Ornidazole (ORZ, 1-(2-hydroxy-3-chloropropyl)-2-methyl-5-nitroimidazole)), a kind of third generation nitroimidazole drug used to treat a variety of infections caused by bacteria, protozoa, and fungi, is increasingly detected in water bodies [1]. Due to the high water-solubility and low biodegradability of ORZ, it is not easy to be removed in the environment and finally enrich in the water bodies [2]. In addition, it has also been reported that ORZ not only has potential carcinogenic and mutagenic properties, but also leads to the growth and reproduction of resistant bacteria, which does great harm to human health and ecosystem [2-4].Therefore, an efficient way to degrade ORZ is urgently needed.
Advanced oxidation processes (AOPs) are used to remove organic pollutants [5-7], among which, peroxymonosulfate (PMS) is considered to be an efficient oxidant owing to its low cost, high stability and the production of reactive oxidation species (ROS) with high redox potentials [8-10]. Various ways including thermal, ultraviolet, alkaline, and transition metal ionsetc. are used to active PMS [10-12]. However, thermal and ultraviolet activation both require additional energy, while alkaline and transition metal ions activation are both easily affected by the initial value of pH and unable to be recycled [13-15]. Therefore, it is especially important to find a PMS activation method that is energy-efficient and easy to be recycled.
Piezoelectric materials with both mechanical and electrical behaviors begin to be paid more attention in the application of contaminant degradation in recent years [16-22]. Under a certain force like wind, sound and tide pressure, the positive charges and negative charges generated and further separated on the surface of materials. It has been reported that the generated charges can contribute to the decomposition of organic pollutants [23-26]. For example, as a traditional piezoelectric material, BaTiO3 (BTO) with high piezoelectric coefficient and dielectric constant is widely employed to degrade contaminants [27-31]. Recently, our group novelty used the local accumulated charges on the surface of BTO to break O-O bond of PMS to generate sulfate radical (SO4•‒), hydroxyl radical (•OH), and singlet oxygen (1O2) for the degradation of benzothiazole in water [23]. This result showed a new strategy to promote PMS activation by using a piezoelectricity for the removal of organic pollutants. However, because of its poor conductivity, the degradation ability of BTO is greatly limited [32]. In addition, odd-numbered layers MoS2 (MS) as another classic piezoelectric material has the defect of fast recombination of electron-hole pairs, which may also restrict the application due to poor electron migration [33-35]. It is reported that synthesizing heterojunction to reduce the possibility of carrier recombination for effectively transferring the abundant electrons is an effective way to improve the piezoelectric effect [34, 36]. Thus, synthesizing BTO/MS heterojunction composite for piezoelectric-PMS activation may be an effective mean to degrade pollutants.
In this work, BTO/MS heterojunctions with different compound ratio are synthesized to investigate the ability of piezo-activation of PMS for ORZ degradation. The purpose of this study is to (i) select the optimal ratio of the BTO/MS composite for piezoelectric-PMS activation to degrade ORZ; (ii) evaluate the mechanism of piezoelectric-PMS activation; (iii) propose the degradation pathways and products of ORZ.
The main reagents, preparation and characterization of BTO, MS, and BTO/x%MS (x=10, 20, 50, wherex is the compound ratio of MS), experimental procedure, and analysis methods are exhibited in Text S1 (Supporting information). As shown in Texts S2-4 and Figs. S1-3 (Supporting information), the synthesized BTO, MS, and BTO/MS were analyzed by transmission electron microscope (TEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS), which suggest the successful composite of BTO/MS.
In order to obtain an optimal BTO/x%MS, the degradation rates of ORZ were measured to investigate the piezoelectric property of the catalyst. As shown in Fig. 1a, the ability of piezoelectric catalytic degradation of BTO, MS, and BTO/x%MS under ultrasonic condition were evaluated. It can be seen that pure BTO and MS both exhibited a restrict piezo ability to remove ORZ, and the degradation ratios were 14.9% and 41.4% within 30min, respectively. When compositing BTO and 10%MS, about 59.6% degradation ratio was observed, indicating a collaboration of BTO and MS. Moreover, with thex increasing to 20%, the degradation ratio of ORZ enhanced to 69.8%, which is 55.1%, 28.4%, 10.2% and 32.4% higher than that of BTO, MS, BTO/10%MS and BTO/50%MS, respectively. The improved ORZ degradation efficiency may be attributed to the heterojunction between BTO and MS, which improved the separation efficiency of piezoelectric induced electrons and holes in the composite.

|
Download:
|
| Fig. 1. (a) Degradation ratios and (b) reaction rate constant (k) of ORZ using various piezoelectric systems. (c) Degradation ratios and (d) first-order kinetics model of ORZ using various BTO/MS-based systems. Experimental conditions: [PMS] = 0.25 g/L, [catalyst] = 0.10 g/L, [ORZ] = 0.050 g/L, pH 5.0, T = 25 ℃, mechanical force from ultrasonic wave: 40 kHz, 100 W. | |
First-order kinetic model -ln(C/C0) = kt is used to further evaluate the degradation efficiency of ORZ. As shown in Fig. 1b, the k of BTO/20%MS displayed the highest value (0.0282min‒1), which is 7.0, 1.8, 1.7, and 2.7 times higher than that of BTO, MS, BTO/10%MS, and BTO/50%MS, respectively. Thus, the first-order kinetic model further supports that the BTO/20%MS composite displayed a higher piezocatalytic activity, which was selected as the optimal ratio for piezoelectric-PMS activation. The BTO/20%MS composite is abbreviated as BTO/MS in the following description.
The degradation efficiency of ORZ under various BTO/MS-based systems were displayed in Fig. 1c and Fig. S4 (Supporting information). It can be observed that almost no degradation of ORZ can be observed within 40min by PMS alone, ultrasound alone or PMS-ultrasound coupling systems. Therefore, the contribution of ultrasound and PMS on ORZ removal was both negligible. In addition, BTO/MS can hardly absorb ORZ. The degradation capability of ORZ was further evaluated in the (BTO/MS)/PMS, (BTO/MS)/piezo and (BTO/MS)/piezo/PMS processes. Only 4.1% ORZ was removed in the (BTO/MS)/PMS process, implying that PMS is hardly activated by BTO/MS without piezo. With a piezoelectric force on BTO/MS, 69.8% removal ratio of ORZ was obtained, insinuating a considerable piezoelectric catalysis performance of BTO/MS. Moreover, with the presence of piezo and PMS simultaneously, a clearly enhancement of ORZ degradation ratio (90.9%) was found within 40min, which is 84.6% and 21.1% higher than that of only PMS and piezo, respectively. The first-order kinetics model of ORZ degradation were performed, and thek of the (BTO/MS)/piezo/PMS process is 139, 62.1 and 2.0 times higher than that of the BTO/MS, (BTO/MS)/PMS, and (BTO/MS)/piezo, respectively, suggesting the synergistic effect of piezocatalysis and PMS activation in the (BTO/MS)/piezo/PMS process (Fig. 1d). In addition, cycle experiment was also conducted to indicate the stability of BTO/MS. As shown in Fig. S5 (Supporting information), the degradation ratio of ORZ was still maintained about 90% in 5 cycles, which proved the outstanding stability of BTO/MS.
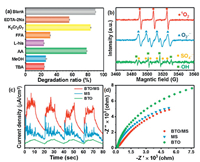
In order to identify the ROS and their contribution to the degradation of ORZ in the (BTO/MS)/piezo/PMS process, radical capture experiments and electron paramagnetic resonance (ESR) tests were performed. Radical capture experiments were carried out using different scavengers (Fig. 2a). Methanol (MeOH), tert-butanol (TBA), l-ascorbic acid (AA), l-histidine (l-His), furfuryl alcohol (FFA), K2Cr2O7, and EDTA-2Na are used to capture •OH and SO4•−, •OH, superoxide radical (O2•−), 1O2, 1O2, electrons (e−) and holes (h+), respectively [23]. As shown in Fig. 2a, the ORZ degradation ratios decreased to 24.3% and 26.9% in the presence of TBA and MeOH, respectively, indicating that •OH may play more important role than SO4•− in the (BTO/MS)/piezo/PMS process. The addition of FFA and l-His dramatically inhibited the degradation efficiency of ORZ by 59.1% and 67.2%, respectively, which both exhibited the higher inhibitory effect compared to other scavengers, confirming 1O2 appears to be the dominant ROSs for ORZ decomposition. In addition, the removal ratios of ORZ decreased to 79.0%, 56.6% and 84.0% in the presence of AA, EDTA-2Na and K2Cr2O7, respectively, suggesting the co-existence of O2•−, h+ and e− in the (BTO/MS)/piezo/PMS process. Therefore, above results demonstrate that •OH and 1O2 play a dominant role in the reaction, while other reactive species may be also involved in ORZ removal with a minor contribution. To further determine the reactive species in the (BTO/MS)/piezo/PMS process, ESR experiments were carried out using DMPO and TEMP as capture reagents to detect the radicals and non-radicals. As shown in Fig. 2b, the signal of DMPO-SO4•‒, DMPO-•OH, DMPO-O2•− and TEMP-1O2 were observed, implying that SO4•‒, •OH, O2•− and 1O2 were produced in the (BTO/MS)/piezo/PMS process, which is consistent with the results of quenching experiments. In addition, ESR experiments with only BTO/MS process, (BTO/MS)/PMS process and (BTO/MS)/piezo process were also carried out for comparison (Fig. S6 in Supporting information). Specific discussion was displayed in Text S5 (Supporting information).
|
Download:
|
| Fig. 2. (a) Degradation ratios of ORZ using various scavengers and (b) ESR in the (BTO/MS)/piezo/PMS system. (c) Transient piezoelectric current responses and (d) EIS Nyquist plots of BTO, MS, and BTO/MS. Experimental conditions: [PMS] = 0.25 g/L, [catalyst] = 0.10 g/L, [ORZ] = 0.050 g/L, pH 5.0, T = 25 ℃, [scavenger]=10 mmol/L, mechanical force from ultrasonic wave: 40 kHz, 100 W. | |
Generally, the faster charges separation leads to the higher PMS activation capability for generating more SO4•‒, •OH, O2•− and 1O2 [23]. The transient piezoelectric current responses and electrochemical impedance spectroscopy (EIS) were carried out to investigate the piezo-charges transport properties of the samples. The transient piezoelectric current responses of BTO, MS, and BTO/MS are shown in Fig. 2c. Under the applying force, the piezoelectric current intensity of BTO/MS enhanced obviously, which was higher than that of BTO and MS alone, indicating the highest piezoelectric-induced electron-hole separation efficiency in BTO/MS. In Fig. 2d, compared with BTO and MS alone, BTO/MS exhibited the smallest arc in the EIS Nyquist plots, indicating the minimum charge transfer resistance and the solid state interface layer resistance in BTO/MS, which is beneficial to activate PMS to generate SO4•‒, •OH, O2•− and 1O2 for ORZ removal. These results suggest a heterojunction between BTO and MS, which increases the charge separation of piezo charges.
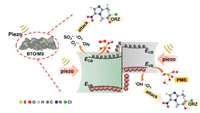
It is known that the Mott-Schottky diagrams display positive slopes of n-type semiconductors, the flat charge potential (EFB) of which corresponds to the conduction band (CB) edge potential (ECB). The EFB of BTO and MS were ‒1.14 and ‒1.51eVvs. SCE (Fig. S7 in Supporting information). According to ECB = EFB + 0.197 (where 0.197 is the standard electrode potential of Ag/AgCl electrode) [37], theECB of BTO and MS were calculated to be −0.94 and −1.31eVvs. NHE. Moreover, the valence band (VB) of BTO and MS were calculated to be 2.11 and 0.06eVvs. NHE through VB-XPS spectra (Fig. S8 in Supporting information), respectively. Therefore, the BTO/MS may follow a typical type Ⅱ heterostructure charge transfer system, as shown in Fig. 3. In this charge transfer mechanism, the piezoelectric-induced e− of MS transfer to the CB of BTO, while the piezoelectric-induced h+ of BTO transfer to the VB of MS, resulting in the charge separation of BTO and MS. Thus, the separation of piezoelectric-induced charges in BTO and MS are accelerated in the way of type Ⅱ heterostructure charges transfer mechanism, thereby inhibiting their recombination rate to improve the PMS activation for generating more ROS. The h+ of BTO and e− of MS can attack the O-O bond of PMS, leading the generation of SO4•−, •OH, O2•− and 1O2 (Eqs. 1-6). Under the applying force, a large number of e− and h+ are provided on the surface of BTO/MS (Eq. 1). The e− accumulated on the surface of BTO not only react with PMS to form •OH and SO4•−, but also reduce O2 to O2•− (Eqs. 2-4) while h+ accumulated on the surface of MS react with PMS and H2O to form PMS anion radical (SO5•−) and •OH (Eqs. 5-6). Then 1O2 would be further produced by the reaction of •OH and O2•− and the self-reaction of SO5•− (Eqs. 7 and 8). These ROS are responsible for the degradation of ORZ in the (BTO/MS)/piezo/PMS process.
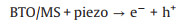
|
(1) |

|
(2) |
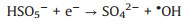
|
(3) |
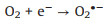
|
(4) |
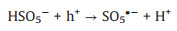
|
(5) |
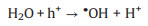
|
(6) |
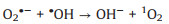
|
(7) |

|
(8) |
|
Download:
|
| Fig. 3. Proposed mechanism of ORZ degradation in the (BTO/MS)/piezo/PMS system. | |
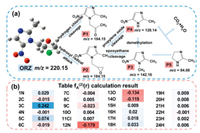
In order to investigate the degradation pathways and products of ORZ, the a dual descriptor (DD) value fA(2) was calculated based on density functional theory (DFT) to predict the most vulnerable sites by nucleophilic and electrophilic attack on the molecular structure of ORZ [38]. The labeled ORZ molecule is displayed in Fig. 4a and the values of fA(2) are provided in Fig. 4b. As can be seen that 3C (fA(2) = 0.242) and 5C (fA(2) = 0.074) displayed higher value than that of other atoms, suggesting that 3C and 5C may tend to be attacked by nucleophilic species (SO4•‒ and •OH). Meanwhile, 12N (fA(2) = ‒0.179), 13O (fA(2) = ‒0.134), 14O (fA(2) = ‒0.119) and 9C (fA(2) = ‒0.023) displayed lower value than that of other atoms, implying that 12N, 13O, 14O, and 9C are more likely to be attacked by electrophilic species (SO4•‒, •OH, O2•− and 1O2). Thus, ORZ may be decomposed by the cleavages of the 9C-11Cl, 8C-10O, and 3C-12N bonds.
|
Download:
|
| Fig. 4. (a) Pathways proposed for ORZ degradation by the (BTO/MS)/piezo/PMS system; (b) DFT calculation for ORZ molecule. | |
Therefore, according to the DFT calculation, liquid chromatography quadrupole time-of-flight tandem mass spectrometer (LC-TOF-MS) analysis, and previous studies, five degradation products and two degradation pathways were identified [39, 40]. ProductsP1, P2, P3, and P4 were formed by the cleavage of 9C-11Cl and 8C-10O bonds. Specifically, P1 was generated through demethylation, dehydrogenation, and hydrogen chloride cleavage. P2 as one of the most frequently identified substances in previous studies, was yielded by •OH attack, which further changed into P3 by epoxyethane cleavage. The emergence of P4 would be caused by the propylene oxide cleavage of P2 and demethylation of P3. Finally, due to the frangibility of 3C-12N, P5 emerged by dehydrogenation and -NO2 cleavage. In summary, ORZ was transformed into these five products, and mineralized into H2O and CO2 at last.
In summary, a heterojunction of BTO/MS with enhanced piezoelectric effect was prepared to activate PMS for ORZ degradation. The optimal ratio of BTO/MS composite was determined to 5/1 and the reaction rate constant of ORZ degradation by the (BTO/MS)/piezo/PMS process was 13.9, 3.6, 62.1 and 2.0 times higher than that of the BTO/piezo, MS/piezo, (BTO/MS)/piezo, and (BTO/MS)/PMS processes, respectively, indicating the excellent piezoelectric effect and significant synergy of BTO/MS for piezo-activation of PMS. Scavenging experiments and ESR detection proved the generation of SO4•−, •OH, O2•−, 1O2, h+ and e−, while •OH and 1O2 were the main reactive substances in the reaction. The BTO/MS follow a type Ⅱ heterostructure charge transfer system, in which the recombination of e—h+ pairs is suppressed, contributing to the PMS activation for ORZ degradation. Moreover, ORZ was transformed into five by-products based on the DFT calculation and LC-TOF-MS analysis. This study proves that combining BTO and MS can greatly enhance the piezoelectric effect, thereby promoting the efficiency of PMS activation, which provides a new way for enhancing piezo-activation of PMS by constructing heterojunctions in piezoelectric materials.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by Guangdong Basic and Applied Basic Research Foundation, China (No. 2020B1515020038) and the Pearl River Talent Recruitment Program of Guangdong Province (No. 2019QN01L148), China. The authors are also grateful to Prof. W. Guo from the Harbin Institute of Technology for assistance of DFT calculation.
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.11.016.
| [1] |
W. Ahmad, A. Singh, K.K. Jaiswal, P. Gupta, J. Inorg. Organomet. P. 31 (2021) 614-623. DOI:10.1007/s10904-020-01703-6 |
| [2] |
R. Tan, Z. Lv, J. Tang, et al., Sci. Rep. 9 (2019) 1091. DOI:10.1038/s41598-018-37645-w |
| [3] |
D. Li, W. Shi, Chinese J. Catal. 37 (2016) 792-799. DOI:10.1016/S1872-2067(15)61054-3 |
| [4] |
T. Bergan, Scand. J. Infect. Dis. 46 (1985) 64-71. |
| [5] |
R. Yin, Y. Chen, S. He, et al., J. Hazard. Mater. 388 (2020) 121996. DOI:10.1016/j.jhazmat.2019.121996 |
| [6] |
C. Qi, X. Liu, J. Ma, et al., Chemosphere 151 (2016) 280-288. DOI:10.1016/j.chemosphere.2016.02.089 |
| [7] |
Q. Chen, L. Chen, J. Qi, et al., Chin. Chem. Lett. 30 (2019) 1214-1218. DOI:10.1016/j.cclet.2019.03.002 |
| [8] |
S. Xiao, M. Cheng, H. Zhong, et al., Chem. Eng. J. 384 (2020) 123265. DOI:10.1016/j.cej.2019.123265 |
| [9] |
J. Wang, S. Wang, Chem. Eng. J. 334 (2018) 1502-1517. DOI:10.1016/j.cej.2017.11.059 |
| [10] |
M. Zhang, J. He, Y. Chen, et al., Chin. Chem. Lett. 31 (2020) 2721-2724. DOI:10.1016/j.cclet.2020.05.001 |
| [11] |
S. Nimai, H. Zhang, Z. Wu, N. Li, B. Lai, Chin. Chem. Lett. 31 (2020) 2657-2660. DOI:10.1016/j.cclet.2020.08.008 |
| [12] |
H. Zhang, Q. Ji, L. Lai, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 1129-1132. DOI:10.1016/j.cclet.2019.01.025 |
| [13] |
S. Yang, P. Wang, X. Yang, et al., J. Hazard. Mater. 179 (2010) 552-558. DOI:10.1016/j.jhazmat.2010.03.039 |
| [14] |
S.Y. Oh, H.W. Kim, J.M. Park, H.S. Park, C. Yoon, J. Hazard. Mater. 168 (2009) 346-351. DOI:10.1016/j.jhazmat.2009.02.065 |
| [15] |
G. Zhen, X. Lu, L. Su, et al., Water Res. 134 (2018) 101-114. DOI:10.1016/j.watres.2018.01.072 |
| [16] |
S. Lan, J. Feng, Y. Xiong, et al., Environ. Sci. Technol. 51 (2017) 6560-6569. DOI:10.1021/acs.est.6b06426 |
| [17] |
Y. Chen, X. Deng, J. Wen, J. Zhu, Z. Bian, Appl. Catal. B: Environ. 258 (2019) 118024. DOI:10.1016/j.apcatb.2019.118024 |
| [18] |
Y. Feng, L. Ling, Y. Wang, et al., Nano Energy 40 (2017) 481-486. DOI:10.1016/j.nanoen.2017.08.058 |
| [19] |
Z.L. Wang, J. Song, Science 312 (2006) 242-246. DOI:10.1126/science.1124005 |
| [20] |
Z.L. Wang, Adv. Mater. 24 (2012) 4632-4646. DOI:10.1002/adma.201104365 |
| [21] |
L. Pan, S. Sun, Y. Chen, et al., Adv. Energy Mater. 10 (2020) 2000214. DOI:10.1002/aenm.202000214 |
| [22] |
H. Huang, S. Tu, C. Zeng, et al., Angew. Chem. Int. Edit. 56 (2017) 11860-11864. DOI:10.1002/anie.201706549 |
| [23] |
S. Lan, Y. Chen, L. Zeng, et al., J. Hazard. Mater. 393 (2020) 122448. DOI:10.1016/j.jhazmat.2020.122448 |
| [24] |
S. Lan, X. Zeng, R.A. Rather, I.M.C. Lo, Environ. Sci. Nano 6 (2019) 554-564. DOI:10.1039/C8EN01192C |
| [25] |
Y. Feng, H. Li, L. Ling, et al., Environ Sci. Technol. 52 (2018) 7842-7848. DOI:10.1021/acs.est.8b00946 |
| [26] |
S. Tu, Y. Guo, Y. Zhang, et al., Adv. Funct. Mater. 30 (2020) 2005158. DOI:10.1002/adfm.202005158 |
| [27] |
P. Wang, X. Li, S. Fan, et al., Appl. Catal. B: Environ. 279 (2020) 119340. DOI:10.1016/j.apcatb.2020.119340 |
| [28] |
X. Liu, L. Xiao, Y. Zhang, H. Sun, J. Materiomics 6 (2020) 256-262. DOI:10.1016/j.jmat.2020.03.004 |
| [29] |
J. Wu, N. Qin, D. Bao, Nano Energy 45 (2018) 44-51. DOI:10.1016/j.nanoen.2017.12.034 |
| [30] |
S. Xu, L. Guo, Q. Sun, Z.L. Wang, Adv. Funct. Mater. 29 (2019) 1808737. DOI:10.1002/adfm.201808737 |
| [31] |
W. Lv, L. Kong, S. Lan, et al., J. Chem. Technol. Biot. 92 (2017) 152-156. DOI:10.1002/jctb.4981 |
| [32] |
X. Zhou, S. Wu, C. Li, et al., Nano Energy 66 (2019) 104127. DOI:10.1016/j.nanoen.2019.104127 |
| [33] |
X. Zhao, Y. Lei, P. Fang, et al., Nano Energy 66 (2019) 104168. DOI:10.1016/j.nanoen.2019.104168 |
| [34] |
M. Dai, W. Zheng, X. Zhang, et al., Nano lett. 20 (2019) 201-207. |
| [35] |
H. Zhang, J. He, C. Zhai, M. Zhu, Chin. Chem. Lett. 30 (2019) 2338-2342. DOI:10.1016/j.cclet.2019.07.021 |
| [36] |
Q. Wang, Y. Qiu, D. Yang, et al., Appl. Phys. Lett. 113 (2018) 053901. DOI:10.1063/1.5035309 |
| [37] |
J. Wang, Q. Zhang, F. Deng, X. Luo, D.D. Dionysiou, Chem. Eng. J. 379 (2020) 122264. DOI:10.1016/j.cej.2019.122264 |
| [38] |
C. Morell, A. Grand, A. Toro-Labbe, J. Phy. Chem. A 109 (2005) 205-212. DOI:10.1021/jp046577a |
| [39] |
J. Zhao, B. Yao, Q. He, T. Zhang, J. Hazard. Mater. 229 (2012) 151-158. |
| [40] |
R. Changotra, J.P. Guin, S.A. Khader, A. Dhir, J. Environ. Chem. Eng. 8 (2020) 104423. DOI:10.1016/j.jece.2020.104423 |
 2021, Vol. 32
2021, Vol. 32 

