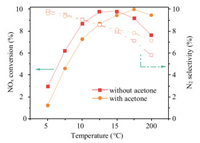
3Mn1Ce-Ac catalyst still exhibits an excellent SCR activity when gaseous acetone is in the feed gas (Fig. S8 in Supporting information). The impact of gaseous acetone on NOx conversion and N2 selectivity are then evaluated over the 3Mn1Ce-Ac catalyst (Fig. 3). When the temperature is below 150 ℃, the NOx conversion and N2 selectivity decrease when acetone is introduced into the flue gas. However, when the temperature is higher than 150 ℃, these two indicators are improved.
|
Download:
|
| Fig. 3. The impact of gaseous acetone on NOx conversion and N2 selectivity over the 3Mn1Ce-Ac catalyst. Reaction conditions: [NH3] = [NO]=500 ppm, [acetone]=50 ppm (when used), O2=10 vol%, GHSV=60, 000 mL g−1h−1. | |
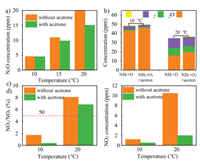
Fig. 4a shows the byproduct N2O concentration in the SCR reaction without/with gaseous acetone. It is obvious that N2O concentration decreases when gaseous acetone is added. The NSCR reaction (4NH3 + 4NO + 3O2 → 4N2O + 6H2O) is suppressed by gaseous acetone. As a consequence, more NH3 can participate in the standard SCR reaction when gaseous acetone is present, resulting in a positive effect on the N2 selectivity. At the same time, gaseous acetone restrains the NH3 catalytic oxidation reaction (C-O reaction: 4NH3 + 5O2 → 4NO + 6H2O and 2NH3 + 2O2 → N2O +3H2O). The concentration of NH3 oxidation by-products (N2O+NO) significantly decrease, resulting in more available NH3 in the SCR reaction (Fig. 4b). The conjunction of these results reveals that gaseous acetone inhibits the SCR side reactions over the 3Mn1Ce-Ac catalyst.
|
Download:
|
| Fig. 4. (a, c, d) N2O concentration, NO2/NOx percentage and NO2 concentration in the NH3-SCR reaction without/with acetone, and (b) the effect of acetone on the NH3 catalytic oxidation over the 3Mn1Ce-Ac catalyst. Reaction conditions: [NH3] = [NO]=500 ppm (when used), [acetone]=50 ppm (when used), O2=10 vol%, GHSV=60, 000 mL g−1h−1. | |
On the other hand, the NO2 fraction affects the reaction paths of NH3-SCR [27]. As is generally accepted, the deNOx activity will be improved through the so-called 'fast-SCR' process with NO2 concentration increasing. However, when NO2/NOx > 50%, NO reduction is mainly controlled by 'slow-SCR' reaction (NO 2-SCR reaction), and higher NO2 concentration will limit the deNOx activity. As illustrated in Fig. 4c, a condition of NO2/NOx < 50% is identified at 100 ℃, suggesting that the so-called 'fast-SCR' process determines the NH 3-SCR reaction. On the contrary, the percentage of NO2/NOx increases to more than 50% at 200 ℃, and the NH3-SCR process shifts to 'slow-SCR'. In addition, gaseous acetone inhibits the NO2 formation as shown in Fig. 4d. Thus, it is clear that lower NO2 concentration in the NH3-SCR reaction with acetone at 100 ℃ reduces the percentage of the 'fast-SCR' process, leading to a decrease of SCR activity. In contrast, slow SCR reaction is limited by lower NO2 concentration at 200 ℃, thus, results in the enhancement of deNOx activity.
For the purpose of comparison, the impacts of gaseous acetone on SCR performance and SCR side reactions over the 3Mn1Ce-N catalyst are also investigated (Fig. S9 in Supporting information). The tendency of NOx conversion is in consistent with the 3Mn1Ce-Ac catalyst. However, the N2 selectivity exhibits an opposite trend over the 3Mn1Ce-N catalyst compared with the 3Mn1Ce-Ac catalyst. Gaseous acetone facilitates the NSCR process to produce more N2O, leading to a decrease of N2 selectivity (Fig. S10 in Supporting information).
Overall, three different 3Mn1Ce catalysts synthesized by hydrolysis driving redox method (3Mn1Ce-Ac, and 3Mn1Ce-N) and NH3·H2O co-precipitation method (Cop-3Mn1Ce) are used for NH3-SCR. Among them, the 3Mn1Ce-Ac catalyst with some nanorod structures prepared by acetate (Ac) precursor exhibits the greatest SCR performance. Such a favorable deNOx behavior of 3Mn1Ce-Ac catalyst is mainly attributed to its large surface area for more available active sites, plentiful Lewis acid sites, and excellent low-temperature reducibility. Gaseous acetone inhibits the SCR undesired side reactions (NSCR & C-O reactions) and limits the "slow-SCR" process over 3Mn1Ce-Ac catalyst above 150 ℃, resulting in the enhancement of NO x conversion and N2 selectivity. Different from the 3Mn1Ce-Ac catalyst, the 3Mn1Ce-N catalyst displays weak capacity against NSCR reaction, leading to poor N2 selectivity.
Declaration of competing interestThe authors report no declarations of competing interest.
AcknowledgmentsThis work was supported by the Key Laboratory of Water and Air Pollution Control of Guangdong province, China (No. 2017A030314001), the National Key Research and Development Plan (No. 2019YFC0214303), Central Public-Interest Scientific Institution Basal Research Fund (No. PM-zx703-202002-015), and the National Natural Science Foundation of China (No. 22076224).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.12.040.
| [1] |
L.P. Han, S.X. Cai, M. Gao, et al., Chem. Rev. 119 (2019) 10916-10976. DOI:10.1021/acs.chemrev.9b00202 |
| [2] |
C. Liu, J.W. Shi, C. Gao, et al., Appl. Catal. A 522 (2016) 54-69. DOI:10.1016/j.apcata.2016.04.023 |
| [3] |
C. He, J. Cheng, X. Zhang, et al., Chem. Rev. 119 (2019) 4471-4568. DOI:10.1021/acs.chemrev.8b00408 |
| [4] |
Y. Geng, W.P. Shan, F.D. Liu, et al., J. Hazard. Mater.. |
| [5] |
G.S. Qi, R.T. Yang, Chem. Commun. (2003) 848-849. DOI:10.1039/b212725c |
| [6] |
J. Chen, X. Chen, W.J. Xu, et al., Chem. Eng. J. 330 (2017) 281-293. DOI:10.1016/j.cej.2017.07.147 |
| [7] |
J. Chen, X. Chen, X. Chen, et al., Appl. Catal. B 224 (2018) 825-835. DOI:10.1016/j.apcatb.2017.11.036 |
| [8] |
J.H. Li, J.J. Chen, K. Rui, et al., Catal. Commun. 8 (2007) 1896-1900. DOI:10.1016/j.catcom.2007.03.007 |
| [9] |
F. Kapteijn, L. Singoredjo, M. Vandriel, et al., J. Catal. 150 (1994) 105-116. DOI:10.1006/jcat.1994.1326 |
| [10] |
H. Huang, H. Hu, J.J. Zhang, et al., Environ. Res. 188 (2020) 109802. DOI:10.1016/j.envres.2020.109802 |
| [11] |
J.M. Chen, C.L. Li, Z. Ristovski, et al., Sci. Total Environ. 579 (2017) 1000-1034. |
| [12] |
M.L. Wang, S.Y. Li, R.C. Zhu, et al., Atmos. Environ. 223 (2020) 117294. DOI:10.1016/j.atmosenv.2020.117294 |
| [13] |
Q. Zhao, Q.L. Liu, J.F. Han, et al., J. Chem. Technol. Biotechnol. 94 (2019) 3753-3762. DOI:10.1002/jctb.5868 |
| [14] |
L.M. Ye, P. Lu, X.B. Chen, et al., Appl. Catal. B 277 (2020) 119257. DOI:10.1016/j.apcatb.2020.119257 |
| [15] |
L.N. Gan, K.Z. Li, S.C. Xiong, et al., Catal. Commun. 117 (2018) 1-4. DOI:10.23919/acess.2018.8669260 |
| [16] |
J. Chen, X. Chen, D.X. Yan, et al., Appl. Catal. B 250 (2019) 396-407. DOI:10.1016/j.apcatb.2019.03.042 |
| [17] |
Q. Shen, L.Y. Zhang, N.N. Sun, et al., Chem. Eng. J. 322 (2017) 46-55. DOI:10.1016/j.cej.2017.02.148 |
| [18] |
J. Chen, X. Chen, Z. Xu, et al., ChemistrySelect 1 (2016) 4052-4056. DOI:10.1002/slct.201600921 |
| [19] |
X.J. Yao, K.L. Ma, W.X. Zou, et al., Chinese J. Catal. 38 (2017) 146-159. DOI:10.1016/S1872-2067(16)62572-X |
| [20] |
P.F. Zhang, H.F. Lu, Y. Zhou, et al., Nat. Commun. 6 (2015) 8446. DOI:10.1038/ncomms9446 |
| [21] |
W.L. Cen, Y. Liu, Z.B. Wu, et al., Phys. Chem. Chem. Phys. 14 (2012) 5769-5777. DOI:10.1039/c2cp00061j |
| [22] |
K. Qi, J.L. Xie, Z. Zhang, et al., Powder Technol. 338 (2018) 774-782. DOI:10.1016/j.powtec.2018.07.073 |
| [23] |
D.S. Zhang, L. Zhang, L.Y. Shi, et al., Nanoscale 5 (2013) 1127-1136. DOI:10.1039/c2nr33006g |
| [24] |
Z.B. Wu, R.B. Jin, Y. Liu, et al., Catal. Commun. 9 (2008) 2217-2220. DOI:10.1016/j.catcom.2008.05.001 |
| [25] |
R.B. Jin, Y. Liu, Z.B. Wu, et al., Chemosphere 78 (2010) 1160-1166. DOI:10.1016/j.chemosphere.2009.11.049 |
| [26] |
L. Chmielarz, P. Kuśtrowski, M. Zbroja, et al., Appl. Catal. B 53 (2004) 47-61. DOI:10.1016/j.apcatb.2004.04.019 |
| [27] |
M. Iwasaki, H. Shinjoh, Appl. Catal. A. 390 (2010) 71-77. DOI:10.1016/j.apcata.2010.09.034 |
 2021, Vol. 32
2021, Vol. 32 


