b Shandong Cancer Hospital and Institute, Shandong First Medical University & Shandong Academy of Medical Sciences, Ji'nan 250117, China
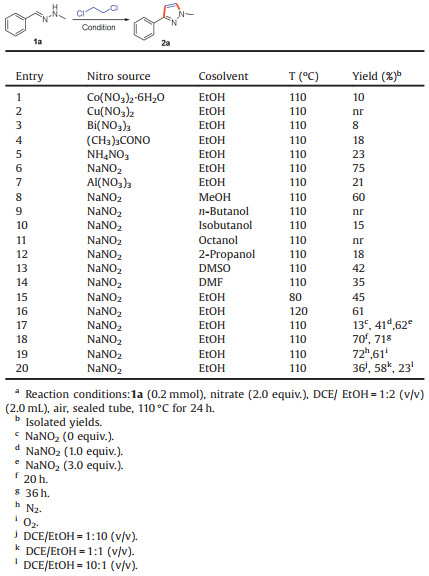
1H-Pyrazoles are highly valuable compounds and not only are prevalent in pharmaceuticals such as anthelmintic activity [1], 5-HT2C receptor agonists [2], modulators of the M1 muscarinic acetylcholine receptor [3], and inhibition of trypanosoma brucei and trypanosoma cruzi [4], monoamine oxidase-A inhibitors [5] but also can serve for catalytic materials [6] as ligands (Fig. 1). In view of their great importance, development an efficient method to formation of 1H-pyrazoles is attracting more attention in the past decades [7].
|
Download:
|
| Fig. 1. Representative compounds of 1H-pyrazoles. | |
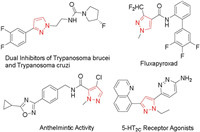
Hydrazones possess an excellent reactivity for cyclization reaction. Comas-barcel's group [8] has reported the synthesis of pyrazole compounds from alkynes and sydnones catalyzed by Cu(OAc)2·H2O with excellent yield (Scheme 1a). Meanwhile, pyrazole compounds synthesized by ethane-1, 2-diol derivatives react with (E)-1-benzylidene-2-phenylhydrazine compounds under the catalysis of FeCl3 have been reported by Panda's group [9] (Scheme 1b). In the past few decades, pyrazole derivatives prepared by transition metals has obtained a remarkable achievement. However, the catalysis of transition metals may hinder their practical application in chemical synthesis. Therefore, developing a new type of mild, metal-free catalytic reaction is challenging and indispensable. Herein, inspired by these works and continuing efforts in our previous work [10, 11], we design and synthesize an metal-free annulation onto (E)-1-benzylidene-2-methylhydrazine, which was achieved by cynalization and aromatization of 1, 2-dichloroethane with NaNO2 catalysis (Scheme 1c). This method provides a simple and convenient way for the construction of double C–C/C–N bonds and reduces the generation of by-products in the reaction process.
|
Download:
|
| Scheme 1. Reaction from hydrazones. | |
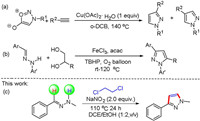
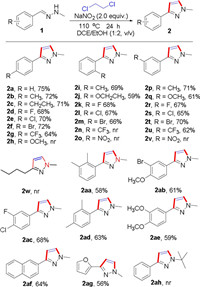
(E)-1-Benzylidene-2-methylhydrazine (1a) was chosen as the model substrates for the optimization of the reaction conditions, which include the nitro source, cosolvent, amount of raw materials, temperature and atmosphere. The optimization results showed that the reaction is very sensitive to nitro source effects (Table 1, entries 1−7), and the best result was obtained by using NaNO2 as nitro source (Table 1, entry 6). Next, cosolvents were screened, EtOH gave a better yield than other cosolvents including MeOH, n-butanol, isobutanol, octanol, 2-propanol, DMSO and DMF (Table 1, entries 8−14). Considering the influence of the reaction temperature, we observed that the yield was slightly decreased when decreasing to 80 ℃ or increasing to 120 ℃ (Table 1, entries 15 and 16). As nitrate can improve the reaction and the NaNO2 evidently facilitate the transformation, different amount of the NaNO2 were examined (Table 1, entry 17). Further investigations on the reaction did not improve the yield of product (Table 1, entries 18–20). With the optimized conditions in hand, we investigated the substrate scope of this reaction, various substituents on the benzene ring of the aromatic aldehyde were examined and the results are summarized in Scheme 2. Substrates with para substituents such as 4-Me(2b), 4-CH2CH3 (2c), 4-F (2d), 4-Cl (2e), 4-Br (2f), 4-CF3 (2g) afforded the corresponding products in good yields. Furthermore ortho-substituents bearing electron donating (2i and 2j) and halogen substituents (2k–2m) were well tolerated, but withdrawing character (2n and 2o) could not be converted into corresponding products. Meta substituents with diverse electronics also worked well, with electron-donating groups (2p and 2q), halogens (2r–2t) and electron-withdrawing groups (2u) all providing the pyrazole in good yields. What we regret is that the target compound cannot be obtained when using alkyl aldehydes (2w). Similarly, many disubstituted compounds such as 2aa-2af also afforded the corresponding products in synthetically useful yields (58%–68%). In addition, 2-furaldehyde (2ag) gave a 56% yield. Unfortunately, the methodology failed to produce any products when bearing 2-OCH3 (2h) and 3-NO2 (2v) group. t-Butylhydrazine replaced of methylhydrazine, we failed to produce the requisite pyrazole. Unfortunately, under standard conditions, when 1, 2-dichloropropane was used instead of 1, 2-dichloroethane, we did not get the expected compound.
|
|
Table 1 Optimization of reaction conditions.a |
|
Download:
|
| Scheme 2. Scope of various substituents of hydrazones 1. Reaction conditions: 1 (0.2 mmol), NaNO2 (2.0 equiv.) and DCE/ EtOH = 1:2 (v/v) (2.0 mL), air, sealed tube, 110 ℃ for 24 h. Isolated yields. | |
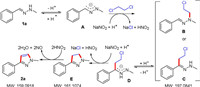
To study the mechanism of cyclization reaction, some control experiments were conducted. Firstly, it was known that no target product (2a) was produced in the absence of NaNO2 (Scheme 3a). Similarly, we have not detected 1a, 3 and 4 when using many bases such as K2CO3, Na2CO3, CsCO3 and NaOH replace NaNO2 (Scheme 3b). These results indicate that NaNO2 are crucial for facilitating this reaction. Secondly, we obtained successfully the intermediate (H) about 67% yield (Scheme 3c) or desired product 2a (Scheme 3d) about 15% yield from (Z)-1-(3-chloro-1-phenylpropylidene)-2-methylhydrazine 4 as starting material when reaction temperature is 20 ℃ or 110 ℃. When compound 4 was subjected to NaNO2 under the standard reaction conditions, the desired product 2a was formed about 85% yield (Scheme 3e). Thus, the last step of this tandem reaction is successfully promoted by NaNO2. Further investigations found that (Z)-1-(2-chloroethyl)-1-methyl-2-(1-phenylethylidene)hydrazine 6 and (Z)-3-chloro-1-phenyl-N-(piperidin-1-yl)propan-1-imine 8 were not obtained under the standard conditions respectively, by using (Z)-1-methyl-2-(1-phenylethylidene)hydrazine 5 and (Z)-N-morpholino-1-phenylmethanimine 7 as starting material (Schemes 3f and g). But, when the radical scavenger TEMPO (3.0 equiv.) was added under standard conditions, the target compound 2a was obtained in 68% yield. This suggested that it may not be a free radical mechanism (Scheme 3h). Excitingly, we have detected of intermediate (B), (C), (E) and 2a (Scheme 4) from reaction mixture about 10 min after the start of the reaction by HPLC-MS (Supporting information).

|
Download:
|
| Scheme 3. Mechanistic studies. | |
|
Download:
|
| Scheme 4. Postulated reaction mechanism. | |
On the basis of the results of our group and others [5, 7], a plausible mechanism is proposed (Scheme 4). Firstly, 1a released a hydrogen ion to generate intermediate (A), which react with 1, 2-dichloroethane to generate intermediate (B) and intermediate (C) under the participation of NaNO2. Secondly, intermediate (D) was generated from intermediate (C) released a hydrogen ion. After this, a cyclization of intermediate (D) can occur to give the intermediate (E). Finally, the target product 2a is obtained by oxidation of intermediate (E).
In summary, we have developed a method of sodium nitrite promoted dual C–N, C–C coupling reaction using hydrazones and 1, 2-dichloroethane as starting materials under mild conditions providing 1H-pyrazoles as products. In these processes, 1, 2-dichloroethane serve as alkylation reagent in good to excellent yields. Moreover, this protocol would be anticipated to construct diversified 1H-pyrazoles for the screening of the potential pharmaceuticals in future.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThe authors gratefully acknowledge financial support for this research from Shandong Provincial Natural Science Foundation (No. ZR2017LB006) and Academic promotion programme of Shandong First Medical University (No. 2019LJ003).
| [1] |
T.G. Le, A. Kundu, A. Ghoshal, et al., J. Med. Chem. 62 (2019) 3367-3380. DOI:10.1021/acs.jmedchem.8b01790 |
| [2] |
J. Carpenter, Y. Wang, G. Wu, et al., J. Med. Chem. 60 (2017) 6166-6190. DOI:10.1021/acs.jmedchem.7b00385 |
| [3] |
J.C.C. Dallagnol, E. Khajehali, E.T. van Der Westhuizen, et al., J. Med. Chem. 61 (2018) 2875-2894. DOI:10.1021/acs.jmedchem.7b01812 |
| [4] |
S. Varghese, R. Rahmani, S. Russell, et al., ACS Med. Chem. Lett. 11 (2020) 278-285. DOI:10.1021/acsmedchemlett.9b00218 |
| [5] |
F. Chimenti, A. Bolasco, F. Manna, et al., Chem. Biol. Drug Des. 67 (2006) 206-214. DOI:10.1111/j.1747-0285.2006.00367.x |
| [6] |
L.L. De Oliveira, R.R. Campedelli, M.C.A. Kuhn, J.F. Carpentier, O.L. Casagrande, J. Mol. Catal. A Chem. 288 (2008) 58-62. DOI:10.1016/j.molcata.2008.03.020 |
| [7] |
(a) S. Peruncheralathan, T.A. Khan, H. Ila, H. Junjappa, J. Org. Chem. 70 (2005) 10030-10035; (b) C.M. Thompson, J.L. Poole, J.L. Cross, I. Akritopoulou-Zanze, S.W. Djuric, Molecules 16 (2011) 9161-9177; (c) G.W. Zhang, Y. Zhao, H.B. Ge, Angew. Chem. Int. Ed. 52 (2013) 2559-2563; (d) F. Punner, Y. Sohtome, M. Sodeoka, Chem. Commun. (Camb. ) 52 (2016) 14093-14096; (e) J. Chen, R. Properzi, D.P. Uccello, et al., Org. Lett. 16 (2014) 4146-4149; (f) J.D. Kirkham, S.J. Edeson, S. Stokes, J.P.A. Harrity, Org. Lett. 14 (2012) 5354-5357; (g) J. Schranck, X.F. Wu, H. Neumann, M. Beller, Chem. Eur. J. 18 (2012) 4827-4831. |
| [8] |
J. Comas-Barcel, R.S. Foster, B. Fiser, E. Gomez-Bengoa, J.P.A. Harrity, Chem. Eur. J. 20 (2014) 1-8. DOI:10.1002/chin.201527170 |
| [9] |
N. Panda, A.K. Jena, J. Org. Chem. 77 (2012) 9401-9406. DOI:10.1021/jo301770k |
| [10] |
G.D. Wang, J. Sun, K. Wang, et al., Org. Chem. Front. 6 (2019) 1608-1612. DOI:10.1039/c9qo00367c |
| [11] |
L.Q. Hao, G.D. Wang, J. Sun, et al., Adv. Synth. Catal. 362 (2020) 1657-1662. DOI:10.1002/adsc.201901563 |
 2021, Vol. 32
2021, Vol. 32