b CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
Macrocyclic materials are characteristic of specific molecular recognition capability for analytes due to their unique structures and properties, and have found their use as selective stationary phases for gas chromatographic (GC) analyses, mainly including crown ethers [1], cyclodextrins (CD) [2-6], calixarenes [7-12] and cucurbit[n]urils (CB) [13-18], known as the first to fourth generation of macrocycles, respectively. Often, their modified derivatives of a macrocyclic scaffold were utilized or applied in combination with other materials such as polysiloxanes [1-3, 5, 9, 11] in order to improve their selectivity towards specific analytes.
Triptycene (TP) has a three-dimensional (3D) rigid π-rich structure with three arene units fused to the [2.2.2]bicycle-octatriene bridgehead. Its unique structure, easy functionality and high thermal stability facilitate the advancement of TP-based materials in supramolecular chemistry and materials chemistry [19, 20]. Also, they have shown good potential in chromatographic analyses in our previous work [21-26]. Recently, triptycene-derived heterocalixarenes with a fixed conformation have attracted growing attention thanks to their fascinating molecular recognition capabilities [27, 28]. One typical example is TP-derived oxacalixarene composed of TP and 1, 8-naphthyridine moieties (denoted as TDOC) [29, 30] (Fig. 1a), which differs from the conventional calixarenes in composing units, linkers, portal sizes and properties. TDOC contains two different moieties (triptycene, 1, 8-naphthyridine) linked via oxygen atoms while the conventional calixarenes consist of phenolic units connected by methylene bridges. The 3D rigid triptycene units in TDOC serve as anchors to stabilize its scaffold conformation and thus enhance its molecular recognition capabilities for guest molecules. On contrary, the conventional calixarenes have several conformational isomers resulting from the free rotation of the methylene bonds [7]. Moreover, TDOC has two semi-cavities encircled by triptycene moieties and naphthyridine moieties, respectively. As proven, the triptycene semi-cavity is aploar, moreπ-rich and crowded than the naphthyridine semi-cavity [30]. In contrast, the conventional calixarenes vary in shapes and sizes owing to their conformation variation.
|
Download:
|
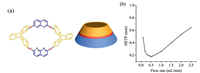
| Fig. 1. (a) Structure of TDOC and (b) the Golay curve of the TDOC capillary column determined by n-dodecane at 120 ℃. | |
On the basis of its distinctive structure and properties, TDOC is an ideal candidate as the stationary phase for GC analyses. To date, however, triptycene-derived macrocyclic materials have not been reported in the area of chromatography, which are quite worth exploration to advance their researches and applications in this area. Herein, we present the first example of the triptycene-derived macrocycles for chromatographic analyses.
First, TDOC was synthesized by the one-pot coupling reaction procedure described in Ref. [29]. Briefly, 2, 7-dichloro-1, 8-naphthyridine (2 mmol) and 2, 7-dihydroxytriptene (2 mmol) were refluxed in a 1, 4-dioxane solution for 48 h in the presence of Cs2CO3. Afterwards, the mixture was cooled to room temperature and extracted with CH2Cl2 and water (5:4). The product obtained from the organic layer was further purified by gel chromatography. As a result, the final product of TDOC was obtained as a white solid and confirmed by the characterization data from 1H NMR, 13C NMR and MALDI-TOF MS [29].1H NMR (300 MHz, CDCl3): δ 5.23 (s, 2H), 5.41 (s, 2H), 6.94-7.00 (m, 16H), 7.30-7.37 (m, 8H), 8.00 (d, 4H, J = 8.7 Hz).13C NMR (75 MHz, DMSO-d6): δ 163.5, 153.3, 150.6, 146.6, 145.8, 145.1, 141.3, 140.9, 125.1, 124.9, 124.3, 123.7, 123.4, 117.2, 116.6, 116.1, 112.2, 52.1, 51.4. MALDI-TOF MS: m/z 825.5 [M+H]+, 847.5 [M+Na]+, 863.4 [M+K]+.
Then, the TDOC capillary columns (10 m × 0.25 mm, i.d.) were prepared with its solution in dichloromethane (0.05%, w/v) at room temperature by the static coating method [21]. The coated column was conditioned from 40 ℃ to 200 ℃ at 1 ℃/min and held at 200 ℃ for 7 h under nitrogen. Next, the TDOC column was investigated in terms of column efficiency, polarity, separation performance and thermal stability. Its separation performance was evaluated by several mixtures consisting of a wide range of analytes and isomers, respectively.
Efficiencies of the TDOC column at different flow rates were measured by n-dodecane at 120 ℃ and then its Golay curve (Fig. 1b) was obtained by plotting the height equivalent to a theoretical plate (HETP) against flow rate. The column efficiency was 5679 plates/m at the minimum HETP, indicating the high efficiency of the TDOC column. Also, McReynolds constants were determined to evaluate the column polarity. The constants were measured at 120 ℃ by the five probe compounds, i.e., benzene (X'), 1-butanol (Y'), 2-pentanone (Z'), 1-nitropropane (U') and pyridine (S'). The general and average polarities were obtained by summing up and averaging the five constants, respectively. Table 1 provides the results, indicating the weak polarity of the TDOC column close to that of the commercial HP-5MS column.
|
|
Table 1 McReynolds constants of the TDOC and HP-5MS columns. |
Afterwards, the separation performance of the TDOC column was investigated by employing several mixtures consisting of a wide range of analytes and different types of isomers (skeletal, positional, cis-/trans-isomers). Meanwhile, the HP-5MS column was used for reference. High-resolution separation of diverse analytes and isomers in various samples is essential for their accurate determinations [31-34]. Figs. 2a and b shows the separation results for the mixture of 16 analytes with apolar to polar nature on the given columns. Notably, the TDOC column achieved good separation of all the analytes in contrast to two pairs of them coeluted on the HP-5MS column, i.e., methyl octoate/1, 6-dichlorohexane (peaks 9/10) and p-diisopropylbenzene/1, 2, 4-trichlorobenzene (peaks 11/12). High resolution of the pairs on the TDOC column results from its longer retention for the halogenated analytes via the halogen-bonding interactions (X⋯N=) between the analyte chlorine atoms and the nitrogen atoms on the naphthyridine rings. In addition, it is interesting to note that the TDOC column exhibited reversal elution for most of the analytes in contrast to the reference column. Observably, all the aromatics (peaks 2, 4, 6, 8, 10, 12) and the alcohols (peaks 14, 16) eluted later on the TDOC column because of their stronger π-π and H-bonding interactions (O-H⋯N=) with the stationary phase, respectively. Also, the halogen-bonding interactions contribute to the retention of those halogenated analytes. The above findings proved the different selectivity and retention behaviour of the TDOC stationary phase from the classic polysiloxane despite their comparable polarity. Fig. 2c presents a brief illustration on the selective interactions of the TDOC stationary phase with the typical analytes described above.

|
Download:
|
| Fig. 2. Separations of the mixture of 16 analytes on the TDOC column (a) and the HP-5MS column (b) and schematic illustration on the selective interactions of the TDOC stationary phase with the indicated analytes (c). Peaks: (1) 3-methylheptane, (2) toluene, (3) n-nonane, (4) o-xylene, (5) bromohexane, (6) 1-bromobenzene, (7) n-decane, (8) 1, 2, 4-trimethylbenzene, (9) methyl octoate, (10) 1, 6-dichlorohexane, (11) p-diisopropylbenzene, (12) 1, 2, 4-trichlorobenzene, (13) n-tridecane, (14) 1-nonanol, (15) n-tetradecane, (16) 1-decanol. GC conditions: 40 ℃ (1 min) to 160 ℃ at 10 ℃/min, flow rate at 1 mL/min. | |
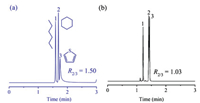
Another mixture was employed to testify the distinguishing capability of the TDOC stationary phase for aromatics from aliphatics. The mixture consisted ofn-hexane, cyclohexane and thiophene that share in common with either the carbon number (n-hexane, cyclohexane) or the molecular weight (cyclohexane, thiophene). As shown in Fig. 3, the TDOC column well resolved cyclohexane and thiophene in contrast to their partially overlapping on the reference column. These results further prove the advantageous performance of the TDOC stationary phase through its selective π-π interactions with aromatics.
|
Download:
|
| Fig. 3. Separations of the mixture of hexane, cyclohexane and thiophene on the TDOC column (a) and the HP-5MS column (b). Peaks: (1) n-hexane, (2) cyclohexane, (3) thiophene. GC conditions: 60 ℃, flow rate at 0.5 mL/min. | |
Further, its separation performance was investigated by the mixtures of different types of isomers (skeletal, positional, cis-/trans-). Figs. 4a-f show the separations of the isomer mixtures of alkanes (C6-C8), propyl/butylbenzenes, α-/β-pinene, cis-/trans- naphthane, nerol/geraniol and pentanols on the TDOC column, respectively. As known, separating alkane isomers presents a big challenge to the selectivity of a GC stationary phase owing to their high resemblance in structures and properties. From Fig. 4a, the TDOC column well resolved the alkane isomers, indicating its high selectivity towards these apolar isomers. Notably, the column showed preferential retention for linear alkanes to their branched isomers, suggesting its shape-fitting selectivity for the alkanes. On the principle "like attracts like", the apolar alkanes have higher affinity to the apolar triptycene portal via the CH-π interactions. The steric hindrances of the branched alkanes prevent their close contact with the triptycene moieties, leading their relatively shorter retention than their linear counterparts.

|
Download:
|
| Fig. 4. Separations of the mixtures of (a) alkane isomers, (b) propylbenzene and butylbenzene isomers, (c) pinene isomers, (d) cis-/trans-naphthane isomers, (e) nerol and geraniol isomers, and (f) pentanol isomers on the TDOC column. Peaks for (a): (1) 2, 2-dimethylbutane, (2) 3-methylpentane, (3) n-hexane, (4) 2, 4-dimethylpentane, (5) cyclohexane, (6) iso-octane, (7) n-heptane, (8) 2, 2-dimethylhexane, (9) 3-methylheptane, (10) n-octane. Oven temperatures: (a) 30 ℃, (b) 40 ℃ to 160 ℃ at 10 ℃/min, (c) 120 ℃, (d) 70 ℃, (e) 110 ℃ and (f) 40 ℃ to 140 ℃ at 10 ℃/min. Flow rate: 0.5 mL/min for (a, b, e, f) and 1.0 mL/min for (c, d). | |
Fig. 4b shows baseline separations of propylbenzenes and butylbenzenes, proving the high distinguishing capability of the TDOC stationary phase for alkylbenzenes with linear alkyl groups from those with branched alkyl groups. Its high-resolution performance can be ascribed to its different strengths of π-π and CH-π interactions with the alkylbenzenes resulting from their differences in steric hindrances. Fig. 4c shows the separation of α-pinene and β-pinene with a difference in the location of the double bond. β-pinene retained longer because of its stronger CH-π interaction with the stationary phase through its=CH2 group located outside the cyclohexane ring. In addition, the TDOC column achieved baseline separation of cis-/trans-naphthane isomers (Fig. 4d). Observably, the less bulky molecule of trans-naphthane than its cis-isomer allows its easier access to the TDOC cavity and thus has stronger CH-π interaction with the stationary phase, leading to their good separation. In brief, the shape-fitting differences of cis-/trans-isomer molecules with the cavity mainly contribute to their high-resolution separations on the stationary phase. Figs. 4e and f present the separations of nerol and geraniol and pentanol isomers, respectively, proving the high selectivity of the TDOC column for the polar analytes. Regarding the retention behaviours, the linear alcohol isomers retained longer due to their stronger H-bonding interactions with the nitrogen and oxygen atoms in the stationary phase. The above results demonstrate the high resolving performance of the TDOC column for a wide range of analytes with apolar to polar nature. Additionally, its column thermal stability was assessed by its bleeding profile. As shown in Fig. 5, the column was thermally stable up to 310 ℃, indicating its high thermal stability. The advantageous features of the TDOC column described above demonstrate its good potential for practical GC analyses.

|
Download:
|
| Fig. 5. Bleeding profile of the TDOC column determined from 40 ℃ to 380 ℃ at 3 ℃/min under nitrogen. | |
In summary, we report a new type of macrocyclic materials, i.e., a triptycene-derived heterocalixarene, as the stationary phase for gas chromatography. This is the first example of employing TDOC-based macrocycles for chromatographic analyses. As demonstrated, the TDOC column exhibits advantageous separation performance towards a wide range of analytes and isomers and high column thermal stability. This work demonstrates their good potential as a new class of selective macrocyclic stationary phases for chromatographic analyses.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentThe authors are grateful for the financial support by the National Natural Science Foundation of China (No. 21575013).
| [1] |
R.M. Kakhki, J. Incl. Phenom. Macrocycl. Chem. 75 (2013) 11-22. DOI:10.1007/s10847-012-0158-0 |
| [2] |
Y. Xiao, S. Ng, T. Tan, et al., J. Chromatogr. A 1269 (2012) 52-68. DOI:10.1016/j.chroma.2012.08.049 |
| [3] |
S. Fox, H. Strasdeit, S. Haasmann, et al., J. Chromatogr. A 1411 (2015) 101-109. DOI:10.1016/j.chroma.2015.07.082 |
| [4] |
X. Shi, F. Liu, J. Mao, Anal. Chim. Acta 912 (2016) 156-162. DOI:10.1016/j.aca.2016.01.037 |
| [5] |
H. Schmarr, M. Mathes, K. Wall, et al., J. Chromatogr. A 1516 (2017) 135-141. DOI:10.1016/j.chroma.2017.08.010 |
| [6] |
X. Shi, Y. Zhou, F. Liu, et al., J. Chromatogr. A 1596 (2019) 161-174. DOI:10.1016/j.chroma.2019.02.063 |
| [7] |
B. Mokhtari, K. Pourabdollah, N. Dalali, Chromatographia 73 (2011) 829-847. DOI:10.1007/s10337-011-1954-1 |
| [8] |
O. Gezici, M. Bayrakci, J. Incl. Phenom. Macrocycl. Chem. 83 (2015) 1-18. DOI:10.1007/s10847-015-0553-4 |
| [9] |
L. Lin, C. Wu, Z. Yan, et al., Chromatographia 47 (1998) 689-694. DOI:10.1007/BF02467455 |
| [10] |
J. Shi, Q. Jia, S. Xu, Chromatographia 75 (2012) 779-787. DOI:10.1007/s10337-012-2256-y |
| [11] |
G. Delahousse, V. Peulon-Agasse, J. Debray, et al., J. Chromatogr. A 1318 (2013) 207-216. DOI:10.1016/j.chroma.2013.10.007 |
| [12] |
T. Sun, L. Qi, W. Li, et al., J. Chromatogr. A 160 (2019) 310-318. |
| [13] |
P. Zhang, S. Qin, M. Qi, et al., J. Chromatogr. A 1334 (2014) 139-148. DOI:10.1016/j.chroma.2014.01.083 |
| [14] |
L. Wang, X. Wang, M. Qi, et al., J. Chromatogr. A 1334 (2014) 112-117. DOI:10.1016/j.chroma.2014.01.070 |
| [15] |
T. Sun, N. Ji, M. Qi, et al., J. Chromatogr. A 1343 (2014) 167-173. DOI:10.1016/j.chroma.2014.03.084 |
| [16] |
X. Wang, M. Qi, R. Fu, J. Chromatogr. A 1371 (2014) 237-243. DOI:10.1016/j.chroma.2014.10.066 |
| [17] |
Y. Zhang, M. Qi, R. Fu, Chin. Chem. Lett. 27 (2015) 88-90. |
| [18] |
J. He, J. Ran, J. Yao, et al., Front. Chem. 8 (2020) 31. DOI:10.3389/fchem.2020.00031 |
| [19] |
J. Chong, M.J. MacLachlan, Chem. Soc. Rev. 38 (2009) 3301-3315. DOI:10.1039/b900754g |
| [20] |
Y. Jiang, C. Chen, Eur. J. Org. Chem. 2011 (2011) 6377-6403. DOI:10.1002/ejoc.201100684 |
| [21] |
Y. Yang, Q. Wang, M. Qi, et al., Anal. Chim. Acta 988 (2017) 121-129. DOI:10.1016/j.aca.2017.07.070 |
| [22] |
L. Yu, J. He, M. Qi, et al., J. Chromatogr. A 1599 (2019) 239-246. DOI:10.1016/j.chroma.2019.04.033 |
| [23] |
J. He, M. Qi, Chin. Chem. Lett. 30 (2019) 1415-1418. DOI:10.1016/j.cclet.2019.03.008 |
| [24] |
T. Shi, M. Qi, X. Huang, J. Chromatogr. A 1614 (2019) 460714. |
| [25] |
Y. He, M. Qi, J. Chromatogr. A 1618 (2020) 460928. DOI:10.1016/j.chroma.2020.460928 |
| [26] |
Q. Yuan, M. Qi, J. Chromatogr. A 1621 (2020) 461084. DOI:10.1016/j.chroma.2020.461084 |
| [27] |
Y. Ma, Y. Han, C. Chen, J. Incl. Phenom. Macrocycl. Chem. 79 (2014) 261-281. DOI:10.1007/s10847-013-0372-4 |
| [28] |
C. Chen, Y. Han, Acc. Chem. Res. 51 (2018) 2093-2106. DOI:10.1021/acs.accounts.8b00268 |
| [29] |
S. Hu, C. Chen, Chem. Commun. 46 (2010) 4199-4201. DOI:10.1039/c002944k |
| [30] |
H. Wang, Z. Meng, J. Xiang, et al., Chem. Sci. 7 (2016) 469-474. DOI:10.1039/C5SC03511B |
| [31] |
J. Chen, Y. Huang, X. Wei, et al., Chem. Commun. 55 (2019) 10908-10911. DOI:10.1039/C9CC05307G |
| [32] |
W. Tang, J. Xu, Z. Gu, Chem. Asian J. 14 (2019) 3462-3473. DOI:10.1002/asia.201900738 |
| [33] |
Y. He, M. Qi, Chin. J. Chromatogr. 38 (2020) 409-413. |
| [34] |
Y. He, M. Qi, Sci. Sin. Chim. 50 (2020) 1142-1150. DOI:10.1360/SSC-2020-0078 |
 2021, Vol. 32
2021, Vol. 32 


