b Engineering Research Center of Clinical Functional Materials and Diagnosis & Treatment Devices of Zhejiang Province, Wenzhou Institute, University of Chinese Academy of Sciences, Wenzhou 325001, China
For decades, electrochemical detection method has proven to be a powerful tool in the fields of analysis because of its simple operation, cost efficiency, rapid detection, and low detection limit [1-3]. So far, numerous materials have been investigated as electrochemical sensor for analyte detection, such as transition metal oxides [4, 5], metal nanoparticles (Au, Pd, Pt) [6-8], carbon materials [9, 10]. Although these materials received extensive attention due to their high surface area, porosity and good conductivity, a lack of tunable porosity, atomically precise structures and possibility for surface functionalizations resulted in undesirable performance for electrode materials. Thus, the development of novel electrode materials to improve the electrochemical performances is highly sought after.
Recently, covalent organic framework (COF), a promising crystalline material, has engendered enormous scientific interest [11, 12]. In comparison with traditional materials, (ⅰ) the pore size of COF can be pre-designed to selectively identify and adsorb target analytes; (ⅱ) the high surface area with rich π-electron density and functional groups within COF is beneficial for the interactions with target molecules; (ⅲ) the self-assembled 2 D pore channels constructed by π-π stacking of COF are conducive to charge-carrier transport thus effectively minimize the diffusion resistance. These unique features of COF have attracted researchers for many applications including catalysis, energy storage, adsorption, sensing, and catalysis [13-16]. Unfortunately, the poor intrinsic electrical conductivity of COF has greatly restricted its further application in electrochemical sensing. In this regard, incorporating COF with a specific conductive material can be considered as an effective post-synthetic strategy to modulate the limited sensing ability, especially when it comes to introduction of intrinsically conductive polymers into the frameworks.
Polyaniline (PANI) was one of the conductive polymers that has been extensively applied in electrochemical sensor due to its highly electrical conductivity, environmental stability, low cost and different redox states [17]. Numerous researchers have reported that PANI hybrid material could yield highly conductive electrode with significant property enhancements [18, 19]. On the other hand, the imine-based COF has comparable crystallinity than that of boron-containing COF and possesses superior structural regularity compared with triazine-based COF [20]. In particular, TAPB-DMTP-COF (TAPB, 1, 3, 5-tris(4-aminophenyl)benzene; DMTP, 2, 5-dimethoxyterephaldehyde) features relatively high stability and easy synthesis without involving rigorous experimental conditions and longtime consumption [21].
With this background in mind, we propose to synthesize a PANI coated TAPB-DMTP-COF (TAPB-DMTP-COF@PANI) composite via a facile chemical oxidative polymerization method. Characterizations were made by X-ray diffraction patterns (XRD), fourier transform infrared spectra (FTIR), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM) to verify their structure and morphology. Then we applied TAPB-DMTP-COF@PANI as an electrochemical amplifier for the determination of drug molecule. Acetaminophen, as an analgesic and antipyretic drug, is mainly used for the treatment of fever, cancerous pain and postoperative pain. Overdosing and long-term usage of acetaminophen can cause accumulation of toxic metabolites, leading to rashes and inflammation of the pancreas [22, 23]. Thus, it is vital to monitor the levels of acetaminophen. TAPB-DMTP-COF not only offers a high electroactive surface area to amplify current response, but also plays the role of backbone for PANI to prevent it from aggregation. PANI serves as a charge collector to effectively accelerate charge transport from COF framework and accordingly increases the sensitivity of the electrochemical sensor. Consequently, the electrochemical performances of the TAPB-DMTP-COF@PANI hybrid material for detecting acetaminophen were thoroughly investigated in terms of detection limit, linear range, and selectivity in real samples.
Powder XRD was utilized to verify the phase composition, as displayed in Fig. S1A (Supporting information). PANI with low crystallinity has two main peaks at 19.31° and 25°, which are related to the repetition of benzenoid and quinoid rings in PANI chains [24]. The XRD pattern of pure TAPB-DMTP-COF diffracted at an angle of 2.79°, 4.84°, 5.60°, 7.39°, 9.73° and 25.42° are assignable to the (100), (110), (200), (210), (220) and (001) facets, respectively [21]. For the TAPB-DMTP-COF@PANI composite, the diffraction peaks are similar to that of pure TAPB-DMTP-COF due to the relatively weak crystallinity of PANI, suggesting that the coating of PANI does not change the highly crystallized structure of TAPB-DMTP-COF. As illustrated in Fig. S1B (Supporting information), FTIR spectrum was measured in the range of 400−4000 cm−1 for the component identification of all synthesized materials. In the FTIR spectrum of PANI sample, the main absorption peaks at 1579 and 1495 cm−1 are attributed to the C=C stretching vibration of quinoid and benzenoid aromatic rings, respectively, indicating the presence of an emeraldine salt state in PANI [25]. Two peaks at 1310 and 1240 cm−1 are associated with the characteristic N−H bending vibration corresponding to the aromatic secondary amine of PANI. The band at 1137 cm−1 was assignable to the stretching vibration of the quinoid N=Q=N unit of doped PANI. As for TAPB-DMTP-COF, several essential peaks implied the presence of functional groups: 3482 cm−1(N−H stretching vibrations of TAPB), 1680 cm−1 (C=O stretching vibrations of DMTP), 1614 cm−1 (C=N stretching vibrations), providing compelling evidence of the covalent condensation of TAPB to DMTP. The emergence of PANI in the TAPB-DMTP-COF@PANI composite is determined by the presence of the N−H and the quinoid N=Q=N absorption bands originating from PANI, while other peaks of TAPB-DMTP-COF remain retained. It is noticeable that these two peaks are red shifted in comparison with pure PANI, which indicates π-π stacking and electrostatic interactions and hydrogen bonding between−NH group of PANI film and TAPB-DMTP-COF [26, 27].
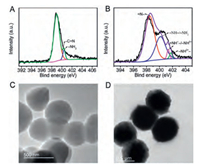
XPS was employed to further characterize chemical composition of TAPB-DMTP-COF and TAPB-DMTP-COF@PANI. In N 1s spectrum of TAPB-DMTP-COF, the binding energy centered at 400.55 eV was assigned to the residual −NH2, which was consistent with the result of FTIR (Fig. 1A). The other peak at 398.8 eV was corresponded to the imine nitrogen formed by a Schiff-base condensation reaction [28]. In the N 1s spectrum of TAPB-DMTP-COF@PANI (Fig. 1B), four peaks located at 398.5, 400.1, 401.2 and 401.8 eV were attributed to the neutral imine (=N−), neutral amine (−NH−and−NH2), positively charged imine (=NH+− and−NH3+), and positively charged amine (−NH2+−), respectively [29, 30]. The area fraction of =N− of the total peak area of N 1s was 0.65, meanwhile, the peak area of TAPB-DMTP-COF@PANI of imine nitrogen increased compared to that of TAPB-DMTP-COF. The incremental =N− content was beneficial to increase the sp2 nitrogen, resulting in improvement of the π-π stacking interaction between PANI chains and TAPB-DMTP-COF. Therefore, the electron transfer rate might be facilitated and a synergistic effect on electrochemical properties of the hybrid material was carried out. In addition, as revealed by TEM measurements, the prepared TAPB-DMTP-COF has a spherical shape with a rough surface and the diameter is about 500 nm (Fig. 1C). After growth of PANI on TAPB-DMTP-COF, the morphology of TAPB-DMTP-COF was well retained with a PANI shell (Fig. 1D), indicating the successful formation of the core-shell structure of TAPB-DMTP-COF@PANI.
|
Download:
|
| Fig. 1. N 1s XPS spectra and TEM images of TAPB-DMTP-COF (A, C) and TAPB-DMTP-COF@PANI (B, D). | |
Electrochemical impedance spectroscopy (EIS) method was employed to investigate the electron transport characteristics of bare glassy carbon electrode (GCE), PANI/GCE, TAPB-DMTP-COF/GCE and TAPB-DMTP-COF@PANI/GCE (Fig. 2A). The small semicircle at high frequency section represents a low electron transfer resistance (Rct). The slope at low frequency is relevant to the diffusion process on the modified electrodes. It is obvious that the bare TAPB-DMTP-COF/GCE performs largest interface electron impedance value, while the Rct of GCE was only 278 Ω. This result is due to the TAPB-DMTP-COF film formed on the electrode impedes the electron transfer of the redox probe on the electrode interface. However, TAPB-DMTP-COF@PANI exhibits a smaller Rct than GCE, which signify that the combination of PANI contributes to improve the electric conductivity and electrocatalytic property of electrodes. Meanwhile, these results are in a good consistent with the cyclic voltammogram (CV) data (Fig. S2 in Supporting information). The redox peak currents remarkably increased when GCE modified with the PANI and TAPB-DMTP-COF@PANI. Thus, it is believed that TAPB-DMTP-COF@PANI has potential to serve as a good electrochemical sensing platform for acetaminophen detection.

|
Download:
|
| Fig. 2. (A) EIS Nyquist plots of 5 mmol/L K3[Fe(CN)6]3−/4− containing 0.1 mol/L KCl and (B) DPV voltammograms of 50 μmol/L acetaminophen in phosphate buffer solution (PBS) (0.1 mol/L, pH 7.0) at bare GCE, PANI/GCE, TAPB-DMTP-COF/GCE, and TAPB-DMTP-COF@PANI/GCE. | |
Furthermore, the electrochemical responses of acetaminophen at bare GCE, PANI/GCE, TAPB-DMTP-COF/GCE and TAPB-DMTP-COF@PANI/GCE were investigated using differential pulse voltammetry (DPV). As shown in Fig. 2B, the DPV response of bare GCE displayed a quite weak oxidation peak of 0.26 μA. In contrast, the oxidation peak current increased to 0.58 μA, while TAPB-DMTP-COF was coated on the electrode surface, which indicates the high affinity of TAPB-DMTP-COF to the adsorbed acetaminophen molecules. Improved current response with a current value of 0.98 μA was also obtained using the PANI/GCE. This is because high conductivity of PANI can effectively promote the electron transfer and improve the electrochemical performance. It was obvious that PANI@TAPB-DMTP-COF/GCE displayed a strongest electrochemical signal among these four electrodes, which might origin from the synergistic amplification of PANI and TAPB-DMTP-COF.
To further check the electron transfer mechanism between electrode and electrolyte solution interface, CV response at different scan rate was examined. As depicted in Fig. S3A (Supporting information), acetaminophen oxidation peak current was enhanced with increasing scan rate, and the peak potentials shifted towards more positive values. In inset of Fig. S3A, a good linear relationship was observed between the peak currents and scan rates which indicates a typical adsorption-controlled process for electrocatalytic oxidation of acetaminophen. The corresponding linear regression equations can be expressed as: Ipa (μA)=0.4098+0.0012v (V/s) (R2=0.9944). The relation between the peak potential (Ep) and logarithm of scan rate was linear as: Ep (V)=0.3290+0.0515logv (log V/s), (R2=0.9987).Ep can be defined by Laviron's equation [31]:

|
Where α represents the charge transfer coefficient. For a totally irreversible electrode process, α is assumed to be 0.5. According to the slope of Ep-log v plot, the value of electron transfer number (n) can be calculated to be 2.29, which is appropriate to 2.
The impact of pH value on TAPB-DMTP-COF@PANI/GCE was also investigated in the pH range from 5.0 to 9.0. As shown in Fig. S3B (Supporting information), the peak current of acetaminophen gradually increased at a higher pH and peak potential moved negatively by raising pH value from 5.0 to 9.0. The maximum peak current was obtained at a pH of 7.0. Hence, the PBS at pH 7.0 is selected as the optimum supporting electrolyte. It is confirmed that the electrochemical process involves proton transfer in the rate determining step. The relationship between the peak potential and pH was investigated and the linear regression equation Epa=−0.0522 pH + 0.6708 (R2=0.9951). The value of the slope is similar to the theoretical Nernst value of 59 mV/pH, indicating the electrocatalytic oxidation of acetaminophen refers to an equal number of electrons and protons process. Based on the result of the scan rate data, we can conclude that two-proton and two-electron are participated in the electrochemical reaction. This was in accordance with that reported in the literature [32].
In addition, accumulation is an effective way to improve the determination sensitivity and efficiency. Hence, the impacts of accumulation time and accumulation potential on the oxidation peak currents of acetaminophen were studied. Note that the oxidation peak currents of acetaminophen increased with rising the accumulation time from 60 s to 150 s, and then leveled off for further prolonging accumulation time (Fig. S3C in Supporting information). This phenomenon was the consequence of the saturated adsorption of the analytes on the electrode surface. Thus, the optimized accumulation time was selected to be 150 s for further experiments. In addition, the effect of accumulation potential on the peak intensities was also researched. The peak currents increased when the accumulation potential increased in the range of −0.4 to −0.1V, and then decreased when the accumulation potential exceeded −0.1V (Fig. S3D in Supporting information). Therefore, −0.1V of accumulation potential was adopted in this work.
Under the optimized conditions, the DPV responses of different concentration of acetaminophen at TAPB-DMTP-COF@PANI/GCE were investigated. Fig. 3 portrayed the enhanced oxidation peak current with gradually increasing concentration of acetaminophen. Two calibration curves were acquired with corresponding linear regression equations of I1 (μA)=0.0062+0.1229C (C: μmol/L, R2=0.9997, 0.10−3 μmol/L) and I2 (μA)=0.6136+0.0065C (C: μmol/L, R2=0.9966, 3−2500 μmol/L), respectively. The limit of detection (LOD) was calculated to be 0.032 μmol/L, according to the principle of 3 S/N (signal/noise). The reason for obtaining two segments with different gradients could be associated with different sensitivities of acetaminophen at the modified electrode. In detail, under the condition of low concentration of acetaminophen, even subtle changes in concentration could make great difference towards the current response due to the existence of the diffusion effect. Thus, the local concentration of acetaminophen on the surface of modified electrode was quickly depleted owing to the conversion of acetaminophen into product, resulting in high sensitivity in the ranges of 0.10−3.0 μmol/L. With the addition of acetaminophen, a saturation level of absorption was achieved and excessive acetaminophen molecules accumulated on the surface of polymer membrane, leading to the covering of active sites. Therefore, the transformation process could not be effectively preceded and a lower sensitivity of this proposed sensor was obtained [33, 34]. Furthermore, in contrast with other reported literatures for the detection of acetaminophen (Table S1 in Supporting information) [35-43], the constructed electrochemical sensor possesses superior detection performance with a lower LOD and wider linear range. The excellent sensing performance of TAPB-DMTP-COF@PANI is a consequence of the following facts: (1) The highly regular TAPB-DMTP-COF with a large surface area can offer an effectively electroactive surface area and then promote the electrochemical response; (2) the existence of π-π stacking effect and hydrogen bonding interaction between TAPB-DMTP-COF@PANI and analyte are suitable for acetaminophen adsorption onto the electrode surface; (3) the high conductivity of PANI makes a contribution to accelerate the electron transfer rate of core-shell composite.

|
Download:
|
| Fig. 3. (A) DPVs of acetaminophen at the TAPB-DMTP-COF@PANI modified electrode in the pH 7.0 PBS. Acetaminophen concentrations (from a to q): 0.1, 0.8, 1.2, 2.0, 5.0, 20, 40, 60, 100, 200, 300, 400, 500, 800, 1200, 2000, 2500 μmol/L. (B) The calibration curve of acetaminophen. Inset: the tendency of current intensity when acetaminophen concentrations are low. | |
Further, the repeatability of the TAPB-DMTP-COF@PANI was investigated by DPV for the six repetitive assays of 50 μmol/L acetaminophen. The relative standard deviation (RSD) was calculated to be 3.6%. Seven independent TAPB-DMTP-COF@PANI/GCE was used to evaluate the reproducibility under the same conditions, and a satisfactory RSD of 4.2% was achieved, indicating the great reproducibility. Moreover, the storage stability of TAPB-DMTP-COF@PANI based electrochemical sensor was also validated. A batch of TAPB-DMTP-COF@PANI modified electrodes was stored in a refrigerator at 4 ℃ under dry conditions. Continuous measurement was performed every day for 14 days. No evident decrease of current response for the first week was observed and 95% of its original value was obtained after two weeks, verifying a desirable storage stability of the sensor. The outstanding stability is probably due to the stable skeleton of TAPB-DMTP-COF and strong interaction between the PANI and TAPB-DMTP-COF.
The interferences of potentially interfering substances on the determination of 50 μmol/L acetaminophen were evaluated, and a deviation on the response currents less than ± 5% was taken as the tolerance limit. It was observed that common ions such as Na+, K+, Ca2+, Cu2+, Fe3+, Al3+, NO3−, Cl−, SO42− and CO32− with 1000-fold of concentrations caused negligible influence on electrochemical signals. No substantial response change was obtained by addition of 300-fold concentrations of caffeine, vitamin A, vitamin E and vitamin C, 200-fold concentrations of glucose, 50-fold concentrations of ascorbic acid and dopamine, 20-fold concentrations of uric acid. All these results (Table S2 in Supporting information) revealed that the sensor had favorable selectivity towards acetaminophen detection. The possible reason is due to the kinetic dynamic diameter of acetaminophen molecule is smaller than the pore sizes of TAPB-DMTP-COF. Thus, acetaminophen can penetrate into the TAPB-DMTP-COF channels. And the mesoporous structure of TAPB-DMTP-COF@PANI permits the size- and shape-selectivity over the guests that were adsorbed on the electrode surface.
To testify the practical feasibility of TAPB-DMTP-COF@PANI/GCE for real sample analysis, the prepared sensor was applied to detect the content of acetaminophen in commercial tablet, human blood serum and urine. As shown in Table S3 (Supporting information), the recoveries of commercial tablet, human blood serum and urine samples were between 97.5% and 103.6%, indicating an excellent accuracy and reliability for the analysis of acetaminophen in real samples.
In summary, a core-shell structured TAPB-DMTP-COF@PANI composite has been successfully synthesized by a simple chemical oxidative polymerization method. The TAPB-DMTP-COF@PANI based electrochemical sensor exhibited remarkable electrocatalytic performance toward acetaminophen detection, such as a low detection limit (0.032 μmol/L) and wide linear range (0.10−2500 μmol/L). Additionally, it also presented excellent anti-interference ability, good repeatability and reproducibility. The excellent current response towards acetaminophen oxidation may result from the large electroactive surface area of TAPB-DMTP-COF and good intrinsic electrical conductivity of PANI. To the best of our knowledge, it is the first time that the surface modification of COF with conducting polymer was employed as electrochemical electrode materials in electrochemical field. Besides, the proposed sensor has been utilized to detect acetaminophen in commercial tablet, human blood serum and urine with satisfactory results. Our research made a useful contribution to explorations of more applications of COF materials in the electrochemical sensor.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21205103), Jiangsu Provincial Natural Science Foundation (No. BK2012258), Young and Middle-aged Academic Leaders Foundation of Yangzhou University, Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.10.001.
| [1] |
J. Luo, T. Li, M.H. Yang, Chin. Chem. Lett. 31 (2020) 202-204. DOI:10.1016/j.cclet.2019.05.051 |
| [2] |
W.Z. Zhang, X.X. Jiang, S.P. Liu, et al., Chin. Chem. Lett. 31 (2020) 459-462. DOI:10.1016/j.cclet.2019.08.050 |
| [3] |
Y. Liu, T.T. Li, C.X. Ling, et al., Chin. Chem. Lett. 30 (2019) 2211-2215. DOI:10.1016/j.cclet.2019.05.020 |
| [4] |
Z.Z. Yang, X.C. Huang, R.C. Zhang, et al., Electrochim. Acta 70 (2012) 325-330. DOI:10.1016/j.electacta.2012.03.075 |
| [5] |
J.L. Huang, G.J. Yang, W.J. Meng, et al., Biosens. Bioelectron. 25 (2010) 1204-1211. DOI:10.1016/j.bios.2009.10.036 |
| [6] |
J. Li, J. Yang, Z.Z. Yang, et al., Anal. Methods 4 (2012) 1725-1728. DOI:10.1039/c2ay05926f |
| [7] |
Y. Zhang, Y.H. Zhao, S.S. Yuan, et al., Sens. Actuator. B: Chem. 185 (2013) 602-607. DOI:10.1016/j.snb.2013.05.059 |
| [8] |
F.F. Wen, Y. Zhang, J. Tan, et al., J. Electroanal. Chem. 822 (2018) 10-16. DOI:10.1016/j.jelechem.2018.05.013 |
| [9] |
A.R. Mao, H.B. Li, Z.S. Cai, X.Y. Hu, J. Electroanal. Chem. 751 (2015) 23-29. DOI:10.1016/j.jelechem.2015.04.034 |
| [10] |
Q.F. Shi, G.W. Diao, S.L. Mu, Electrochim. Acta 133 (2014) 335-346. DOI:10.1016/j.electacta.2014.04.043 |
| [11] |
Y. Yusran, H. Li, X.Y. Guan, Q.R. Fang, S.L. Qiu, Energy Chem. 2 (2020) 100035. DOI:10.1016/j.enchem.2020.100035 |
| [12] |
T. Zhang, Y.L. Chen, W. Huang, Y. Wang, X.Y. Hu, Sens. Actuator. B: Chem. 276 (2018) 362-369. DOI:10.1016/j.snb.2018.08.132 |
| [13] |
T. Zhang, C.W. Gao, W. Huang, et al., Talanta 188 (2018) 578-583. |
| [14] |
C.J. Jin, J. Han, F.Y. Chu, X.X. Wang, R. Guo, Langmuir 33 (2017) 4520-4527. DOI:10.1021/acs.langmuir.7b00640 |
| [15] |
Y. Yuan, Y.J. Yang, G.S. Zhu, Energy Chem. 2 (2020) 100037. DOI:10.1016/j.enchem.2020.100037 |
| [16] |
H.C. Ma, J.L. Kan, G.J. Chen, C.X. Chen, Y.B. Dong, Chem. Mater. 29 (2017) 6518-6524. DOI:10.1021/acs.chemmater.7b02131 |
| [17] |
L. Yu, Y.P. Huang, Z. Wei, et al., J. Org. Chem. 80 (2015) 8677-8683. DOI:10.1021/acs.joc.5b01358 |
| [18] |
J. Han, P. Fang, J. Dai, R. Guo, Langmuir 28 (2012) 6468-6475. DOI:10.1021/la300619d |
| [19] |
Y.Y. Tan, X.X. Guo, J.H. Zhang, J.Q. Kan, Biosens. Bioelectron. 25 (2010) 1681-1687. DOI:10.1016/j.bios.2009.12.007 |
| [20] |
F.J. Uribe-Romo, J.R. Hunt, H. Furukawa, et al., J. Am. Chem. Soc. 131 (2009) 4570-4571. DOI:10.1021/ja8096256 |
| [21] |
H. Xu, J. Gao, D.L. Jiang, et al., Nat. Chem. 7 (2015) 905-912. DOI:10.1038/nchem.2352 |
| [22] |
A.R. Mao, H.B. Li, D.Q. Jin, L.Y. Yu, X.Y. Hu, Talanta 144 (2015) 252-257. DOI:10.1016/j.talanta.2015.06.020 |
| [23] |
L. Liu, H.Y. Lv, C.Y. Wang, Z.M. Ao, G.X. Wang, Electrochim. Acta. 206 (2016) 259-269. DOI:10.1016/j.electacta.2016.04.123 |
| [24] |
Y. Chen, S. Lu, W.J. Liu, J. Han, Colloid Polym. Sci. 293 (2015) 2301-2309. DOI:10.1007/s00396-015-3619-3 |
| [25] |
W.J. Liu, S.S. Wang, Q.H. Wu, et al., Chem. Eng. Sci. 156 (2016) 178-185. DOI:10.1016/j.ces.2016.09.025 |
| [26] |
J. Singh, A.P. Bhondekar, M.L. Singla, A. Sharma, ACS Appl. Mater. Interfaces 5 (2013) 5346-5357. DOI:10.1021/am401257q |
| [27] |
H.L. Wang, Q.L. Hao, X.J. Yang, L.D. Lu, X. Wang, ACS Appl. Mater. Interfaces 2 (2010) 821-828. DOI:10.1021/am900815k |
| [28] |
Y. Hu, N. Goodeal, Y. Chen, et al., Chem. Commun. 52 (2016) 9941-9944. DOI:10.1039/C6CC03895F |
| [29] |
D. Raffa, K.T. Leung, F.A. Battaglini, Anal. Chem. 75 (2003) 4983-4987. DOI:10.1021/ac0341620 |
| [30] |
Y. Wang, L. Wang, W. Huang, et al., J. Mater. Chem. A 5 (2017) 8385-8393. DOI:10.1039/C7TA01066D |
| [31] |
E. Laviron, J. Electroanal. Chem. 101 (1979) 19-28. DOI:10.1016/S0022-0728(79)80075-3 |
| [32] |
W.Q. Zhang, L.K. Zong, S.Q. Liu, et al., Biosens. Bioelectron. 131 (2019) 200-206. DOI:10.1016/j.bios.2019.01.069 |
| [33] |
Y.L. Chen, X. Sun, S. Biswas, et al., Biosens. Bioelectron. 141 (2019) 11470. |
| [34] |
Y. Wang, L. Wang, H.H. Chen, X.Y. Hu, S.Q. Ma, ACS Appl. Mater. Interfaces 8 (2016) 18173-18181. DOI:10.1021/acsami.6b04819 |
| [35] |
A.A. Ensafi, H. Karimi-Maleh, S. Mallakpour, M. Hatami, Sens. Actuator. B: Chem. 155 (2011) 464-472. DOI:10.1016/j.snb.2010.12.048 |
| [36] |
J.C. Song, J. Yang, J.F. Zeng, J. Tan, L. Zhang, Sens. Actuator. B: Chem. 155 (2011) 220-225. DOI:10.1016/j.snb.2010.11.051 |
| [37] |
S.F. Wang, F. Xie, R.F. Hu, Sens. Actuator. B: Chem. 123 (2007) 495-500. DOI:10.1016/j.snb.2006.09.031 |
| [38] |
Y. Fan, J.H. Liu, H.T. Lu, Q. Zhang, Colloids Surf. B Biointerfaces 85 (2011) 289-292. DOI:10.1016/j.colsurfb.2011.02.041 |
| [39] |
M.Q. Li, L.H. Jing, Electrochim. Acta 52 (2007) 3250-3257. DOI:10.1016/j.electacta.2006.10.001 |
| [40] |
F. Cao, Q.C. Dong, C.L. Li, et al., Sens. Actuator. B: Chem. 256 (2018) 143-150. DOI:10.1016/j.snb.2017.09.204 |
| [41] |
W.M. Si, W. Lei, Z. Han, et al., Sens. Actuator. B: Chem. 193 (2014) 823-829. DOI:10.1016/j.snb.2013.12.052 |
| [42] |
M.P.N. Bui, C.A. Li, K.N. Han, X.H. Pham, G.H. Seong, Sens. Actuator. B: Chem. 174 (2012) 318-324. DOI:10.1016/j.snb.2012.08.012 |
| [43] |
M. Sivakumar, M. Sakthivel, S.M. Chen, RSC Adv. 6 (2016) 104227-104234. DOI:10.1039/C6RA23114D |
 2021, Vol. 32
2021, Vol. 32 

