5-HMF is a significant platform compound from cellulose degradation and an essential raw material for industry. 5-HMF and its derivatives are widely used in fine chemical industry, medicine, degradable plastics, energy and other scopes [1]. Thus, developing various catalysts for cellulose hydrolysis [2-6] has always been a broad research subject. Lai et al. reported that one-pot production from cellulose was achieved with an excellent yield of 5-HMF over stable heteropoly acid catalysis ChH4CeW12O40 at 140 ℃ for 6 h, and other carbohydrates were converted efficiently, including fructose, glucose, sucrose, cellobiose and starch in the H2O/DMSO/MIBK biphasic system [5]. But the reaction time was too long to gain ideal production. Besides, N. Sweygers et al. discovered that a maximum HMF yield of 33.65 ± 0.46 wt% (or 43.27 mol%) and a maximal furfural yield of 33.30 ± 1.30 wt% (or 45.79 mol%) could be achieved in aqueous phase and methyl isobutyl ketone (MIBK) phase catalyzed by hydrochloric acid [7]. However, the use of hydrochloric acid as the main catalyst would corrode equipment and the entire reaction needed to be carried out under microwave heating conditions. In addition, S. Xu et al. reported that using the chromium phosphate (CrPO4) with Lewis and Brønsted acidity as a catalyst, the production of furfural from xylose and wheat straw in the water/tetrahydrofuran (THF) biphasic system gained excellent yield. Meanwhile, 67% yield of furfural and 32% yield of 5-HMF were achieved from wheat straw at 180 ℃ for 90 min [8]. Halogenated compounds [9-12] who possessed mild reaction conditions, environmental pollution-free, low toxicity, controllability and inexpensive price were discovered to solve above weaknesses of other catalysts. RuCl3 was found to be the most effcient on pursuing high output of 5-HMF in NaCl-aqueous/butanol biphasic system [10]. Subsequently, B. Saha et al. found that Zr(O)Cl2/CrCl3 mixed catalysts were much more effective for the conversion of sugarcane bagasse to HMF and 5-ethoxymethyl-2-furfural (EMF) than single metal chloride [11].
However, due to the instability of the five-membered heterocyclic ring of 5-HMF, metal chlorides may lead to the further rehydration reaction to generate levulinic acid and char so that the yield of 5-HMF is subsided a lot. The emergence of the two-phase system copes with this problem. It was reported that the most HMF yield of 39.7% was gained in 2 h at 200 ℃ under the NaCl-H2O/THF catalytic system with InCl3 [12]. Other biphasic systems [7, 9, 10] have been selected to achieve high yield of 5-HMF, furan derivatives and other platform chemicals. In the bi-phase system, the aqueous phase underwent the process that transformed cellulose or lignocellulose into high value-added products such as monosaccharide, 5-HMF and furan derivatives [13-16]. The organic phase is used to complete the fractional extraction of value-added products, preventing the aqueous phase reaction from continuing, which avoids the extent of generating of byproducts. With regard of the selecting of the organic phase and the reaction phase, the basic regulations are mentioned as follows: (1) using an aprotic solvent with similar polarity to the product as organic phase, for example DMSO; (2) providing a suitable solvent that dissolves cellulose completely, for instance, high concentration NaCl solution.
Our work aimed to find the optimal conditions to gain the highest concentration of 5-HMF and other furan derivatives in various biphasic systems with FeCl3-CuCl2 mixed catalysts, and explore cellulose depolymerization kinetics. We specifically carried out the following investigation: (1) exploring the effects of temperature, time, volume ratio of extraction solvent to reaction solvent on the target product; (2) calculating the activation energy and the reaction rate constant of biphasic system with optimal catalyst; (3) proposing the probable kinetic reaction mechanism.
Above all, 0.1 g α-cellulose and combined catalysts were dissolved in high concentration NaCl aqueous phase with trace 12mol/L hydrochloric acid. Then one of 1-butanol, THF, DCM was regarded as the extraction phase in each run. Next, the above materials were introduced into the reaction kettle at a preset volume ratio (R) of extraction solvent to reaction solution. Subsequently, the reactor was heated to a specified temperature range of 443−503 K under stirring conditions at 500 rpm, and then the temperature was raised to a specified temperature for a certain period of time. During this process, experiments have been completed in a high-temperature, high-pressure and corrosion-resistant batch reactor. Condensate water was always passed through the reactor to maintain a specific temperature. After the reaction, the reactor was quenched by circulating cooling water.
For the analyses of products, the conversion rate of reactants and yield of target product are calculated by the following equation as shown in Table 1.
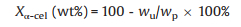
|
(1) |

|
(2) |
|
|
Table 1 Catalytic performance under various catalysts and solvents. |
Among them, Xα-cel refers to the conversion rate of cellulose. Ytp refers to the yield of target products. wu is the mass of unreacted cellulose. wp is the mass of added cellulose. wt is the target product quality.
From Table 1, we found the reaction with combined FeCl3 and CuCl2 (mg/mg=1:3, c =0.02 mol/L) gained the maximum yield of 5-HMF (49.13 wt%). Compared with single transition metal chloride salt, bimetal metal salts devoted to higher yield of target products. CuCl2 with the same substance and solvent could only obtain trace output, and FeCl3 with the same substance concentration could only obtain 6.31 wt% yield in their best experiment conditions. (190 ℃, 60 min, 2 MPa, NaCl/butanol). Besides, the two-phase reaction system tended to much higher yield. If using a single solvent and same catalysts, for instance, n-butanol, THF, DCM, the yield of 5-HMF was found to be only 4.22 wt%, 4.15 wt%, trace respectively. The results showed a single solvent had not been achieved the desired effect yet. Too much char and solvent coupling occurred in single solvent. However, in bi-phasic system, high concentration NaCl saturated solution as reaction phase dissolved more α-cellulose based on principle of salting out. THF, n-butanol and DCM as extraction phase were aprotic dipolar solvents to extract target chemicals in time. Therefore, conversion rate of single solvents was lower than two-phase system.
It could be seen from Fig. 1 that within the selected time range, when the temperature and time improved, the output of HMF increased sharply high and then decreased in general. Multiple two-phase systems had different optimal temperatures and time. In detail, the NaCl/n-butanol system obtained the highest yield of 49.13 wt% at 190 ℃ for 45 min. Meanwhile, the NaCl/THF, NaCl/DCM systems achieved the best yield 23.15 wt%, 18.92 wt% respectively at 210 ℃ for 45 min. In addition, furan derivatives gradually increased with ascendant temperature, reaching the highest value of 24 wt% in NaCl/THF system under 250 ℃ lasting for 45 min. It could be speculated that the higher the temperature rose, the more favorable it was for the formation of furan derivatives from Fig. 1d. It could be seen from Fig. 1c that the prolongation of time also had a certain influence on the selectivity of furan derivatives. Theoretically speaking, the prolongation of time and the rising of temperature would continue to increase the production of furan derivatives. However, further increasing the experimental temperature found that the content of char mounted sharply, the conversion rate dropped and the 5-HMF content reduced a lot, which was not conducive to the overall economic view of conversion.

|
Download:
|
| Fig. 1. Effect of time and temperature of the yield of (a, b) 5-HMF and (c, d) furan derivatives. | |
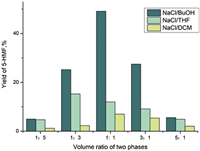
Moreover, the ratio of the organic phase to the reaction phase also had a critical influence on the reaction (Fig. 2). R was short for the ratio of the volume of organic phase and the reaction phase. Based on the effect of the volume of the reactor and the distribution coefficient, the R was adjusted to include 1:5, 1:3, 1:1, 3:1 and 5:1 (The volume of the container is 30 mL). when R was 1, the three systems could achieve the best yield except for the NaCl/THF system. Affected by the partition coefficient, the organic phase was an aprotic polar solvent with a polarity similar to that of the product, which had an outstanding extraction effect. As R improved, the extraction efficiency rose firstly and then decreased. This phenomenon occurred because the extraction efficiency increased as the extraction volume ascended. But the ratio of the reaction phase reduced, the content of dissolved cellulose lowered as the conversion rate dropped. Ultimately, the output lowered.
|
Download:
|
| Fig. 2. Effect of ratio of the organic phase and the reaction phase on the yield of 5-HMF. | |
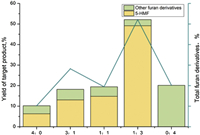
When the unit concentration was 0.005 mol/L, the catalyst was chosen in 5 ratios for comparison which included 4:0, 3:1, 1:1, 1:3, 0:4. The concentration of combined catalysts in the solvent was around 0.02 mol/L. Fe3+ and Cu2+ were hydrolyzed in the solution, which resulted in the addition of trace amount of hydrochloric acid. It could be seen that the proportion of Fe3+ rose up, 5-HMF presented a trend of sharp growth to the maximum in c(Fe3+)/c(Cu2+)=1:3. However, the output of furan derivatives was on the contrary. When no Fe3+ only 0.02 mol/L Cu2+ played a catalytic role in the aqueous phase, the yield of furan derivatives rose rapidly, and a very small amount of 5-HMF was detected. The results confirmed that the selectivity of the products was related to the ratio of the two ions. Within a certain concentration range, Fe3+ was added a bit, which might promote the production of 5-HMF and inhibit the production of other furan derivatives. Cu2+ had the opposite effect. As shown in Fig. 3, when the procedure was performed in NaCl/butanol at 190 ℃ for 45 min, mixed catalysts (c(Fe3+) /c(Cu2+)=1:3) could transform cellulose into the maximum output of 5-HMF (49.13 wt%) with 2.98 wt% furan derivatives, the maximum output of high value-added chemicals is 52.11%, compared with the other four kinds of total output 10.21 wt%, 28.2 wt%, 19.45 wt%, 20.16 wt%, respectively.
|
Download:
|
| Fig. 3. Effect of various concentration ratio of mixed catalysts on yields of 5-HMF in NaCl/butanol. | |
As reported in the literature, the radius of Fe3+ was small and hydration was strong, which could further improve the concentration of target products in the aqueous phase, and increase the concentration difference between the two phases, which was convenient for extraction of organic phase. However, excessive Fe3+ could not improve the selectivity of HMF, which could explain the optimal catalysts with a small proportion of mixed catalysts. It had been reported that Cu2+ improved the selectivity of furfural and HMF in specific ionic liquids, which might be related to the formation of [CuCl4]2− [17] complex in high concentration of sodium chloride, and the complex participated in cellulose. Based on experimental phenomena and literature, we explored the reaction path of cellulose in the NaCl/butanol system and verified it by XRD, FTIR, Raman and UV–vis spectra.
It could be found in the UV-spectra (Fig. 4a) that the characteristic peaks of CuCl2 and FeCl3 at approximately 240 nm and 330 nm respectively disappeared after reaction, which might result from n→σ* transition of the complex [18, 19]. The peak of the dissolved product in the water phase appeared on the left side of the characteristic peak. The absence of 280 nm belonging to 5-HMF indicated the high efficiency extraction of the two-phase system [11]. Additionally, the complex ion [CuCl4]2− could be detected by UV–vis and Raman. Appearance of two strong peaks 287 nm, 400 nm proved the formation of the complex in UV–vis spectrum (Fig. 4b) [20]. In the XRD spectrum (Fig. 5a), the peak at about 14°–16° 2θ represented the characteristic plane (101), which was original from the sub ordered part of samples. The characteristic at 22° 2θ of the crystalline plane (002) could be attributed to the highly crystalline phase of cellulose. The crystallinity plane (040) was the characteristic of low crystallinity. After reaction, the intensity of crystalline plane decreased greatly, which could be confirmed that the distance between cellulose chains increased obviously due to the formation of hydrogen bond in between cellulose chain and [CuCl4]2−, which resulted in the loss of crystallization order [21]. In FT-IR (Fig. 5b), the most intense signal between 1030 cm-1 and 1060 cm-1 that corresponded to stretching of C-O glycosidic bond became inconspicuous as the reaction temperature increased. And the absorption peak at 1165 cm-1 which was the stretching vibrations of C–O–C (1160 cm-1) disappeared [20]. The results showed that oligosaccharides had undergone further reactions catalyzed by [CuCl4]2−. Besides, the Cu-Cl vibration presented under 350 cm-1 to confirm the existence of the [CuCl4]2− in Raman as well (Fig. 5c) [20, 22].

|
Download:
|
| Fig. 4. (a) The variation of UV–vis between mixed catalysts solution and aqueous phase after reaction. (b) The UV–vis of [CuCl4]2− from water phase after reaction (190 ℃, 45 min, FeCl3-CuCl2 (mg/mg=1:3), NaCl/bunatol). | |

|
Download:
|
| Fig. 5. (a) The difference of XRD between raw cellulose and char. (b) The FTIR of char. (c) The Raman of [CuCl4]2− from water phase after reaction (190 ℃, 45 min, FeCl3-CuCl2 (mg/mg=1:3), NaCl/bunatol). | |
Based on the experiments, reaction rate constant of catalyst at diverse temperatures were calculated according to the first-order reaction as shown in Eq. 3. Where C0 represented initial cellulose concentration (g/L), C was symbol of content of cellulose (g/L) at time t, k was short for rate constant (h−1). Apparent activation energy of reaction with specific catalyst was defined through Arrhenius equation (Eq. 4). In the equation, k was rate constant at specific temperature T, T was reaction temperature (K), Ea was apparent activation energy of reaction (J/mol of cellulose), and R was universal gas constant (8.31446J K−1 mol−1). To determine the value of Ea, the logarithm of both sides of the Arrhenius equation was taken in Eq. 5. Then the function image of lnk pair 1/T had been done. The slope was the value of -Ea/RT.
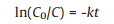
|
(3) |
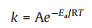
|
(4) |
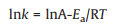
|
(5) |
The kinetic reaction was used to calculate the degradation of cellulose with mixed catalysts (Fig. 6). According to the results of linear fitting, a linear equation of 0−90 min in the range of 170−230 ℃ was completed. The value of k was derived from the slope of the linear equation. As the temperature rose, the slope that represented k ranged from 0.0122 min−1 at 170 ℃ to 0.0248 min−1 at 230 ℃, which indicated that the combined catalysts FeCl3-CuCl2 were greatly affected by temperature. The linear equation was y=0.0145x - 0.0545, R2 = 0.987 at 190 ℃ for 45 min. The reaction rate constant found was 0.0145 min−1. Then with the Arrhenius equation, the apparent activation energy of combined catalysts under various temperature was conducted. The linear equation was given, which was y = -2604.09x + 1.42578, R2 = 0.9495. The data gave the result, lnk = -2604.09/T + 1.4258, R2 = 0.9495. The apparent activation energy in the range of 170–230 ℃ was 21.65 kJ/mol, which was lower than the pyrolysis of cellulose used diluted acid as catalyst (170−180 kJ/mol) [23]. This also clarified that the acid catalyst could only break through the barrier of activation energy at high temperature. As if the reaction took place at the moderate temperature, high concentrations of acid would not be able to obtain better returns owing to the high apparent activation energy.

|
Download:
|
| Fig. 6. Kinetic analysis and reaction rate constants for the hydrolysis of α-cellulose by combined catalysts. (a) The first order reaction quasi-equation of mixed catalysts; (b) lnk vs. 1/T of mixed catalysts. | |
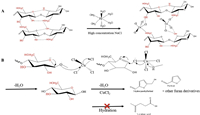
As shown in Fig. 7, we proposed mechanism of pyrolysis of cellulose in biphasic system with mixed catalysts FeCl3-CuCl2. Above all, the strong hydration of Fe3+ led to the coordination of water molecules in concentrated NaCl solution to generate stable complex [Fe(H2O)6]3+. In this process, Cu2+ reacted with high concentration Cl− to form a two-dimensional planar tetrahedral deformation structure of [CuCl4]2- that could be detected by UV–vis and Raman. It bound to the −OH group of the cellulose chain to break the previous hydrogen bond. Subsequently, the β-1−4 glycoside bond was destroyed to the complex by electron transfer to form monosaccharide. In the presence of Cu2+, monosaccharide was dehydrated to form 5-HMF and furan derivatives. The conjecture mentioned was consistent with the results of UV–vis, XRD and FTIR.
|
Download:
|
| Fig. 7. The probable process for hydrolysis of cellulose with FeCl3-CuCl2. | |
In conclusion, the mixed catalysts FeCl3-CuCl2 (mg/mg=1:3, 0.02 mol/L, 190 ℃, 45 min) were found to be high efficiency to generate maximum yield of 5-HMF (49.13 wt%) along with furan derivatives (2.98 wt%) from cellulose degradation in NaCl/n-butanol two-phase medium. During the reaction, char was avoid generating from the side reaction of 5-HMF in two-phase system that experienced a controllable selective formation of 5-HMF process. Metal chloride salts resulted in the fracture of hydrogen bond through binding −OH groups in the structure of cellulose. Cu2+ gave the decent dehydration ability to generate 5-HMF and furan derivatives. The apparent activation energy is 21.65 kJ/mol in the range of 170−230 ℃, which is far lower than that of inorganic acid catalysis. Therefore, the reaction had a faster reaction rate under moderate temperature.
Declaration of competing interestThe authors declared no conflict of interest.
AcknowledgmentsThis work was finally supported by the National Natural Science Foundation of China (No. 22078103), National Key Research and Development Project (No. SQ2019YFE011926), the Key Project of Science and Technology Commission of Shanghai Municipality (No. 18DZ1112700) and Shanghai Natural Science Fund (No. 18ZR1411100).
| [1] |
R. van Putten, J.C. van der Waal, E. de Jong, et al., Chem.Rev. 113 (2013) 1499-1597. DOI:10.1021/cr300182k |
| [2] |
A.S. Amarasekara, Chem. Rev. 116 (2016) 6133-6183. DOI:10.1021/acs.chemrev.5b00763 |
| [3] |
Y. Chen, G. Li, F. Yang, S. Zhang, Polym. Degrad. Stabil. 96 (2011) 863-869. DOI:10.1016/j.polymdegradstab.2011.02.007 |
| [4] |
Y. Zhang, W. Guan, H. Song, et al., Microporous Mesoporous Mater. 305 (2020) 110328. DOI:10.1016/j.micromeso.2020.110328 |
| [5] |
F. Lai, F. Yan, P. Wang, et al., Chem. Eng. J. 396 (2020) 125282. DOI:10.1016/j.cej.2020.125282 |
| [6] |
M. Wada, M. Ike, K. Tokuyasu, Polym. Degrad. Stabil. 95 (2010) 543-548. DOI:10.1016/j.polymdegradstab.2009.12.014 |
| [7] |
N. Sweygers, J. Harrer, R. Dewil, L. Appels, J. Clean. Prod. 187 (2018) 1014-1024. DOI:10.1016/j.jclepro.2018.03.204 |
| [8] |
S. Xu, D. Pan, Y. Wu, et al., Fuel Process. Technol. 175 (2018) 90-96. DOI:10.1016/j.fuproc.2018.04.005 |
| [9] |
T. Deng, X. Cui, Y. Qi, et al., Chem. Commun. (Camb.) 48 (2012) 5494. DOI:10.1039/c2cc00122e |
| [10] |
L. Yan, R. Ma, H. Wei, et al., Bioresour. Technol. Rep. 279 (2019) 84-91. DOI:10.1016/j.biortech.2019.01.120 |
| [11] |
S. Dutta, S. De, M.I. Alam, M.M. Abu-Omar, B. Saha, J. Catal. 288 (2012) 8-15.
|
| [12] |
Y. Shen, J. Sun, Y. Yi, et al., Bioresour. Technol. Rep. 172 (2014) 457-460. DOI:10.1016/j.biortech.2014.09.077 |
| [13] |
Y. Li, X. Cao, S. Sun, et al., Ind. Crop. Prod. 147 (2020) 112173. DOI:10.1016/j.indcrop.2020.112173 |
| [14] |
Z. Chen, S. Liao, L. Ge, et al., Chem. Eng. J. 379 (2020) 122284. DOI:10.1016/j.cej.2019.122284 |
| [15] |
B. Xue, Y. Yang, M. Zhu, Y. Sun, X. Li, Bioresour. Technol. Rep. 270 (2018) 55-61. DOI:10.1016/j.biortech.2018.09.028 |
| [16] |
Q. Zhang, Y. Deng, X. Tan, et al., Ind. Crop. Prod. 145 (2020) 112091. DOI:10.1016/j.indcrop.2020.112091 |
| [17] |
H. Zhao, J.E. Holladay, H. Brown, et al., Science 316 (2007) 1597-1600. DOI:10.1126/science.1141199 |
| [18] |
N. Zhang, D. Zeng, G. Hefter, Q. Chen, J. Mol. Liq. 198 (2014) 200-203. DOI:10.1016/j.molliq.2014.06.025 |
| [19] |
Y. Chen, Y. Zhou, R. Zhang, C. Hu, Mol. Catal. 484 (2020) 110729. |
| [20] |
I.B.H. Sadok, F. Hajlaoui, K. Karoui, et al., J. Mol. Struct. 1186 (2019) 118-126. DOI:10.1016/j.molstruc.2019.03.025 |
| [21] |
V. Flores-Velázquez, G.E. Córdova-Pérez, A.A. Silahua-Pavón, et al., Fuel 265 (2020) 116857. |
| [22] |
A. Kaiba, M.H. Geesi, P. Guionneau, et al., J. Mol. Struct. 1204 (2020) 127380. |
| [23] |
V.V. Bravo, M.P. Paez, M. Aoulad, A. Reyes, Enzyme Microb. Technol. 26 (2000) 614-620. DOI:10.1016/S0141-0229(00)00136-8 |
 2021, Vol. 32
2021, Vol. 32