Pesticide, brought agriculture to a new milestone, has kept a large variety of crops and products away from disease and insect pests, promoting the development of society to some extent [1]. Yet, in long-term pesticide abuse situations, the harmful substances in the environment have greatly increased, which have led to adverse consequences for the biosphere [2]. Most recently, nitenpyram (NTP) have been extensively used in agricultural industry by virtue of their high insecticidal activity, and wide spectrum [3]. However, the NTP is difficult to remove by water self-purification and simultaneously retain a high residue rate, which has an extremely detrimental effect on the ecological environment and human health [4, 5]. Concerning the serious pollution of NTP in actual water, many strategies, such as physical adsorption, electrocatalysis, and low temperature plasma treatment, have been adopted to address the problem [6-8]. Compared with those methods, photocatalysis has intrigued much attention due to its environmental benignity and high-efficiency [9, 10]. Currently, only a few literatures have reported photocatalytic degradation of neonicotinoid pesticides leveraging semiconductor materials, which only can use ultraviolet light that accounts for 5% of the sunlight [11-16]. To develop low energy pesticide removal technology, it is thus highly imperative to explore photocatalytic materials that can efficiently utilize visible light.
As a kind of metal-free polymer semiconductor, graphite carbon nitride (g-C3N4) is sought-after and considered to be a rather promising visible-light-responsive photocatalyst with a band gap of −2.7 eV [17-20]. Nevertheless, the challenging issue facing g-C3N4 is still low migration rate of charge carriers, which is restrict the practical application [21-23]. To overcome kinetic limitations and prevent the recombination of photogenerated electron-hole pairs, the construction of heterojunction shows great potential [24-26]. In traditional heterostructures, the photoinduced electrons migrate from more negative CB potential to another CB potential and holes from more positive VB potential migrate to another VB potential [27]. As a result, the holes and electrons are spatially separated, which greatly impedes their recombination [28]. However, the demerit is that the redox ability of photoexcited electrons and holes is weakened after charge transfer. In sharp contrast to traditional g-C3N4-based heterostructure, Z-scheme photocatalysts inherit the nature photosynthesis virtues, i.e., possessing the strong reducibility of g-C3N4, and the giant enhancement of photogenerated charge carriers separation efficiency at the same time [29, 30]. In recently, a large number of g-C3N4-based Z-scheme heterojunction have been successfully established to enhance photocatalytic activity [31]. For example, She et al. [32] reported that 2Dα-Fe2O3/g-C3N4 Z-scheme catalysts presented a high H2 evolution rate. Xia et al. [33] demonstrated that the Z-scheme g-C3N4/MnO2 photocatalysts exhibited greatly enhanced photocatalytic activities for phenol removal and dye degradation. To the best of our knowledge, the use of g-C3N4-based Z-scheme photocatalyst materials (especially materials with visible light activity) to remove neonicotinoids in water is still relatively rare, and the influence of water environmental factors on the activity is rarely mentioned. Additionally, the in-situ growth technology in the composites system can assist in forming tight contact interface, which is more favorable for the transmission of photoinduced carriers.
Bearing the aforementioned discussion in mind, it is rather more promising to explore the reasonable design and controllable preparation of efficient Z-scheme photocatalyst, and ulteriorly obtain in-depth study on NTP degradation performance and mechanism in the actual water body. In this work, Z-scheme WO3/g-C3N4 photocatalyst was synthesized by in situ calcination approach. The degradation of NTP was utilized to evaluate the photocatalytic performance of WO3/g-C3N4 under visible light irradiation. Compared with pure WO3 and g-C3N4, the prepared Z-scheme WO3/g-C3N4 composite photocatalyst has higher photocatalytic efficiency. The structure, morphology, optical and photoelectrochemical properties of the prepared Z-scheme composite photocatalyst was tested by various characterization methods, and its catalytic mechanism was further discussed.
Fig. 1a shows the overall synthetic process of WO3/g-C3N4 sample, the specific prepartion process is in the Supporting information. The morphology of samples was studied by transmission electron microscopy (TEM). As shown in Figs. 1b and c, the clear laminar structure is observed for WO3 sample. Fig. 1d depicts the clear and obvious lattice figure of WO3, and the lattice spacing for 0.374 nm is consistent with the (020) crystal plane of WO3. It can be observed from Figs. 1e and f that the flexible nanosheet with large transverse size is very thick, which is a typical feature of g-C3N4 nanosheets. Visually, the WO3 has grown successfully on the surface of g-C3N4 nanosheets. Moreover, there is a clear tight contact interface between WO3 and g-C3N4 in Fig. 1g. This interface structure is very conducive to charge carriers transmission between WO3 and g-C3N4. In addition, the corresponding Transmission Electron Microscope (TEM) elemental mapping analysis was employed to indicate the composition and distribution of different components in the composite. As exhibited in Fig. 1h, C, N, O and W elements distribut homogeneous in the CW-3. Finally, the morphology of samples, in combination with XRD (Fig. S1 in Supporting information) and FT-IR (Fig. S2 in Supporting information) anlysis, collectively reveal the successful construction of WO3/g-C3N4 composites.

|
Download:
|
| Fig. 1. Schematic illustration of the synthesis of WO3/g-C3N4 nanocomposites (a), TEM images of WO3 (b-d) and CW-3 (e-g) and TEM elemental mapping images (h) of CW-3. | |
As shown in Fig. 2a, the degradation efficiency of g-C3N4 sample was up to 51% after 30 min irradiation, and WO3 had no degradation effect on NTP. There is no doubt that the recombination rate of photogenerated electron-hole pairs is very fast for pure semiconductor, so it exhibits relatively lower activity. The composite samples have shown enhanced photocatalytic degradation efficiency with low proportion of WO3, while the efficiency decreased at high proportion. It can be seen that the photocatalytic degradation efficiency could achieve 68% for CW-3. Ulteriorly, the kinetics reaction constant are obtained by a pseudo-first-order kinetics model as follows (Eq. 1):

|
(1) |
where C0 represents the initial concentration of NTP and C refers to the concentration at different irradiation times t, and k is the reaction rate constant [34]. An obvious volcano-type trend between different proportions samples and the reaction rate constant k is observed (Fig. 2b). The reaction rate constant k of CW-3 nanocomposite was 0.036 min−1, which was ~1.7 and ~25 times higher than those of g-C3N4 and WO3, respectively. Therefore, it is reasonable to believe that there is heterojunction interface between g-C3N4 and WO3, which contribute to the improvement of photocatalytic performance. Simultaneously, as presented in Figs. 2c and d, the capture experiment result indicates that h+ and ·O2− act as main active substances in the experiment. Given the complexity in the water, the different type of water and the inorganic salts were employed to figure out the effect for the photocatalytic degradation of NTP from simulation experiment. It is clearly observed in Fig. S3 (Supporting information) that the different type of water and the inorganic salts have little effect on the removal of NTP, indicating that the CW-3 have good universality for NTP removal in actual water body. In addition, the pilot process and products analysis can contribute to understand the overall pathway for degradation of NTP, as shown in Fig. S5 (Supporting information).

|
Download:
|
| Fig. 2. The photocatalytic degradation performance of WO3/g-C3N4 composites with different content of WO3 (a); the reaction rate constant with different sample (b); capture experiment of CW-3 for NTP degradation (c) and the reaction rate constant with trapping agent (d). | |
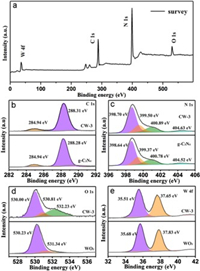
The X-ray photoelectron spectroscopy (XPS) analysis was used to elucidate surface composition and chemical states. From the Fig. 3a, the CW-3 hybrid showed all the expected elements, including C, N, O, and W. For the high-resolution C 1 s spectrum (Fig. 3b), the two peaks at binding energies of 284.94 eV and 288.28 eV could be attributed to C-C and N-C=N [35]. As shown in Fig. 3c, three peaks at 398.64 eV, 399.37 eV and 400.78 eV, can be attributed to C-N=C, N-(C)3 and C=N-H, respectively, and the small peak at about 404.52 eV is caused by π excitation [36]. For the O 1 s spectrum (Fig. 3d), the strong main peak at a binding energy of 530.23 eV belongs to the oxygen atom that forms the strong W-O bond, equal to the lattice O atoms of WO3, while the weak shoulder peak at 531.34 eV can be assigned to the terminal hydroxyl groups (OH−) on surface. Additionaly, from the O 1 s spectrum of the CW-3 heterostructure, the peaks at 530.81 and 532.23 eV are assigned to the oxygen atoms in g-C3N4, due to the oxidation effect during thermal condensation [37]. For the high-resolution W4f spectrum (Fig. 3e), the two peaks at binding energies of 35.68 eV and 37.83 eV could be attributed to W 4f7/2 and W 4f5/2 [38]. Interestingly, compared with the pure g-C3N4, the peaks of C 1 s and N 1 s in the CW-3 heterostructure move towards higher binding energy. On the contrary, it is noted that W 4f peaks in CW-3 heterostructure shift to lower binding energy in comparison with WO3. This demonstrates that WO3 have a strong chemical interaction with g-C3N4, in favor of improving separation of charge carriers, thus enhancement of photocatalytic degradation NTP performance [39].
|
Download:
|
| Fig. 3. The XPS survey spectra (a) and high-resolution C 1 s (b), N 1 s (c), O 1 s (d) and W 4f (e) spectra of CW-3. | |
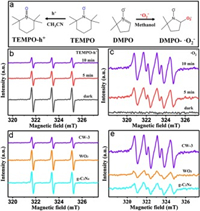
Electron spin resonance (ESR) experiments were performed to directly detect short-lived active species in the photocatalytic experiment [40]. As illustrated in Fig. 4a, it is very reliably to research the utility of 2, 2, 6, 6-tetramethylpiperidine-1-oxyl (TEMPO) to marks h+ and the employment of 5, 5-dimethyl-1-pyrroline N-oxide (DMPO) to determine ·O2− in-situ formed during photoexcitation of semiconductors. With the extension of illumination time, the signal intensity of TEMPO gradually decreased, which is ascribed to the oxidation of TEMPO to TEMPO + via h+ with strong oxidizing power. However, the increasing characteristic bands can be assigned to DMPO-·O2− adducts for DMPO. It is clearly shown in Figs. 4b and c that the obtained CW-3 samples can produce more photogenerated carriers with the extension of illumination time, which is important for the photocatalytic reaction. Compared with the CW-3, owing to the fast recombination of charge carriers, the h+ and ·O2− signals of pure sample are severely inhibited (Figs. 4d and e). In addition, Photoluminescence spectroscopy (PL) spectra (Fig. S4a in Supporting information), photocurrent (Fig. S4b in Supporting information) and electrochemical impedance spectra (EIS) plots (Fig. S4c in Supporting information) were preformed to verify that the CW-3 composite has lower electron-hole recombination rate and more efficient charge transfer. Obviously, the ESR experiments results further indicate that construction of WO3/g-C3N4 heterojunction by tight connection between WO3 and g-C3N4 is conducive to the separation of charge carriers, thereby enhancing the photocatalytic activity.
|
Download:
|
| Fig. 4. Spin-trapping mechanism of TEMPO and DMPO (a); ESR signal of TEMPO radical reacting with photogenerated holes in acetonitrile dispersion of CW-3 (b); ESR signal of DMPO-·O2― adducts in methanol dispersion over CW-3 (c); ESR signal of TEMPO radical (d) and DMPO-·O2― adducts of as-prepared different samples (e). | |
The above investigations included degradation activities, crystal structure, chemical environment, detailed morphological features, and charge carriers' transmission and separation. It is proposed that a heterojunction forms at the interface between g-C3N4 and WO3, where accelerated electron-hole separation and improved carriers charge transfer efficiency, were witnessed by these evidence. Based on the analysis of UV–vis DRS (Fig. S4d), both traditional heterojunction and Z-scheme mechanism may be involved for the constructed WO3/g-C3N4 heterojunction materials, as shown in Fig. 5. According to the above capture experimental results and ESR analysis, the possible Z-scheme WO3/g-C3N4 heterojunction degradation mechanism is suggested. In such Z-scheme heterojunction photocatalysts, photogenerated electrons accumulated on the CB position of WO3 are more likely to recombine with the holes from the VB position of g-C3N4. Simultaneously, positively charge holes that remained in the VB position of WO3 demonstrate strong oxidizing power toward photocatalytic degradation NTP, while the electrons kept on the CB position of g-C3N4 enable the occurrence of reduction reaction.

|
Download:
|
| Fig. 5. Scheme diagram of proposed possible photocatalytic mechanism of CW-3. | |
To sum up, Z-scheme WO3/g-C3N4 photocatalyst was synthesized by in-situ calcination approach. The hybrids show improvement of photocatalytic activity for the degradation NTP compared with the pure sample, and the CW-3 heterostructure possess the highest photocatalytic activity. The rate constant value for NTP degradation over the Z-scheme CW-3 heterojunction is 0.036 min−1, which is about 1.7 and 25 times higher than that of pure g-C3N4 and WO3, respectively. The excellent photocatalytic degradation activity mainly attributes to tight heterojunction interface in the CW-3 Z-scheme structure and fast transfer of photogenerated carriers. The capture experiment and ESR results demonstrate that h+ and ·O2− as main active species play the important roles in degradation of NTP. It may provide advanced insights into the construction of Z-scheme heterojunctions for effective pesticide removal in environment systems.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by the National Science Funds for Creative Research Groups of China (No. 51421006), National Natural Science Foundation of China (No. 51679063), the Key Program of National Natural Science Foundation of China (No. 91647206), the National key Plan for Research and Development of China (No. 2016YFC0502203), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (No. 51479064).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.12.002.
| [1] |
A.M.Chara-Serna, L.B. Epele, C.A. Morrissey, et al., Sci. TotalEnviron. 692 (2019) 1291-1303. |
| [2] |
M. Samy, M.G. Ibrahim, M.G. Alalm, et al., Chem. Eng. J. 395 (2020) 124974. DOI:10.1016/j.cej.2020.124974 |
| [3] |
M.L. Tang, Y.H. Ao, C. Wang, et al., Appl. Catal. B: Environ. 270 (2020) 118918. DOI:10.1016/j.apcatb.2020.118918 |
| [4] |
X. Liu, C.S. Li, B.J. Zhang, et al., Chemosphere 253 (2020) 126672. DOI:10.1016/j.chemosphere.2020.126672 |
| [5] |
T. Hirano, S. Minagawa, Y. Furusawa, et al., Toxicol Appl Pharm 383 (2019) 114777. DOI:10.1016/j.taap.2019.114777 |
| [6] |
F.Y. Suo, X.W. You, Y.Q. Ma, et al., Chemosphere 235 (2019) 918-925. DOI:10.1016/j.chemosphere.2019.06.158 |
| [7] |
Y.J. Sun, S.C. Zhu, W.Q. Sun, et al., J. Environ. Chem. Eng. 7 (2019) 103276. DOI:10.1016/j.jece.2019.103276 |
| [8] |
Y.L. Wang, L. Xu, H. Zhu, et al., Anal Methods-UK 11 (2019) 5421-5430. DOI:10.1039/C9AY01978B |
| [9] |
H.X. Zhang, Q.L. Hong, J. Li, et al., Angew. Chem. Int. Ed. 58 (2019) 11752-11756. DOI:10.1002/anie.201905869 |
| [10] |
P.Y. Kuang, P.X. Zheng, Z.Q. Liu, et al., Small 12 (2016) 6735-6744. DOI:10.1002/smll.201602870 |
| [11] |
R. Fiorenza, Di Mauro A., M. Cantarella, et al., Chem. Eng. J. 379 (2020) 122309. DOI:10.1016/j.cej.2019.122309 |
| [12] |
T.X. Xu, J.P. Wang, Y. Cong, et al., Chin. Chem. Lett. 31 (2020) 1022-1025. DOI:10.1016/j.cclet.2019.11.038 |
| [13] |
P.L. Ji, X.Z. Kong, J.G. Wang, et al., Chin. Chem. Lett. 23 (2012) 1399-1402. DOI:10.1016/j.cclet.2012.10.021 |
| [14] |
M. Wang, Y.X. Liu, D. Li, et al., Chin. Chem. Lett. 30 (2019) 985-988. DOI:10.1016/j.cclet.2019.01.017 |
| [15] |
M.B. Kralj, E.G. Dilcan, G. Salihoglu, et al., J. Anal. Chem. 74 (2019) 1371-1377. DOI:10.1134/S1061934819140077 |
| [16] |
Z.P. Yan, W.C. Wang, L.L. Du, et al., Appl. Catal. B: Environ. 275 (2020) 119151. DOI:10.1016/j.apcatb.2020.119151 |
| [17] |
X.H. Wu, D.D. Gao, P. Wang, et al., Carbon 153 (2019) 757-766. DOI:10.1016/j.carbon.2019.07.083 |
| [18] |
M.L. Tang, Y.H. Ao, C. Wang, et al., Appl. Catal. B: Environ. 268 (2020) 118395. DOI:10.1016/j.apcatb.2019.118395 |
| [19] |
K.L. He, J. Xie, Z.Q. Liu, et al., J. Mater. Chem. A 6 (2018) 13110-13122. DOI:10.1039/C8TA03048K |
| [20] |
X. Zhang, X.Z. Yuan, L.B. Jiang, et al., Chem. Eng. J. 390 (2020) 124475. DOI:10.1016/j.cej.2020.124475 |
| [21] |
X.H. Wu, H.Q. Ma, W. Zhong, et al., Appl. Catal. B: Environ. 271 (2020) 118899. DOI:10.1016/j.apcatb.2020.118899 |
| [22] |
Y.H. Wu, Q. Chen, S. Liu, et al., Chin. Chem. Lett. 30 (2019) 2186-2190. DOI:10.1016/j.cclet.2019.08.014 |
| [23] |
J.X. Huang, D.G. Li, R.B. Li, et al., J. Hazard. Mater. 386 (2020) 121634. DOI:10.1016/j.jhazmat.2019.121634 |
| [24] |
R.H. Mu, Y.H. Ao, T.F. Wu, et al., J. Hazard. Mater. 382 (2020) 121083. DOI:10.1016/j.jhazmat.2019.121083 |
| [25] |
A. Beyhaqi, Q.Y. Zeng, S. Chang, et al., Chemosphere 247 (2020) 125784. DOI:10.1016/j.chemosphere.2019.125784 |
| [26] |
M.X. Liang, Z.S. Zhang, R. Long, et al., Environ. Pollut. 259 (2020) 113770. DOI:10.1016/j.envpol.2019.113770 |
| [27] |
N.C. Zheng, T. Ouyang, Y.B. Chen, et al., Catal. Sci. Technol. 9 (2019) 1357-1364. DOI:10.1039/C8CY02206B |
| [28] |
J.X. Low, J.G. Yu, M. Jaroniec, et al., Adv. Mater. 29 (2017) 1601694. DOI:10.1002/adma.201601694 |
| [29] |
P. Zhou, J.G. Yu, M. Jaroniec, et al., Adv. Mater. 26 (2014) 4920-4935. DOI:10.1002/adma.201400288 |
| [30] |
Y.K. Sun, Q. Zhu, B. Bai, et al., Chem. Eng. J. 390 (2020) 124518. DOI:10.1016/j.cej.2020.124518 |
| [31] |
M. Ding, J.J. Zhou, H.C. Yang, et al., Chin. Chem. Lett. 31 (2020) 71-76. DOI:10.1016/j.cclet.2019.05.029 |
| [32] |
X.J. She, J.J. Wu, H. Xu, et al., Adv. Energy Mater. 7 (2017) 1700025. DOI:10.1002/aenm.201700025 |
| [33] |
P.F. Xia, B.C. Zhu, B. Cheng, et al., ACS Sustain. Chem. Eng. 6 (2018) 965-973. DOI:10.1021/acssuschemeng.7b03289 |
| [34] |
Y.H. Fu, W. Liang, J.Q. Guo, et al., Appl. Surf. Sci. 430 (2018) 234-242. DOI:10.1016/j.apsusc.2017.08.042 |
| [35] |
J.H. Ge, L. Zhang, J. Xu, et al., Chin. Chem. Lett. 31 (2020) 792-796. DOI:10.1016/j.cclet.2019.05.030 |
| [36] |
C.X. Zhao, Z.P. Chen, J.S. Xu, et al., Appl. Catal. B: Environ. 256 (2019) 117867. DOI:10.1016/j.apcatb.2019.117867 |
| [37] |
W.L. Yu, J.X. Chen, T.T. Shang, et al., Appl. Catal. B: Environ. 219 (2017) 693-704. DOI:10.1016/j.apcatb.2017.08.018 |
| [38] |
J.W. Fu, Q.L. Xu, J.X. Low, et al., Appl. Catal. B: Environ. 243 (2019) 556-565. DOI:10.1016/j.apcatb.2018.11.011 |
| [39] |
Q.L. Xu, B.C. Zhu, C.J. Jiang, et al., Sol. RRL. 2 (2018) 1800006. DOI:10.1002/solr.201800006 |
| [40] |
X.F. Yang, L. Tian, X.L. Zhao, et al., Appl. Catal. B: Environ. 244 (2019) 240-249. DOI:10.1016/j.apcatb.2018.11.056 |
 2021, Vol. 32
2021, Vol. 32 

