b School of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China;
c Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, Changsha 410114, China
The primary goal of green chemistry is to make full use of renewable materials while minimizing the generation of chemical waste. For this reason, the development of a sustainable synthesis plan is of particular importance [1]. Much effort has been invested in developing green solvents over the decades [2]. Among these eco-friendly solvents, 2-MeTHF has received considerable attention [3], because it can be obtained from biomass feedstocks as a safe and innocuous solvent, and it is in line with the 3rd, 5th and 7th principles of Green Chemistry. Medical and synthetic chemistry require the construction of complex molecules with biological activity and diversity, for which tandem reaction is a powerful synthetic tool, because they can efficiently reduce the overall steps and minimize the generation of chemical waste [4]. On the other hand, visible light catalysis has attracted much attention in chemical and pharmaceutical industries [5]. However, most photocatalytic processes require precious metals and/or toxic transition metals or harmful organic photosensitizers, which not only generate waste, but also increase production costs, leading to cumbersome purification procedures and environmental problems. Therefore, an important goal of green chemistry is to develop visible light-induced tandem reactions without external photosensitizers [6].
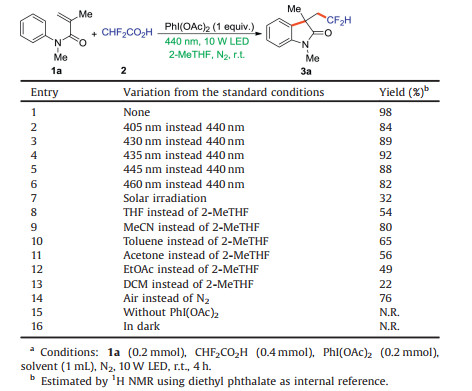
Oxindole motifs exist widely in plenty of natural products, biologically active molecules and synthetic drugs [7]. It is of particular interest to build oxindoles with the difluoromethyl group (CHF2) [8], because CHF2 is a bioisostere of alcohol and thiol groups and may act as a lipophilic hydrogen donor via H-bonding. Accordingly, increasing research efforts have been made in the development of efficient methods for constructing difluoromethylated oxindoles [9]. In 2014, Tan and colleagues [10] pioneered the AgNO3-catalyzed difluoromethylation of N-arylacrylamides with Zn(CHF2SO2)2 in the presence of (NH4)2S2O8 as the oxidant under heating conditions (Scheme 1a). Dolbier [11] and Liu [12] have, respectively, developed the visible-light-induced difluoromethylation of N-arylacrylamides by using different CHF2 sources (CHF2SO2Cl and 2-PySO2CHF2) under alkaline conditions (Schemes 1b and c). The electrochemical difluoroalkylation reactions with CHF2SO2Na and CHF2SO2NHNHBoc under alkaline conditions have been reported by the groups of Xu [13], Ruan and Ackermann [14] (Schemes 1d and e). Despite significant achievements, however, the above-mentioned difluoromethylation reagents not only have limited commercial availability but also are highly costly and harmful. From an eco-friendly point of view, those procedures still suffer from some drawbacks, such as employing expensive and/or harmful metal catalyst, excess chemical oxidants and base additives, toxic organic solvents as well as poor to moderate yields. Therefore, the exploration of environmentally friendly synthetic protocols for synthesizing difluoromethylated 2-oxindoles using cheap and readily available difluoromethylation reagents is of great importance and is highly desirable.
|
Download:
|
| Scheme 1. Synthesis of difluoromethylated oxindoles. | |
Difluoroacetic acid is a low-cost, simple-to-handle and easily available bulk chemical. The direct usage of CHF2CO2H as the difluoromethylation reagent to provide a CHF2 motif is a preferable process [8h]. With our interests on developing green organic synthesis [15], we report herein an eco-friendly protocol for the construction of various difluoromethylated oxindoles via visible-light initiated (Principle 6th) one-pot tandem reaction (Principle 8th) of N-arylacrylamides, CHF2CO2H and PhI(OAc)2 in biomass-derived 2-MeTHF (Principle 5 th) under additive-, base-, metal catalyst-, external photosensitizer-free (Principle 1st) and mild conditions (Scheme 1f).
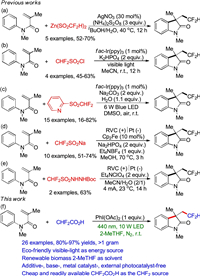
Initially, N-methyl-N-phenylmethacrylamide (1a), CHF2CO2H (2) and PhI(OAc)2 were chosen as the template substrates to screen the reaction conditions (Table 1). Employing PhI(OAc)2 as the promoter, and 2-MeTHF as the solvent, the desired product 3a generated in 98% NMR yield under the irradiation of a 10 W LED (440 nm) at ambient temperature (Table 1, entry 1). We found that the irradiation wavelength of visible-light was of significant importance for the present transformation (entries 2–6), and the irradiation wavelength of 440 nm was the most suitable. Performing the reaction under solar irradiation led to the generation of polycyclic products 3a in 32% yield (entry 7). Replacing 2-MeTHF with THF, MeCN, toluene, acetone, EtOAc or DCM provided a lower yield (entries 8–13). Performing the reaction under air atmosphere instead of nitrogen atmosphere led to a lower yield of 3a (entry 14). The control experiments showed that no reaction took place when this reaction was performed in the absence of PhI(OAc)2 or visible-light irradiation (entries 15 and 16).
|
|
Table 1 Optimization of reaction conditions.a |
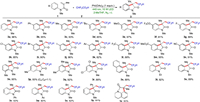
With the optimized procedure developed (Table 1, entry 1), the generality of the visible-light-initiated tandem reaction was then investigated (Scheme 2). A variety of substituted N-arylacrylamides with electron-neutral, electron-donating or electron-withdrawing groups at the para position of phenyl ring were subjected to the present reaction, providing the desired products in excellent yields. Notably, a wide range of valuable functional-groups such as phenyl (3b), alkyl (3c and 3d), alkoxy (3e and 3f), halogens (3g-3j), acetyl (3k), trifluoromethyl (3l), ester (3m) and nitrile (3n) were all tolerated in this approach. N-arylacrylamide substrates containing functional groups at various positions of the phenyl ring were well compatible and provided the target products (3c, 3o-3r) in high yields. When meta-substituted substrate 1p was subjected to the reaction, a 1:1 mixture of two regioisomers 3p and 3p' was obtained. The naphthyl substrate was also suitable substrates for this transformation (3s). Among them, products containing halogens, esters and nitriles may be further functionalized to construct more complex molecules. N-Arylacrylamides containing alkyl, phenyl and acetyl groups at the nitrogen atom underwent the reaction smoothly and provided the corresponding products (3t-3w) in excellent yields. The substrates modified with alkyl and phenyl substituents on the α-position of vinyl group delivered the target products (3x and 3y) in good yields. When N-phenylmethacrylamide was used as the reaction substrate, no desired product was detected. This reaction could also be carried out in the presence of a tetrahydroquinoline moiety, generating the tricyclic oxindole (3z) in 83% yield. No reaction occurred when 2, 2-difluoro-2-phenylacetic acid was instead of difluoroacetic acid.
|
Download:
|
| Scheme 2. Reaction scope. Conditions: 1 (0.3 mmol), CHF2CO2H (0.6 mmol), PhI(OAc)2 (0.3 mmol), 2-MeTHF (1.5 mL), N2, 10 W LED, r.t. 4 h. | |
To investigate the practical applicability of this protocol, the tandem reaction was performed on a gram scale (5 mmol). As expected, the yield of 3a (90%) was similar to that from the small-scale synthesis (Scheme 3).

|
Download:
|
| Scheme 3. Large scale experiment. | |
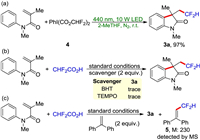
To gain insight into the mechanism of the present tandem reaction, we conducted a series of control experiments. When the model reaction of 1a, 2 and PhI(OAc)2 was performed, we observed the formation of [bis(difluoroacetoxy)iodo]benzene (4) [16]. We envisioned that this reaction probably proceeded via a ligand exchange and a tandem difluoromethylation reaction to provide the product 3a. To prove our hypothesis, we treated 1a and compound 4 in 2-MeTHF under the irradiation of a 10 W LED (440 nm). As expected, the desired product 3a was formed in 97% NMR yield (Scheme 4a). This result suggested that compound 4 was the key intermediate for the present reaction. When 2 equiv. of a radical scavenger (TEMPO or BHT) was added to the reaction system, the desired reaction was seriously inhibited (Scheme 4b). The free-radical capturing adduct (diphenylethylene-CHF2, 5) was also detected by GC-MS (Scheme 4c). The On/Off light illumination revealed that this transformation was fully suppressed in the absence of light irradiation (Fig. 1). The UV–vis absorption experiments revealed that compound 4 could absorb ultraviolet-visible light and serve as the photosensitizer in the present reaction. These results demonstrated that the continuous visible-light illumination is necessary for the developed photocatalytic reaction.
|
Download:
|
| Scheme 4. Control experiments. | |

|
Download:
|
| Fig. 1. On/Off light illumination experiment. | |
On the basis of the above mentioned results and previously reported literatures [7d, 16, 17], a plausible mechanism is proposed (Scheme 5). The ligand exchange between the CHF2CO2H (2) and PhI(OAc)2 first occurred, leading to the formation of bis(difluoroacetoxy)iodo]benzene (4). The intermediate 4 was subsequently excited by visible-light (440 nm) to generate an iodanyl radical A and a difluoroacetic acid radical. The decarboxylation of difluoroacetic acid radical would provide a CHF2 radical, which reacted with substrate 1a to form a carbon-centered radical B. Next, a single-electron-transfer (SET) process occurred between the radical A and the radical B, resulting in the generation of cationic intermediate C, difluoro-acetate anion and PhI. Finally, the difluoroacetate anion promoted the deprotonation of the intermediate C, which would provide the target product 3a and CHF2CO2H.
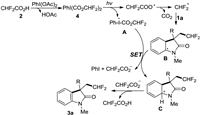
|
Download:
|
| Scheme 5. Plausible reaction mechanism. | |
In summary, we have developed the first example of visible-light induced one-pot tandem reaction of N-arylacrylamides, CHF2CO2H and PhI(OAc)2, providing an eco-friendly and practical method to access various difluoromethylated oxindoles. In contrast to the previous reported protocol, this reaction is promoted by visible light in the absence of any additive, base, metal or external photosensitizer, employing cheap and easily available CHF2CO2H as the difluoromethylation reagent and bulk biomass-derived 2-MeTHF as the reaction medium. Owing to the easily-obtained and low cost starting materials, clean and mild conditions, simple operation procedure and excellent reaction efficiency, this process is expected to be an ideal strategy for preparing CHF2-containing molecules
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe are grateful for financial support from Hunan Provincial Natural Science Foundation of China (Nos. 2018JJ3215 and 2019JJ20008), the Research Foundation of Education Bureau of Hunan Province (No. 18C0147) and Double First-class Construction Project of Hunan Agricultural University (No. SYL2019064).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi: https://doi.org/10.1016/j.cclet.2021.01.021.
| [1] |
(a) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30 (2019) 2132-2138; (b) Y. Kim, C.J. Li, Green Synth. Catal. 1 (2020) 1-11. |
| [2] |
(a) C.J. Clarke, W.C. Tu, O. Levers, A. Bröhl, J.P. Hallett, Chem. Rev. 118 (2018) 747-800; (b) M. Li, F. Wu, Y. Gu, Chin. J. Catal. 40 (2019) 1135-1140; (c) L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30 (2019) 1237-1240; (d) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30 (2019) 2287-2290; (e) Q. Sun, L. Liu, Y. Yang, Z. Zha, Z. Wang, Chin. Chem. Lett. 30 (2019) 1379-1382; (f) F. Gao, R. Bai, F. Ferlin, et al., Green Chem. 22 (2020) 6240-6257; (g) F.H. Qin, X.J. Huang, Y. Liu, et al., Chin. Chem. Lett. 31 (2020) 3267-3270. |
| [3] |
(a) Q. Zhen, L. Chen, L. Qi, et al., Chem. Asian J. 15 (2020) 106-111; (b) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30 (2019) 2259-2262; (c) Y. Wang, J. Shen, Q. Chen, L. Wang, M. He, Chin. Chem. Lett. 30 (2019) 409-412. |
| [4] |
(a) F. Wang, T.Q. Wei, P. Xu, S.Y. Wang, S.J. Ji, Chin. Chem. Lett. 30 (2019) 379-382; (b) Y. Zhang, K. Sun, Q. Lv, et al., Chin. Chem. Lett. 30 (2019) 1361-1368; (c) J. Chen, L. Wu, J. Wu, Chin. Chem. Lett. 31 (2020) 2993-2995; (d) F.S. He, Y. Yao, W. Xie, J. Wu, Chem. Commun. 56 (2020) 9469-9472; (e) Q.W. Gui, F. Teng, S.N. Ying, et al., Chin. Chem. Lett. 31 (2020) 3241-3244. |
| [5] |
(a) X. Mi, Y. Kong, J. Zhang, C. Pi, X. Cui, Chin. Chem. Lett. 30 (2019) 2295-2298; (b) D.Q. Dong, L.X. Li, G.H. Li, et al., Chin. J. Catal. 40 (2019) 1494-1498; (c) M. Fu, X. Ji, Y. Li, G.J. Deng, H. Huang, Green Chem. 22 (2020) 5594-5598; (d) L. Lu, Y. Li, X. Jiang, Green Chem. 22 (2020) 5989-5994; (e) F.S. He, S. Xie, Y. Yao, J. Wu, Chin. Chem. Lett. 31 (2020) 3065-3072; (f) L. Zou, L. Wang, L. Sun, X. Xie, P. Li, Chem. Commun. 56 (2020) 7933-7936; (g) S. He, X. Chen, F. Zeng, et al., Chin. Chem. Lett. 31 (2020) 1863-1867; (h) W. Xiao, J. Wu, Chin. Chem. Lett. 31 (2020) 3083-3094; (i) P. Wang, Q. Zhao, W. Xiao, J. Chen, Green Synth. Catal. 1 (2020) 42-51. |
| [6] |
(a) Y. Liu, X.L. Chen, K. Sun, et al., Org. Lett. 21 (2019) 4019-4024; (b) Q. Liu, L. Wang, H. Yue, et al., Green Chem. 21 (2019) 1609-1613; (c) J. Shi, W. Wei, Chin. J. Org. Chem. 40 (2020) 2170-2172; (d) Z. Gan, G. Li, X. Yang, et al., Sci. China Chem. 63 (2020) 1652; (e) L.Y. Xie, Y.S. Bai, X.Q. Xu, et al., Green Chem. 22 (2020) 1720-1725; (f) J. Xu, H. Zhang, J. Zhao, et al., Org. Chem. Front. 7 (2020) 4031-4042; (g) W.B. He, L.Q. Gao, X.J. Chen, et al., Chin. Chem. Lett. 31 (2020) 1895-1898. |
| [7] |
(a) J. Bergman, Oxindoles, in: E.F.V. Scriven, C.A. Ramsden (Eds.), Advances in Heterocyclic Chemistry, Vol. 117, Academic Press, 2015, pp. 1-81; (b) N. Ye, H. Chen, E.A. Wold, P.Y. Shi, J. Zhou, ACS Infect. Dis. 2 (2016) 382-392; (c) M.Z. Zhang, L. Liu, Q. Gou, et al., Green Chem. 22 (2020) 8369-8374; (d) Y. An, Y. Li, J. Wu, Org. Chem. Front. 3 (2016) 570-573; (e) C. Wang, Q. Chen, Q. Guo, et al., J. Org. Chem. 81 (2016) 5782-5788; (f) K. Zhou, J. Chen, J. Wu, Chin. Chem. Lett. 31 (2020) 2996-2998. |
| [8] |
(a) Z.Z. Han, C.P. Zhang, Adv. Synth. Catal. 362 (2020) 4256-4292; (b) A. Lemos, C. Lemaire, A. Luxen, Adv. Synth. Catal. 361 (2019) 1500-1537; (c) W. Sha, W. Zhang, S. Ni, et al., J. Org. Chem. 82 (2017) 9824-9831; (d) Y. Zhou, Z. Xiong, J. Qiu, L. Kong, G. Zhu, Org. Chem. Front. 6 (2019) 1022-1026; (e) C.F. Meyer, S.M. Hell, A. Misale, A.A. Trabanco, V. Gouverneur, Angew. Chem. Int. Ed. 58 (2019) 8829-8833; (f) R. Xu, C. Cai, Chem. Commun. 55 (2019) 4383-4386; (g) D. Zhang, Z. Fang, J. Cai, et al., Chem. Commun. 56 (2020) 8119-8122; (h) D.Q. Dong, H. Yang, J.L. Shi, et al., Org. Chem. Front. 7 (2020) 2538-2575. |
| [9] |
(a) Y. Xiang, Y. Li, Y. Kuang, J. Wu, Chem. Eur. J. 23 (2017) 1032-1035; (b) J.Y. Wang, Y.M. Su, F. Yin, et al., Chem. Commun. 50 (2014) 4108-4111; (c) J.Y. Wang, X. Zhang, Y. Bao, et al., Org. Biomol. Chem. 12 (2014) 5582-5585. |
| [10] |
J. Liu, S. Zhuang, Q. Gui, et al., Eur. J. Org. Chem. (2014) 3196-3202. |
| [11] |
X.J. Tang, C.S. Thomoson, W.R. Dolbier, Org. Lett. 16 (2014) 4594-4597. DOI:10.1021/ol502163f |
| [12] |
H. Sun, Y. Jiang, Y.S. Yang, et al., Org. Biomol. Chem. 17 (2019) 6629-6638. DOI:10.1039/C9OB01213C |
| [13] |
P. Xiong, H.H. Xu, J. Song, H.C. Xu, J. Am. Chem. Soc. 140 (2018) 2460-2464. DOI:10.1021/jacs.8b00391 |
| [14] |
Z. Ruan, Z. Huang, Z. Xu, et al., Org. Lett. 21 (2019) 1237-1240. DOI:10.1021/acs.orglett.9b00361 |
| [15] |
(a) L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. China Chem. 62 (2019) 460-464; (b) W.M. He, Y.W. Lin, D.H. Yu, Sci. China Chem. 63 (2020) 291-293; (c) L.Y. Xie, T.G. Fang, J.X. Tan, et al., Green Chem. 21 (2019) 3858-3863; (d) K.J. Liu, T.Y. Zeng, J.L. Zeng, et al., Chin. Chem. Lett. 30 (2019) 2304-2308; (e) K.J. Liu, J.H. Deng, T.Y. Zeng, et al., Chin. Chem. Lett. 31 (2020) 1868-1872; (f) Y. Wu, W. He, Chin. J. Org. Chem. 40 (2020) 2597-2599; (g) L.Y. Xie, Y.S. Liu, H.R. Ding, et al., Chin. J. Catal. 41 (2020) 1168-1173; (h) J.Y. Chen, C.T. Zhong, Q.W. Gui, et al., Chin. Chem. Lett. 32 (2021) 475-479; (i) W.T. Wei, Q. Li, M.Z. Zhang, W.M. He, Chin. J. Catal. 42 (2021) 731-742. |
| [16] |
R. Sakamoto, H. Kashiwagi, K. Maruoka, Org. Lett. 19 (2017) 5126-5129. DOI:10.1021/acs.orglett.7b02416 |
| [17] |
(a) Y. Mei, L. Zhao, Q. Liu, et al., Green Chem. 22 (2020) 2270-2278; (b) D. Xia, Y. Li, T. Miao, P. Li, L. Wang, Green Chem. 19 (2017) 1732-1739. |
 2021, Vol. 32
2021, Vol. 32