b School of Pharmaceutical Science, Shanxi Medical University, Taiyuan 030001, China
Fluorescence is a sensitive output signal used to detect biological targets and monitor processes [1]. However, biological targets, except for some native fluorescent and bioluminescent proteins, on their own usually do not have fluorescence signals [2, 3]. Thus a fluorescent probe is needed to illuminate the non-fluorescent biological molecules [4]. According to their response upon detecting targets, fluorescent probes can be divided into three classes, fluorescent light-up probes [5-7], fluorescent light-off probes [8] and always-on probes [9]. Among these three types of probes, fluorescent light-up probes are the most favoured because they have little background fluorescence signal. Therefore, their usage is non-invasive and does not require wash steps. To develop the light-up probe, a fluorescence-quenching group is usually employed. Quenching groups include but are not limited to Disperse Red 1, water-soluble groups and nitro groups (NO2). Disperse Red 1 quenches fluorescence through fluorescence resonance energy transfer (FRET) [5, 6]. In addition to Disperse Red 1, there are various similar quenching groups, such as black hole quenchers (BHQs) [10], QSY quenchers [11] and multi-path quenchers (MPQ) [12]. During the design of FRET-based light-up probes, fluorophores and quenchers should be carefully selected so that the emission spectra of the fluorophores overlap well with the absorption spectra of the quenchers. Water soluble groups could increase the dye's solubility and mobility, allowing quenching of the fluorophore emission with the characteristics of aggregation-induced emission (AIE) [13]. In this unique quenching process, the energy of excited AIE dyes was consumed by molecular motion, but not by irradiative fluorescence emission [14, 15]. The nitro group quenches fluorescence through a photo-induced electron transfer (PET) process. The maximum emission wavelength of fluorophores quenched by nitro groups ranges from 450 nm to 637 nm [16-20]. In addition to its small size and broadly-quenched spectra, another advantage of the nitro group as a quencher is that it can be readily removed via cleavage reaction. Thus, nitro groups are often used as quenchers in the development of fluorescent light-up probes [16-20]. These groups have also frequently been employed together with other quenching groups in the design of dual quenching probes. For example, the combination of a nitro group and a water soluble group rendered the fluorescence of an AIE dye super-quenched [17]. This dual-quenched probe showed a remarkable 4920-fold fluorescence increase upon reaction with formaldehyde. Although NO2 exhibits the three abovementioned advantages, its PET effect can be readily affected by the surrounding environment. Sometimes, it had no quenching effect [21]. To date, there has been no systematic study on the effects of electron-withdrawing groups on the quenching efficiency of nitro groups. Here, we report a fundamental study on the fluorescence-quenching effect of nitro groups in the presence of electron-withdrawing groups. By introducing various groups to nitro group-containing AIE dyes, we found that the electron-withdrawing ability of the introduced groups played an important role in the quenching effect of the nitro groups.
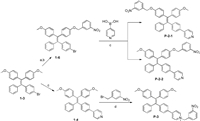
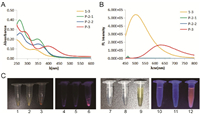
We selected a classical AIE dye (tetraphenylethene, TPE) as the core fluorophore that can be readily synthesized and modified [22, 23]. The first batch of three designed compounds (P-2-1, P-2-2 and P-3) is shown in Scheme 1. P-2-1 and P-2-2 are a pair of cis/trans isomers where the nitro group was introduced via the oxygen atom. P-3 contains a pyridinium salt introduced from the nitrogen atom as the quenching group. All three compounds were readily synthesized according to the Scheme 1 and their structures were verified by high resolution mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy. Notably, P-2-1 and P-2-2 cannot be separated by silica gel chromatography but can be easily separated by reversed-phase HPLC. Then, we measured the optical properties of these samples. P-2-1 and P-2-2 gave rise to similar absorption spectra (Fig. 1A, Table S1 in Supporting information). However, they had no fluorescent emission regardless of whether they were dispersed in aqueous solution or in the solid state (Figs. 1B and C). AIEgens usually emit bright fluorescence because of restricted intramolecular motion (RIM) [14]. The nitro group could therefore efficiently quench the inherent fluorescent signals of P-2-1 and P-2-2. To our surprise, P-3 exhibited significant fluorescence under the same conditions, and the maximum emission wavelength for P-3 in aqueous media was 620 nm (Figs. 1B and C). P-3 actually contained two quenching elements, the nitro group and a water soluble group (pyridinium salts). In principle, P-3 should have lower intensity of fluorescence than P-2-1 and P-2-2 because the additional soluble group could increase the intramolecular motion. This unexpected result indicated that the nitro group in P-3 lost its quenching effect because of the pyridinium salt.
|
Download:
|
| Scheme 1. Synthesis of TPE-based fluorophores with nitro groups at different positions. (a) BBr3, CH2Cl2, r.t., 2 h, 32%; (b)m-nitrobenzyl bromide, K2CO3 in acetonitrile, 60℃ reflux for 8 h, 88%; (c) 4-pyridinylboronic acid, Pd(dppf)Cl2, Bu4NI, K2CO3 in toluene, 110℃ reflux for 18 h, 39% for P-2-1 and P-2-2, 56% for 1-4; (d) m-nitrobenzyl bromide in toluene, 110℃ reflux for 24 h, 83%. | |
|
Download:
|
| Fig. 1. Absorption (A) and fluorescence emission (B) spectra of 1-3, P-2-1, P-2-2 and P-3 in water (10 μmol/L) (excited at 330 nm (1-3), 400 nm with scan slits of 2.00, 4.00, 4.00 and 2.00, respectively). (C) Images of P-2-1 (1, 4, 7, 10), P-2-2 (2, 5, 8, 11) and P-3 (3, 6, 9, 12) in the solid state (1-6) or in aqueous media (7-12) under white light (1-3, 7-9) or 365 nm excitation (4-6, 10-12). | |
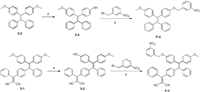
To further investigate the quenching effect of the electron-withdrawing group, we next designed and synthesized a second batch of fluorophores with another quenching group (Scheme 2). In this batch, the dicyanovinyl (DCV) group was conjugated to the TPE core. A control compound without a DCV group was also designed. These compounds were readily synthesized according to Scheme 2. Compound P-5 was also a mixture of cis/trans isomers, and we tried to separate these two isomers but failed. As the separated cis/trans isomers of P-2 had similar optical properties, we used a mixture of cis and trans isomers of P-5 in the following study. As shown in Figs. 2B and C, P-5 and 3-1 had similar absorption and emission spectra. Both samples were strongly fluorescent in aqueous solution and in the solid state. Moreover, P-5 showed typical AIE properties when water was added to a solution of P-5 in ethanol (Fig. S1 in Supporting information). However, the control compound without an electron-withdrawing group (P-4) exhibited little fluorescence under the same conditions. This result verified the abovementioned implication that strong electron-withdrawing group could ablate the quenching effect of the nitro group. This result is consistent with the observation that the probe (DCDHF-βgal) developed by Kim et al. was a ratiometric probe but not a light-up probe [21]. DCDHF-βgal had significant background fluorescence signal even though it contains a nitro group. On the basis of our experimental results, we ascribed the high fluorescence signal of DCDHF-βgal to the DCV group in the probe. This observation also indicated that the phenomenon observed in AIE dyes was also applied to other fluorophores without AIE properties.
|
Download:
|
| Scheme 2. Synthesis of TPE-based fluorophores P-4 and P-5. (a) BBr3, CH2Cl2, r.t., 2 h, 45% for 2-3, 83% for 3-2; (b) m-nitrobenzyl bromide, K2CO3 in acetonitrile, 70℃ reflux for 4 h, 78% for P-4, 89% for P-5. | |

|
Download:
|
| Fig. 2. Absorption (A) and fluorescence emission (B) spectra of 3-1, P-4 and P-5 in water (10 μmol/L) (excited at 400 nm with scan slits of 2.00, 3.50 and 2.00, respectively). (C) Images of 3-1 (3, 6, 9, 12), P-4 (1, 4, 7, 10) and P-5 (2, 5, 8, 11) in the solid state (1-6) and in aqueous media (7-12) under white light (1-3, 7-9) or 365 nm excitation (4-6, 10-12). | |
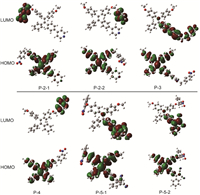
To fully understand the above optical properties, we carried out time-dependent density functional theory (TD-DFT) calculations at the B3LYP/6-31G level to investigate the effect of electron-withdrawing groups. As shown in Fig. 3, all six compounds have separated HOMOs and LUMOs. Their HOMOs were approximately the same and were localized on the TPE core. For P-2-1 and P-2-2 where the nitro group had a fluorescence-quenching effect, their LUMOs were distributed around the nitro group, indicating an efficient intramolecular PET process. However, for P-3 where the nitro group had no quenching effect, the LUMOs were mainly distributed in the vicinity of the pyridinium salts. This result was in line with another report that protonated indoles could cause the nitro group to lose its quenching effect [24]. In addition, the LUMOs of compound P-5 were mainly distributed on the DCV group instead of the nitro group. Together, these strong electron-withdrawing groups changed the LUMO distribution and turned on fluorescence in the presence of nitro groups.
|
Download:
|
| Fig. 3. Molecular orbitals (MOs) involved in transitions S1−S0 of the probes, calculated with TD-DFT at the B3LYP/6-31G level using the polarizable continuum model (PCM) assuming solvent water.P-5-1 and P-5-2 are the trans and cis isomers of P-5. | |
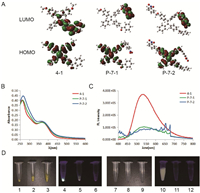
The TD-DFT calculations indicated that the electron-withdrawing groups and nitro groups compete to capture the LUMOs. We therefore assumed that the nitro group may regain its quenching effect if we change the electron-withdrawing group to another group with a lower ability to withdraw electrons. To test this assumption, we changed the electron-withdrawing group to a ketone group and designed compounds P-7-1 and P-7-2. The calculation of the Mulliken atomic charge distribution indicated that the ketone group had a lower electron-withdrawing ability than the pyridinium salt and DCV groups (Table S2 in the Supporting information). We first performed the TD-DFT calculations to investigate the HOMOs and LUMOs of P-7-1/P-7-2 together with compound 4-1. As shown in Fig. 4A, the LUMO of 4-1 without NO2 was primarily distributed on the ketone group, while the LUMOs of P-7-1/P-7-2 were primarily distributed on the nitro groups, suggesting the potential existence of PET and the weak fluorescence of P-7-1 and P-7-2. We next synthesized the P-7-1/P-7-2 to determine whether the calculation results were correct. P-7-1 and P-7-2 were synthesized according to Scheme 3. P-7-1/P-7-2 gave rise to absorption spectra similar to that of compound 4-1 without a nitro group (Fig. 4B, Table S1). However, their emission spectra differed greatly. Compound 4-1 exhibited obvious fluorescence, with an emission maximum of 550 nm. The fluorescence intensity of compounds P-7-1 and P-7-2 at 550 nm decreased by 3.50- and 4.32-fold, respectively (Fig. 4C). Both compounds had rather weak fluorescence in aqueous media and the solid state (Fig. 4D). This result strongly supported the implication of the TD-DFT calculation that the nitro group could quench the fluorescence of TPE in the presence of a ketone group.
|
Download:
|
| Fig. 4. (A) MOs involved in transitions S1−S0 of the probes, calculated with TD-DFT at the B3LYP/6-31G level using a polarizable continuum model (PCM) assuming solvent water. Absorption (B) and fluorescence emission (C) spectra of 4-1, P-7-1 and P-7-2 in water (10 μmol/L) (excited at 372 nm with scan slits of 1.20, 2.50 and 3.00, respectively). (D) Images of 4-1 (1, 4, 7, 10), P-7-1 (2, 5, 8, 11) and P-7-2 (3, 6, 9, 12) in the solid state (1-6) or in aqueous media (7-12) under white light (1-3, 7-9) or 365 nm excitation (4-6, 10-12). | |

|
Download:
|
| Scheme 3. Synthesis of TPE-based fluorophores P-7-1 and P-7-2. (a) BBr3, CH2Cl2, rt., 2 h, 40% for 4-2, 40% for 4-3; (b) m-nitrobenzyl bromide, K2CO3 in acetonitrile, 70℃ reflux for 4 h, 67% for P-7-1, 80% for P-7-2. | |
In conclusion, we have examined the quenching effect of nitro groups in the presence of electron-withdrawing groups. Both the experimental results and TD-DFT calculations indicate that strong electron-withdrawing groups can abolish the quenching effect of nitro groups. Decreasing the electron-withdrawing ability can help the nitro group retain the fluorescent quenching effect. This study helps us to comprehensively understand the quenching effect of nitro groups and will thus provide a guideline for the development of light-up probes with nitro groups as quenching groups.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentWe thank the start-up funding from the State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Peking Union Medical College, CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2017-I2M-4−005).
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi: https://doi.org/10.1016/j.cclet.2021.02.008.
| [1] |
T. Terai, T. Nagano, Pflugers Arch. Eur. J. Physiol. 465 (2013) 347-359. DOI:10.1007/s00424-013-1234-z |
| [2] |
N.C. Shaner, P.A. Steinbach, R.Y. Tsien, Nat. Methods 2 (2005) 905-909. DOI:10.1038/nmeth819 |
| [3] |
D.M. Close, T. Xu, G.S. Sayler, et al., Sensors 11 (2011) 180-206. |
| [4] |
A. Nadler, C. Schultz, Angew. Chem. Int. Ed. 52 (2013) 2408-2410. DOI:10.1002/anie.201209733 |
| [5] |
T. Komatsu, K. Johnsson, H. Okuno, et al., J. Am. Chem. Soc. 133 (2011) 6745-6751. DOI:10.1021/ja200225m |
| [6] |
C.J. Zhang, L. Li, G.Y.J. Chen, et al., Org. Lett. 13 (2011) 4160-4163. DOI:10.1021/ol201430x |
| [7] |
X. Zeng, Z. Chen, L. Tang, et al., Chem. Commun. 55 (2019) 2541-2544. DOI:10.1039/C8CC10286D |
| [8] |
L.D. Newton, S.I. Pascu, R.M. Tyrrell, et al., Org. Biomol. Chem. 17 (2019) 467-471. DOI:10.1039/C8OB02290A |
| [9] |
N. George, H. Pick, H. Vogel, et al., J. Am. Chem. Soc. 126 (2004) 8896-8897. DOI:10.1021/ja048396s |
| [10] |
R.M. Cook, M. Lyttle, D. Dick, US PT 7019129B1 (2006). |
| [11] |
R.P. Haugland, V.L. Singer, S.T. Yue, US PT 6399392 (2002). |
| [12] |
P. Crisalli, E.T. Kool, Bioconjugate Chem. 22 (2011) 2345-2354. DOI:10.1021/bc200424r |
| [13] |
H. Shi, R.T. Kwok, J. Liu, et al., J. Am. Chem. Soc. 134 (2012) 17972-17981. DOI:10.1021/ja3064588 |
| [14] |
J. Mei, N.L.C. Leung, R.T.K. Kwok, et al., Chem. Rev. 115 (2015) 11718-11940. |
| [15] |
Y. Chen, Y. Fang, H. Gu, et al., ACS Appl. Mater. Interfaces 12 (2020) 55094-55106. DOI:10.1021/acsami.0c16585 |
| [16] |
M. Wei, P. Yin, Y. Shen, et al., Chem. Commun. 49 (2013) 4640-4642. DOI:10.1039/c3cc39045d |
| [17] |
H. Yang, F. Wang, J. Zheng, et al., Chem. Asian J. 13 (2018) 1432-1437. DOI:10.1002/asia.201800530 |
| [18] |
A. Sarkar, C. Fouzder, S. Chakraborty, et al., Chem. Res. Toxicol. 33 (2020) 651-656. DOI:10.1021/acs.chemrestox.9b00462 |
| [19] |
Q. Jiang, Z. Zhang, J. Lu, et al., Bioorg. Med. Chem. 21 (2013) 7735-7741. DOI:10.1016/j.bmc.2013.10.019 |
| [20] |
S. Wu, Y. Li, T. Deng, et al., Org. Biomol. Chem. 18 (2020) 2468-2474. DOI:10.1039/D0OB00020E |
| [21] |
E.J. Kim, R. Kumar, A. Sharma, et al., Biomaterials 122 (2017) 83-90. |
| [22] |
L. Ma, C. Li, Q. Yan, et al., Chin. Chem. Lett. 31 (2020) 361-364. DOI:10.1016/j.cclet.2019.07.040 |
| [23] |
Q. Qi, S. Jiang, Q. Qiao, et al., Chin. Chem. Lett. 30 (2020) 1078-1082. |
| [24] |
K. Tateno, R. Ogawa, R. Sakamoto, et al., Org. Lett. 16 (2014) 3212-3215. DOI:10.1021/ol501226x |
 2021, Vol. 32
2021, Vol. 32 

