Cancer immunotherapy is considered to be one of the most promising treatments for cancer, which works by using the patient's own immune system to clear away cancerous cells [1-3]. Cancer vaccines, as a representative type of cancer immunotherapy, show great potential to fight cancer by boosting the body's natural defenses to recognize and destroy antigens [4]. So far, many vaccines have been developed based on proteins, polypeptides or carbohydrates [5-9]. Nevertheless, the immunogenicity of these vaccines is usually poor and cannot induce strong immune responses for tumor elimination. To enhance the immune effect, adjuvants have been developed [10, 11]. Some vaccine adjuvants, such as aluminum salts, MF59, AS01B, AS04 and CpG1018 have been licensed for use in clinical trials [12, 13]. To date, aluminum salts remain the most widely used vaccine adjuvants approved by FDA for humans because of their safety and effectiveness. However, aluminum salts mainly stimulate a humoral immune response rather than cellular immune response and is unfit for developing vaccines against cancers and chronic infections [14]. Since cellular immune response, especially cytotoxic T lymphocytes (CTLs), is required for vaccines to kill cancer cells and inhibit chronic infections, there is a great need to develop immune adjuvants that have the ability to induce both humoral and cellular immune responses.
Stimulator of interferon genes (STING) is a signalling molecule in endoplasmic reticulum. It is crucial to regulate the transcription of multiple genes, including type I interferons (IFNs) and pro-inflammatory cytokines [15]. STING is highly expressed in various cell types, such as T cells, macrophages and dendritic cells (DCs), including myeloid-derived suppressor cells (MDSCs) and plasmacytoid dendritic cells (pDCs) [15, 16]. Initial studies showed that STING can be robustly activated by cyclic dinucleotides CDNs which can be secreted by bacteria [17]. As a kind of CDNs, cyclic dinucleotide adenosine monophosphate (c-di-AMP) can act as a pathogen-associated molecular pattern (PAMP) to activate immunological effect used in vaccination [18]. So far, many CDNs have been investigated as promising agents for the treatment of cancer and chronic infections as vaccine adjuvants [19-27]. Further investigations clarified that STING can also be activated by small molecule agonist, which is different from previous nucleotide STING agonist [28]. Amidobenzimidazole (ABZI)-based compound diABZI can enhance the binding affinity to STING and induced strong anti-tumor activity in the mice with colon tumors. No study has yet demonstrated that the synthetic non-nucleotide small molecule STING agonist can act as vaccine adjuvants to elicit immunity.
Here we report the small molecule STING agonist diABZI that can act as a promising vaccine adjuvant to stimulate humoral and cellular immune responses. STING agonists have been used as vaccine adjuvants or anti-tumor agents [28, 29]. Pioneering works have also presented that the vaccine adjuvants are mainly modified cyclic dinucleotides that simulate the endogenous STING ligand cGAMP [30]. However, the small molecule STING agonist which is not a cyclic dinucleotide to serve as the vaccine adjuvant has not been reported. We constructed an antitumor vaccine by physical mixing glycopeptide antigen with the small molecule STING agonist diABZI. This will be very promising to develop a small molecule STING agonist as the vaccine adjuvant with a simple molecular structure and with a very easy-to-realize formulation mode.
Mucin 1 (MUC1), a glycoprotein which was aberrantly glycosylated and overexpressed on the surface of cancer cells compared with normal cells, was used as the very promising tumor associated antigens for the preparation of cancer vaccines [31-34]. The variable number tandem repeats (VNTR) of MUC1, namely 20 amino acids of the sequence HGVTSAPDTRPAPGSTAPPA, was usually used as the antigen epitope. There are 5 glycosylation sites in serine (S) or threonine (T) residues in each VNTR. We use PAHGVTSAPDT(α-D-N-acetyl galactosamine)RPAPGSTAPPA glycopeptide epitope which possess 22 amino acids as the backbone to cover the full length of one VNTR as the model antigen. To enhance the capacity to stimulate T-cells, the universal human and murine T-cell epitope P2 (QYIKANSKFIGITE) derived from tetanus toxoid was covalently conjugated with the model antigen [35-37]. The small molecule STING agonist diABZI was blended into the vaccine through physical mixing to investigate the immunological effect of diABZI (Fig. 1). Immunological evaluation indicated diABZI could be used as an effective adjuvant for MUC1-based cancer vaccines.
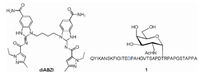
|
Download:
|
| Fig. 1. Structure of the non-nucleotide STING agonist diABZI and MUC1 glycopeptide 1. | |
The agonist diABZI was prepared according to the latest report with a minor revision [28]. It is worth mentioning that the total yield was improved to 64% from 14%, namely the yield rate of the total process was improved 4.6 times after we optimized the synthetic route (Scheme 1). MUC1 based glycopeptide was prepared through the solid-phase peptide synthesis (SPPS) strategy using 2-chlorotrityl resin preloaded with Fmoc-Alanine. The peptide synthesis was carried out with Fmoc amino acids. After finishing the extension of the peptide chain, the acetyl moieties of the Tn antigen were removed by adding MeONa/MeOH (pH 10~11) to give the glycopeptide with the resin. After deprotection of all side chains and cleavage of the resin, the obtained crude glycopeptide was purified by RP-HPLC (Scheme S1 in Supporting information).
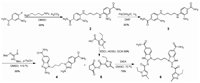
|
Download:
|
| Scheme 1. Synthesis of diABZI 6. | |
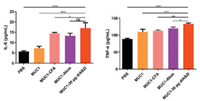
Dendritic cells (DCs) are important regulators of the adaptive immune response, and antitumor responses depend on the DCs that present tumor antigens to lymph nodes or to activate cytotoxic T lymphocytes. DC maturation is the essential step to provide costimulatory signals to T cells. To evaluate the ability of newly synthesized diABZI to stimulate the activation of DCs, the secretion of cytokines IL-6 and TNF-α by bone marrow-derived dendritic cells (BMDCs) stimulated with the vaccine candidates was tested. The production of IL-6 and TNF-α in MUC1−30 μg diABZI group is higher than other groups (Fig. 2), which means diABZI has the potential for the construction of epitope-based vaccines.
|
Download:
|
| Fig. 2. IL-6 and TNF-α were measured in the culture supernatants of mouse BMDCs incubated with PBS, MUC1, MUC1-CFA, MUC1-CFA and MUC1-30 μg diABZI. Data shown as means±SD, and differences among groups were determined by one-way ANOVA with Tukey's test: ns=not significant, *P < 0.05, **P < 0.01, ***P < 0.001. | |
Immunological evaluation was then performed to evaluate the efficacy of the vaccines. To verify the adjuvant activity of diABZI, widely prescribed adjuvants Alum and CFA were used as control. Groups of female Balb/c mice (6–8 weeks old, 6 mice/group) were vaccinated subcutaneously with the mixture of MUC1 glycopeptide and diABZI (different doses of 0.1 μg, 1 μg, 10 μg and 30 μg), MUC1 glycopeptide and Alum, MUC1 glycopeptide and CFA, MUC1 glycopeptide, PBS buffer, respectively. The immunizations were carried out 4 times, and the interval of every two immunizations was 10 days. One week after the last immunization, the sera and spleen were collected for immune evaluation.
IgG antibodies of the antisera were first evaluated by ELISA. As shown in Fig. 3, the IgG response of MUC1-Alum, MUC1-CFA and MUC1 groups was low, while both of the four groups with STING agonist diABZI showed significant IgG responses. Among the STING agonist diABZI adjuvanted groups, MUC1−10 μg diABZI and MUC1−30 μg diABZI elicited almost identical IgG titers, while the titers induced by MUC1−0.1 μg diABZI and MUC1−1 μg diABZI candidates were low. This was consistent with the end-point titers of these vaccine candidates (MUC1−30 μg diABZI, 25, 600; MUC1−10 μg diABZI, 25, 600; MUC1−1 μg diABZI, 12, 800; MUC1−0.1 μg diABZI, 6400) (Table S1 in Supporting information). These results indicated diABZI increased antibody titers in a dose dependent fashion, and 10 μg diABZI might be the optimum dose.

|
Download:
|
| Fig. 3. Titers of the antisera induced by the vaccine candidates analyzed by ELISA. MUC1(Tn) was used to coat the plates. The results are displayed as mean±SD. | |
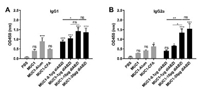
The IgG antibody subtypes of IgG1 and IgG2a were then measured by ELISA. As shown in Fig. 4, both the groups of MUC1−10 μg diABZI and MUC1−30 μg diABZI promoted high IgG1 and IgG2a production. Interestingly, compared with MUC1−30 μg diABZI goup, MUC1−10 μg diABZI group was equivalently potent in inducing IgG1 and IgG2a, this further demonstrated 10 μg diABZI might be the optimum dose. As the antibody IgG1 isotype is considered to be related with Th2 immune responses, IgG2a isotype is associated with Th1 immune responses. The ratio of IgG2a/IG1 represents a relative level of Th1 and Th2 responses [38]. The results revealed the group of MUC1−10 μg diABZI elicited a balanced Th1/Th2 immune responses.
|
Download:
|
| Fig. 4. IgG antibody subtypes IgG1 (A) and IgG2a (B) of the antisera induced by the vaccine candidates evaluated by ELISA. The results were displayed as mean±SD, and differences among groups were determined by one-way ANOVA with Tukey's test: ns=not significant, *P < 0.05, **P < 0.01, ***P < 0.001. | |
To evaluate the therapeutic potential of the antisera induced by the vaccine candidates, flow cytometry was employed to analyze the binding activity of the antibodies to MCF-7 cells. As shown in Fig. 5, compared with MUC1-CFA or MUC1-Alum group, the antisera induced by the groups adjuvanted with diABZI showed stronger binding with MCF-7 cells. Among the diABZI adjuvanted groups, the group of MUC1−30 μg diABZI displayed strongest binding.
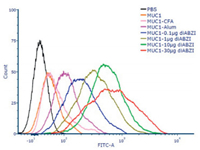
|
Download:
|
| Fig. 5. FACS analysis of the antisera (1:50 dilution) induced by the vaccine candidates binding to MCF-7 cells. | |
Cytokine concentrations in the supernatants of the MUC1(Tn) glycopeptide stimulated splenocytes were then evaluated using ELISA kits. The expression levels of IL-6 and IFN-γ were evaluated. IL-6, secreted by Th2 cells, can induce the maturation of B cells and antibody switch to IgG1; IFN-γ, produced by Th1 cells or CD8+ cytotoxic T cells, can stimulate the activation of macrophages and promote antibody switch to IgG2a. The immunological results showed IL-6 level was significantly increased in diABZI-treated groups, especially in 10 μg diABZI and 30 μg diABZI contained groups (Fig. 6A). Similar results were discovered in the analysis of IFN-γ level (Fig. 6B). These results indicated immunization of diABZI contained glycopeptide vaccines could increase the expression of Th1 and Th2 porarizing cytokines, and the results were consistent with previous antibody subtypes analysis.

|
Download:
|
| Fig. 6. The mice were injected the vaccine candidates on days 0, 10, 20 and 30, splenocytes were harvested on day 37. Splenocytes were restimulated with MUC1(Tn) glycopeptide (10 μg/mL), (A) IL-6 and (B) IFN-γ concentrations in supernatants were measured with ELISA kits. (C) Complement dependent cytotoxicity evaluation of the antisera (1:10 dilution) induced by the vaccine candidates using CCK-8 method. (D) MUC1-specific CTL activities were tested by LDH Cytotoxicity Assay Kit. Data are presented as mean±SD, and differences among groups were determined by one-way ANOVA with Tukey's test: ns=not significant, *P < 0.05, **P < 0.01, ***P < 0.001. | |
To further evaluate the anti-tumor function of the antisera elicited by the vaccine candidates, complement dependent cytotoxicity (CDC) was performed. As shown in Fig. 6C, antisera of the groups of MUC1−10 μg diABZI and MUC1−30 μg diABZI induced obvious cancer cell lysis among all groups. It is worth noting that MUC1−10 μg diABZI group showed comparative CDC activity with MUC1−30 μg diABZI group, through the antisera of MUC1−30 μg diABZI group expressed better binding affinity with tumor cells than MUC1−10 μg diABZI group.
The MUC1-specific killing effect of cytotoxic T lymphocytes in vitro was tested by LDH cytotoxicity assay kit. The mice were immunized with different vaccine candidates as described above, spleens were collected one week after the last vaccination. Splenocytes were isolated as CTL effector cells, and MCF-7 cells were used as target cells. Then the ability to lyse target cells of effector cells was measured. As shown in Fig. 6D, effector cells from MUC1−10 μg diABZI immunized mice showed strong lysis capacity among all groups except for MUC1−30 μg diABZI group which had similar capacity to kill tumor cells. This further demonstrated that 10 μg diABZI might be the appropriate dose to induce in vitro CTL killing.
Encouraged by the above characteristics of humoral and cellular immune responses, we investigated the antitumor effects further in mouse melanoma tumor model. Mice were subcutaneously inoculated with B16-MUC1 cells. The vaccine candidate of MUC1−10 μg diABZI and the control groups were subcutaneously injected seven days after tumor inoculation. The immunization was divided three times, and the interval was four days. Tumor sizes were measured over time along with the calculation of mouse survival rate (Fig. 7A). As shown in Fig. 7B, tumors grew rapidly within the PBS group. In contrast to immunization with MUC1 alone, MUC1-Alum and MUC1-CFA, immunization with MUC1−10 μg diABZI presented significant suppression effect of tumor growth. Meanwhile, vaccination of MUC1−10 μg diABZI can obviously prolong the survival rate of the mice in all of the groups (Fig. 7C). These data demonstrated that immunization of MUC1 vaccine adjuvanted with STING agonist diABZI at a dose of 10 μg could slow tumor growth and prolong the survival.

|
Download:
|
| Fig. 7. Therapeutic anti-tumor activity against melanoma induced by the STING agonist diABZI adjuvanted vaccines. (A) Schematic presentation of the immune process. (B) Volume of tumors over time. Data are presented as mean±SE. (C) Survival curves of tumor-bearing mice. | |
Overall, diABZI can be used as the adjuvant to enhance the immune response of MUC1 glycopeptide antigen. The constructed vaccine has elicited high IgG antibody titers and strong CTL effect to kill tumor cells. Meanwhile, tumor growth was inhibited and survival rate of the mice was improved. Compared with the adjuvant of Alum and CFA, ABZI has showed a better adjuvant effect which provides a new option for the clinical development of tumor vaccines. Moreover, the synthetic route of diABZI has been optimized, which is conducive to the consideration of large-scale preparation. In addition, the dosage of diABZI as an adjuvant has been screened, the minimum dose that gives the best immune effect is 10 μg which provides a reference for its use in the construction of other vaccines. Overall, these results indicate that diABZI can be used as an effective adjuvant for vaccine studies.
In summary, we present that non-nucleotide small molecule STING agonist diABZI can enhance the production of antibodies and T cell immune responses of glycopeptide-based subunit vaccines. The preparation of the vaccines was convenient by just mixing the peptide epitope and diABZI together. The method reported here might be applied to prepare many types of vaccines loaded with tumor-associated antigens, tumor neoantigens, fungal antigens, bacterial antigens and viral antigens for the prevention and treatment of tumors and infectious diseases.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 22077068), the National Key R & D Program of China (No. 2018YFA0507204), the NCC Fund (No. NCC2020FH12), the Natural Science Foundation of Tianjin (No.19JCQNJC05300), the Fundamental Research Funds for the Central Universities.
| [1] |
S.A. Rosenberg, J.C. Yang, N.P. Restifo, Nat. Med. 10 (2004) 909-915. DOI:10.1038/nm1100 |
| [2] |
I. Mellman, G. Coukos, G. Dranoff, Nature 480 (2011) 480-489. DOI:10.1038/nature10673 |
| [3] |
T.N. Schumacher, R.D. Schreiber, Science 348 (2015) 69-74. DOI:10.1126/science.aaa4971 |
| [4] |
I. Melero, G. Gaudernack, W. Gerritsen, et al., Nat. Rev. Clin. Oncol. 11 (2014) 509-524. DOI:10.1038/nrclinonc.2014.111 |
| [5] |
F.O. Nestle, S. Alijagic, M. Gilliet, et al., Nat. Med. 4 (1998) 328-332. DOI:10.1038/nm0398-328 |
| [6] |
N. Gaidzik, U. Westerlind, H. Kunz, Chem. Soc. Rev. 42 (2013) 4421-4442. DOI:10.1039/c3cs35470a |
| [7] |
S.J. Danishefsky, Y.K. Shue, M.N. Chang, et al., Accounts Chem. Res. 48 (2015) 643-652. DOI:10.1021/ar5004187 |
| [8] |
L. Zeng, Z. Liao, W. Li, et al., Chin. Chem. Lett. 31 (2020) 1162-1164. DOI:10.1016/j.cclet.2019.10.015 |
| [9] |
J. Zhao, G. Hu, Y. Huang, et al., Chin. Chem. Lett. 32 (2021) 1331-1340. DOI:10.1016/j.cclet.2020.10.013 |
| [10] |
Z. Zhou, H. Lin, C. Li, et al., Chin. Chem. Lett. 29 (2018) 19-26. DOI:10.1016/j.cclet.2017.09.047 |
| [11] |
F. Liao, H. Wang, Y. Dao, et al., Chin. Chem. Lett. 32 (2021) 1577-1581. |
| [12] |
N. Petrovsky, J.C. Aguilar, Immunol. Cell Biol. 82 (2004) 488-496. DOI:10.1111/j.0818-9641.2004.01272.x |
| [13] |
J.C. Aguilar, E.G. Rodriguez, Vaccine 25 (2007) 3752-3762. DOI:10.1016/j.vaccine.2007.01.111 |
| [14] |
F.C. Zhu, F.Y. Meng, J.X. Li, et al., Lancet 381 (2013) 2024-2032. DOI:10.1016/S0140-6736(13)61049-1 |
| [15] |
G.N. Barber, Nat. Rev. Immunol. 15 (2015) 760-770. DOI:10.1038/nri3921 |
| [16] |
X. Zhou, Z. Jiang, Sci. China-Life Sci. 60 (2017) 563-574. DOI:10.1007/s11427-016-9066-0 |
| [17] |
D.L. Burdette, K.M. Monroe, K. Sotelo-Troha, et al., Nature 478 (2011) 515-U111. DOI:10.1038/nature10429 |
| [18] |
J.J. Woodward, A.T. Iavarone, D.A. Portnoy, Science 328 (2010) 1703-1705. DOI:10.1126/science.1189801 |
| [19] |
T. Ebensen, R. Libanova, K. Schulze, et al., Vaccine 29 (2011) 5210-5220. DOI:10.1016/j.vaccine.2011.05.026 |
| [20] |
E. Van Dis, K.M. Sogi, C.S. Rae, et al., Cell Rep. 23 (2018) 1435-1447. DOI:10.1016/j.celrep.2018.04.003 |
| [21] |
D. Chandra, W. Quispe-Tintaya, A. Jahangir, et al., Cancer Immunol. Res. 2 (2014) 901-910. DOI:10.1158/2326-6066.CIR-13-0123 |
| [22] |
R.D. Junkins, M.D. Gallovic, B.M. Johnson, et al., J. Control. Release 270 (2018) 1-13. DOI:10.1016/j.jconrel.2017.11.030 |
| [23] |
Z. Wang, E. Celis, Cancer Immunol. Immunother. 64 (2015) 1057-1066. DOI:10.1007/s00262-015-1713-5 |
| [24] |
A. Landi, J. Law, D. Hockman, et al., Vaccine 35 (2017) 6949-6956. DOI:10.1016/j.vaccine.2017.10.072 |
| [25] |
J.J. Wu, W.H. Li, P.G. Chen, et al., Chem. Commun. 54 (2018) 9655-9658. DOI:10.1039/C8CC04860F |
| [26] |
A. Sanchez Alberti, A.E. Bivona, N. Cerny, et al., NPJ Vacc. 2 (2017) 9. DOI:10.1038/s41541-017-0010-z |
| [27] |
A. Li, M. Yi, S. Qin, et al., J. Hematol. Oncol. 12 (2019) 35. DOI:10.1186/s13045-019-0721-x |
| [28] |
J.M. Ramanjulu, G.S. Pesiridis, J. Yang, et al., Nature 564 (2018) 439-443. DOI:10.1038/s41586-018-0705-y |
| [29] |
J. Volckmar, L. Knop, S. Stegemann-Koniszewski, et al., Vaccine 37 (2019) 4963-4974. DOI:10.1016/j.vaccine.2019.07.019 |
| [30] |
G. Berger, M. Marloye, S.E. Lawler, T. Trends Mol, Med. 25 (2019) 412-427. |
| [31] |
P. Mukherjee, C.S. Madsen, A.R. Ginardi, et al., J. Immunother. 26 (2003) 47-62. DOI:10.1097/00002371-200301000-00006 |
| [32] |
R. Singh, D. Bandyopadhyay, Cancer Biol. Ther. 6 (2014) 481-486. |
| [33] |
S. Ingale, M.A. Wolfert, J. Gaekwad, et al., Nat. Chem. Biol. 3 (2007) 663-667. DOI:10.1038/nchembio.2007.25 |
| [34] |
J. Taylor-Papadimitriou, J. Burchell, D.W. Miles, et al., BBA-Mol Basis Dis. 1455 (1999) 301-313. DOI:10.1016/S0925-4439(99)00055-1 |
| [35] |
L. Nuhn, S. Hartmann, B. Palitzsch, et al., Angew. Chem. Int. Ed. 52 (2013) 10652-10656. DOI:10.1002/anie.201304212 |
| [36] |
H. Cai, Z.Y. Sun, Z.H. Huang, et al., Chem. Eur. J. 19 (2013) 1962-1970. DOI:10.1002/chem.201203709 |
| [37] |
M. Glaffig, B. Palitzsch, S. Hartmann, et al., Chem. Eur. J. 20 (2014) 4232-4236. DOI:10.1002/chem.201400256 |
| [38] |
N. Yanase, H. Toyota, K. Hata, et al., Vaccine 32 (2014) 5918-5924. DOI:10.1016/j.vaccine.2014.08.059 |
 2021, Vol. 32
2021, Vol. 32 

