b Key Laboratory of Environment Correlative Dietology, Ministry of Education, College of Food Science and Technology, Huazhong Agricultural University, Wuhan 430071, China;
c School of Human and Social Sciences, University of West London, Middlesex TW89GA, United Kingdom
The enantioseparation of chiral compounds plays a vital role in many industries, especially the pharmaceutical industry [1-3]. Although the enantiomers have similar physical and chemical properties in nonchiral environments, their pharmacological characteristics and bioactivities are extremely different in chiral environments. Various methods have been used to realize enantioseparation, including high-performance liquid chromatography (HPLC) [4-9], gas chromatography (GC) [10-12] and capillary electrophoresis (CE) [13-16]. Among them, CE has been accepted as an attractive technique due to its low sample volume, high separation efficiency, and rapid analytical speed.
Capillary electrochromatography (CEC) integration with the high selectivity of HPLC and the high separation efficiency offered by CE, is deemed to be a powerful separation tool. Open tubular CEC (OT-CEC) has also been successfully used to separate enantiomers, featured by the stable stationary phase, easy preparation (no need for end frits and particle packing), no bubble formation and high reproducibility [17, 18].
The chiral stationary phase based on protein offers good capacity and enantioselectivity for a series of chiral compounds due to their multiple binding sites on the surface. Bovine serum albumin (BSA) has been investigated extensively in chiral separation [19-23]. For example, Liang et al. [19] constructed the OT-CEC microdevice based on polydopamine/graphene oxide coating adhering to BSA, which realized the enantioseparation of tryptophan, threonine, and dipeptides enantiomers. Kato et al. [23] developed a BSA-encapsulated monolithic column based on the sol-gel method to realize the enantioseparation of tryptophan, benzoin, eperisone and chlorpheniramine. Although these capillary columns showed good enantioseparation ability, the complicated preparation and limited stability restrained their wide application. Therefore, it is necessary to develop a new and simple method for BSA coating onto capillary columns.
In our previous work [13], the capillary inner wall was coated with phase-transitioned film of lysozyme to realize enantioseparation of three pairs of chiral compounds. The "phase transition" referred to the following process: when added with TCEP (tris(2-carboxyethyl)phosphine), the disulfide bond of lysozyme was broken, then the partial native configuration of lysozyme unfolded, resulting in the exposure of hydrophobic part; the unfolded lysozyme aggregated driven by hydrophobic force, which conformed from water-soluble state to hydrophobic state [24]. The film attachment was precisely immersion-guided, and mere solution-dipped area was covered by the film [25]. It provided a new way to prepare protein film-coated capillary columns. From Yang's group, BSA was also able to form a stable and dense adhesive film when tris(2-carboxyethyl) phosphine (TCEP) was added [26, 27], and the simultaneous high exposure of polar and nonpolar groups on the phase-transition BSA (PTB) surface strengthened the enantioseparation capacity of BSA. Herein, we developed a strategy for applying PTB film coating as stationary phase and chiral selector for enantioseparation in OT-CEC. Chiral drugs and monoamine neurotransmitters (Fig. S1 in Supporting information) were selected as the model analytes to testify the chiral separation capacity of PTB film coated capillary columns. As a result, the good resolution and high stability for eight pairs of enantiomers were realized.
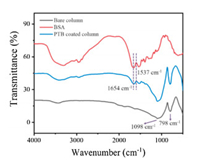
Firstly, the successful assembly of the supramolecular film on the inner wall of the capillary column was characterized. The FT-IR spectra in Fig. 1 verified the typical amino acid absorption bands [28] of symmetrical deformation vibration at 1537 cm−1 and asymmetrical deformation vibration at 1654 cm−1 in the PTB film-coated capillary. The bands of 1098 cm−1 and 798 cm−1 were assigned to the Si-O-Si absorption feature. These characteristic absorption bands indicated the existence of PTB film on the capillary. Fig. 2 presents the scanning electron microscopy (SEM) images of the bare and coated capillary columns. Compared to the smooth bare column (Fig. 2A), the inner wall of the coated column is rough in different enlargement sections (Fig. 2B–D), demonstrating that PTB film successfully adhered onto the capillary inner wall. Moreover, the amyloid-like PTB film could be dyed and assayed with Congo red (Fig. S2A in Supporting information) [24-27], but the bare column treated with BSA was obviously not dyed (Fig. S2B in Supporting information). This phenomenon further indicates that the capillary column was almost entirely coated with PTB film. According to Yang's report, the BSA conformation change occurred after breaking down its disulfide bond by TCEP to induce heterogeneous nucleation, and then the oligomers in the solution/solid interfaces would further propagate quickly to protofibrils, which subsequently aggregated and fused into nanofilm [29]. In our work, the assembly process of the supramolecular film on the inner wall of capillary column should be similar.
|
Download:
|
| Fig. 1. FT-IR spectra of the bare column (black line), BSA (red line) and PTB film modified smashing capillary (blue line). | |

|
Download:
|
| Fig. 2. SEM images of the bare capillary (D) and different enlargement sections of PTB film-coated capillary column. (A), (B) and (C) show the enlargement factor to be 10, 000, 10, 000 and 30, 000, respectively. | |
In OT-CEC, the electroosmotic flow (EOF) is closely related to the functional groups on the inner wall of the capillary column. That is, we can easily determine the change of the capillary inner surface by investigating the variation of EOF under different conditions. In this work, DMSO was used as the EOF marker for bare and coated columns. As shown in Fig. 3, the EOF values of both columns gradually decreased as the methanol content (0%–40%) increased and increased steadily with the increase of buffer pH (7.8–10). However, the EOF values of the PTB film-coated capillary were always smaller than those of the bare capillary column, demonstrating that the EOF of the coated column should mainly depend on the dissociation of BSA assembly. As is known, the closer the solution pH is to the isoelectric point (pI) of the protein, the less charge the protein carries. Although the experimental buffer pH was higher than the pI value (4.7) of BSA, the PTB coating only carried a few negative charges, as a result, the EOF of PTB coated capillary was reduced compared with that of the bare capillary. Furthermore, the EOF values sustainably decreased with the increase of BSA concentration (Fig. S3 in Supporting information). High concentration of BSA resulted in the acceleration of the assembly speed [27], so the activity sites of the PTB film in the capillary inner surface was increased, which led to the gradual decrease in EOF.

|
Download:
|
| Fig. 3. The electroosmotic flow (EOF) changes of bare capillary and PTB film coated capillary with the increase of CH3OH content (%, v/v) (A) and pH (B). CE conditions: (A) 10 mmol/L borate buffer (pH 8.0) with CH3OH content (0%–40%), (B) 10 mmol/L borate buffer (pH 7.8-10.0); EOF marker, DMSO (2%, v/v). | |
Next, the effects of preparation conditions of the coated capillary on the enantioseparation performance were examined using terbutaline enantiomers as the model chiral analytes. A series of PTB film-coated capillary columns with diverse BSA concentrations were prepared to study the effect of the BSA dosage. As illustrated in Fig. S4 (Supporting information), a good separation capacity could be obtained as the BSA concentration ranged from 2 mg/mL to 6 mg/mL, exhibiting BSA concentration-dependent enantioseparation. For 1 mg/mL BSA, the concentration was too low to separate enantiomers, however, as BSA concentration increased to 8 mg/mL, the interaction between PTL film and analytes increased, resulting in peak broadening and separation deterioration. Therefore, 2 mg/mL BSA was chosen in the experiment.
The assembly time is important for the formation of PTB film. To evaluate the effect of the assembly time on the enantioseparation, 2 mg/mL BSA coated capillary columns with different assembly times were investigated. As shown in Fig. S5A–D (Supporting information), the PTB film formed when the assembly time reached half an hour. From Fig. S6 (Supporting information), when the assembly time was more than 1 h, the baseline separation of terbutaline enantiomers was realized. When the assembly time was shorter than 1 h, the PTB film on the capillary inner wall was too thin to realize baseline separation of enantiomers. Thus, 1 h of assembly time was chosen.
To testify the effect of PTB film coating layers on enantioseparation, one to three layers of PTB film-coated capillary columns were prepared. In Figs. S5B, E and F (Supporting information), although the coating layers were different, the capillary inner walls were all coated with the PTB film. In Fig. S7 (Supporting information), the ability of enantioseparation was only slightly enhanced with the increase of coated layers. Therefore, one layer of PTB film was chosen.
Furthermore, the effects of CEC separation conditions including the type and pH of the buffer solution and the addition of the organic modifier on enantioseparation performance were investigated. The effect of pH was checked in the range from 8.0–10.5, and the resulting electropherograms are shown in Fig. S8A (Supporting information). As the pH value increased, the retention time of terbutaline enantiomers increased. The changing pH could affect the ionization degree of chiral analytes and the electrostatic interaction between analytes and PTB coating layer on the inner wall of capillary columns, resulting in the variance of separation efficiency. In this work, the optimum pH for terbutaline enantioseparation was 9.6. Fig. S8B (Supporting information) presents the effect of methanol content (v/v). The resolution of terbutaline enantiomers was greatly improved as the methanol content increased from 0% to 10%, and the migration time was also prolonged. The cause could be attributed to the enhancement of solvent elution ability and the reduction of EOF with the increase of methanol content. Although the resolution of terbutaline enantiomers was higher with the addition of methanol, the migration time was longer and the ratio of enantiomers deviated largely from 1:1. Thus, no addition of methanol was selected for terbutaline enantiomers in the further study.
The optimum separation conditions of the eight pairs of chiral analytes including terbutaline enantiomers are summarized in Table S1 (Supporting information). Obviously, for most enantiomers, the optimum pH values were close to their pKa values.
Finally, to investigate the chiral separation capacity of PTB film-coated capillary columns, eight pairs of enantiomers, including chiral amine drugs and monoamine neurotransmitters were tested. In Fig. 4, the coated capillary shows excellent enantioseparation ability for eight pairs of chiral analytes. The corresponding separation parameters including migration time (t), resolution (Rs) and column efficiency (N) are listed in Table 1. The good separation of eight pairs of enantiomers illustrates that the PTB film-coated capillary column is suitable for the enantioseparation of different types of chiral compounds.

|
Download:
|
| Fig. 4. Electropherograms of eight pairs of enantiomers. The respectively optimum separation conditions are listed in Table S1. Injection: hydrostatic injection for 3 s at 10 cm height; Applied voltage: +20 kV; Detection wavelength: 214 nm; Capillary: 75 μm i.d., 40.0 cm effective length. | |
|
|
Table 1 Separation parameters for eight pairs of enantiomers obtained on the PTB film-coated capillary column under their respective optimum conditions. |
The stability and repeatability of PTB film-coated capillary column for OT-CEC enantioseparation were evaluated through the relative standard deviation (RSD), and the results are compiled in Table S2 (Supporting information). The RSD values of migration time were 0.3%–3.5%, 0.2%–4.9%, and 2.1%–7.7%, respectively, for intra-day, inter-day, and column-to-column precisions. These results indicate that the repeatability of the proposed method was satisfactory although the column preparation was very simple. Moreover, although there was a slight change in the peak shapes of terbutaline enantiomers from 1 run to 300 runs, their migration time kept consistent, as presented in Fig. S9 (Supporting information), indicating that the PTB coating is enough stable despite slight variation in the interaction with analytes.
Then, the PTB film-coated capillary after 300 runs was also characterized by SEM (Fig. S10 in Supporting information) and fluorescence imaging (Fig. S11 in Supporting information). Clearly, the PTB film remained intact in the inner wall of the coated capillary. The possible reason for the excellent stability of PTB film-coated capillary columns was the high interfacial adhesion to resist mechanical and chemical peeling under harsh conditions [26, 27]. As shown in Fig. S12 (Supporting information), none of these extreme treatments including ultrasonic, tearing with adhesive tape and dipping in different organic solvent could detach the PTB film from the inner wall of coated capillary.
In CE, the chiral selector is necessary for chiral recognition. BSA is an effective chiral selector because of its abundant amino acids, and many BSA-based methods have been developed to separate the chiral compounds. However, when BSA was dissolved into the buffer as the additive, the enantiomers could not be separated effectively due to the serious adsorption of BSA on the capillary inner wall. In this work, after self-assembly of BSA, the more exposed amino acids [26, 27] strengthened the recognition capacity of BSA for chiral compounds. Moreover, the enantioseparation capacity could also be attributed to the interaction between the analytes with functional groups on the surface of PTB coating via combinational forces including charge-charge interactions, hydrogen bonds, and hydrophobic interactions, etc. In a word, the excellent enantioseparation ability of PTB coated capillary mainly benefitted from the chiral amino acids and abundant functional groups of PTB film.
To date, many biomacromolecules (BSA, lysozyme, etc.) have been successfully applied in CEC enantioseparation. To further demonstrate the superiority of our proposed method, our results were compared with those of previously reported methods, and the comparison results are illustrated in Table S3 (Supporting information). Similar to our group's previous work [13], the PTB film-coated capillary column had stronger enantioseparation capacity. As shown in Table S3, compared to other chiral CEC columns, the coated column fabricated in this work demonstrated the advantages as follows: (1) the mild, fast and in-situ preparation process, featured by only mixing BSA and TCEP for 1 h at room temperature, (2) the remarkable stability and repeatability, and (3) the good chiral separation ability.
In brief, the low-cost and robust proteinaceous PTB film coated capillary column was prepared based on a rapid, simple and mild method, and utilized as the separation channel for OT-CEC enantioseparation. After coating, the intrinsic disadvantages of BSA as a chiral additive in running buffer including serious adsorption and high consumption were overcome, and the enantioseparation capacity was kept and even improved at the same time. The characterization results of SEM, FT-IR, fluorescence imaging and EOF indicate that PTB film successfully adhered to the inner wall of capillary columns. Diverse model chiral analytes such as amine drugs and neurotransmitters could be well separated by PTB film-coated capillary columns. Besides, PTB coating on the inner wall could resist mechanical and chemical peeling under harsh conditions and be used over 300 runs without an observable change in the separation efficiency and migration time, which indicates the excellent stability of this method. We believe that this work will open a promising avenue for the in-situ fabrication of 2D protein film-coated capillary columns based on the supramolecular assembly in OT-CEC.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThe authors are grateful for the financial support from the National Natural Science Foundation of China (No. 21874060) and the Special Funding for Open and Shared Large-Scale Instruments and Equipments of Lanzhou University (No. LZU-GXJJ-2019C038).
| [1] |
J. Shen, Y. Okamoto, Chem. Rev. 116 (2016) 1094-1138. DOI:10.1021/acs.chemrev.5b00317 |
| [2] |
J.M. Saz, M.L. Marina, J. Chromatogr. A 1467 (2016) 79-94. DOI:10.1016/j.chroma.2016.08.029 |
| [3] |
D. Wu, F. Pan, W. Tan, L. Gao, Y. Tao, Y. Kong, J. Sep. Sci. 42 (2019) 1-11. |
| [4] |
M. Maisuradze, G. Sheklashvili, A. Chokheli, et al., J. Chromatogr. A 1602 (2019) 228-236. DOI:10.1016/j.chroma.2019.05.026 |
| [5] |
X. Yao, H. Zheng, Y. Zhang, X. Ma, Y. Xiao, Y. Wang, Anal. Chem. 88 (2016) 4955-4964. DOI:10.1021/acs.analchem.6b00897 |
| [6] |
L. Fumagalli, L. Pucciarini, L. Regazzoni, et al., J. Sep. Sci. 41 (2018) 1240-1246. DOI:10.1002/jssc.201701308 |
| [7] |
X. Han, J. Huang, C. Yuan, Y. Liu, Y. Cui, J. Am. Chem. Soc. 140 (2018) 892-895. DOI:10.1021/jacs.7b12110 |
| [8] |
M. Zhou, Y. Long, Y. Zhi, X. Xu, Chin. Chem. Lett. 29 (2018) 1399-1403. DOI:10.1016/j.cclet.2017.10.039 |
| [9] |
B. Zhao, L. Li, Y. Wang, Z. Zhou, Chin. Chem. Lett. 30 (2019) 643-649. DOI:10.1016/j.cclet.2018.10.013 |
| [10] |
X. Shi, Y. Zhou, F. Liu, J. Mao, Y. Zhang, T. Shan, J. Chromatogr. A 1596 (2019) 161-174. DOI:10.1016/j.chroma.2019.02.063 |
| [11] |
J. Zhang, S. Xie, L. Chen, et al., Anal. Chem. 87 (2015) 7817-7824. DOI:10.1021/acs.analchem.5b01512 |
| [12] |
S. Xie, J. Zhang, N. Fu, et al., Anal. Chim. Acta 903 (2016) 156-163. DOI:10.1016/j.aca.2015.11.030 |
| [13] |
Y. Sun, C. Li, X. Niu, et al., Talanta 195 (2019) 190-196. DOI:10.1016/j.talanta.2018.11.035 |
| [14] |
H. Guo, Y. Sun, X. Niu, et al., J. Chromatogr. A 1578 (2018) 91-98. DOI:10.1016/j.chroma.2018.10.007 |
| [15] |
C. Pan, W. Lv, X. Niu, et al., J. Chromatogr. A 1541 (2018) 31-38. DOI:10.1016/j.chroma.2018.02.015 |
| [16] |
R. Meng, J. Kang, J. Chromatogr. A 1506 (2017) 120-127. DOI:10.1016/j.chroma.2017.05.010 |
| [17] |
L. Zhao, J. Qiao, H. Zhang, F. Xie, L. Qi, Chin. Chem. Lett. 30 (2019) 349-352. DOI:10.1016/j.cclet.2018.03.007 |
| [18] |
H. Guo, X. Niu, C. Pan, et al., J. Sep. Sci. 40 (2017) 2645-2653. DOI:10.1002/jssc.201700152 |
| [19] |
R. Liang, X. Wang, C. Liu, X. Meng, J. Qiu, J. Chromatogr. A 1323 (2014) 135-142. DOI:10.1016/j.chroma.2013.11.048 |
| [20] |
T. Hong, Y. Zheng, W. Hu, Y. Ji, Anal. Biochem. 464 (2014) 43-50. DOI:10.1016/j.ab.2014.07.015 |
| [21] |
J. Lu, F. Ye, A. Zhang, et al., J. Sep. Sci. 34 (2011) 2329-2336. |
| [22] |
M. Kato, H. Saruwatari, K. Sakai-Kato, T. Toyo'oka, J. Chromatogr. A 1044 (2004) 267-270. DOI:10.1016/j.chroma.2004.04.056 |
| [23] |
M. Kato, K. Sakai-Kato, N. Matsumoto, T. Toyo'oka, Anal. Chem. 74 (2002) 1915-1921. DOI:10.1021/ac0112162 |
| [24] |
P. Yang, Macromol. Biosci. 12 (2012) 1053-1059. DOI:10.1002/mabi.201200092 |
| [25] |
D.H. Wang, Y. Ha, J. Gu, et al., Adv. Mater. 28 (2016) 7414-7423. DOI:10.1002/adma.201506476 |
| [26] |
C. Li, L. Xu, Y. Zuo, P. Yang, Biomater. Sci. 6 (2018) 836-841. DOI:10.1039/C8BM00066B |
| [27] |
X. Hu, J. Tian, C. Li, et al., Adv. Mater. 32 (2020) 2000128. DOI:10.1002/adma.202000128 |
| [28] |
C. Liu, R. Liang, X. Wang, J. Wang, J. Qiu, J. Chromatogr. A 1294 (2013) 145-151. DOI:10.1016/j.chroma.2013.04.022 |
| [29] |
C. Li, L. Xu, Y.Y. Zuo, P. Yang, Biomater. Sci. 6 (2018) 836-841. DOI:10.1039/C8BM00066B |
 2021, Vol. 32
2021, Vol. 32 


