The functions of the brain, including sensation, movement, perception, consciousness, learning, and memory, are all realized through the group activities of neurons. These neurons are related to each other and form a complex network. There is a multi-level process from the activity of single nerve cell to population neurons [1]. Therefore, studying the group activities of neurons is of great significance for understanding the functions of the brain.
Cell membrane potential or voltage, is a key physiological parameter that is critical to all aspects of life, especially for excitable cells, such as neurons [2]. In neural and cardiac tissues, the membrane voltage is usually strictly regulated by intercellular communication such as chemical and electrical synapses. To understand the function of these systems, it is necessary to record the membrane voltage dynamics with high spatial and temporal resolutions [3]. Sensors that report membrane potential more directly will help accurately reflect neuronal activity [4].
The classic potential monitoring technology for cell membrane is based on various electrode design by invasive neural voltage measurement. It mainly includes single channel patch-clamp electrode method, whole-cell patch-clamp electrode method and glass, metal or silicon extracellular electrode method [5]. These electrical recording methods through cell surface or punctured electrodes and patch-clamp technology are widely used at the tissue and cell level [6]. Their advantages are that they have excellent sub-millisecond time resolution, and can provide the most direct information on signal transduction within neural cells or small group of interacting neurons [7]. However, all of these electrode-based technologies are highly invasive, usually limited to a single cell, extremely low-throughput [8], time-consuming, and resource-intensive. At the same time, the limited spatial resolution of cell electrophysiology does not allow precise assessment of conduction disturbances on the micrometer scale [9].
Optical methods for imaging voltage such as conventional fluorescence imaging, two-photon fluorescence imaging, and photoacoustic imaging (PAI) are expected to relieve our dependence on traditional electrode-based methods [10]. They are capable of monitoring the membrane potential at different temporal-spatial resolutions and imaging depths. Optical imaging techniques that use chemically synthesized VSDs or genetically encoded fluorescent indicators make it possible to monitor electrophysiological events in living systems that are inaccessible to various electrode-based methods [11]. They can record the electrical activities of living subject in a minimally invasive, highly parallel, and high-throughput manner. Since calcium concentration in neurons usually increases rapidly and reliably by 10- to 100-fold during action potential discharge, nine different fluorescent calcium sensors have been developed and widely used in reports of neural activities [12]. However, calcium imaging can only provide limited information about natural signal processing in the nervous system, and calcium imaging is only limited to the application of neurons when they stimulate action potentials. This limitation ignores the fluctuation of the subthreshold membrane potential, because the necessary trigger threshold is not reached, thus the action potential will not be directly triggered [13]. Compared with genetically encoded calcium indicators, there are many types of chemically synthesized VSDs, and their voltage response mechanisms are different, which can monitor membrane potential under different circumstances.
In this review, we review VSDs and describe some applications in neuroscience (Fig. 1). Optical imaging technology applied in VSDs can achieve excellent temporal and spatial resolution. Therefore, the study of information processing, memory formation, functional plasticity and pathological processes of the brain in neuronal population and cortical functional columns is helpful to further explain the function of the brain, which makes an important contribution to reveal the underlying mechanism of complex behavior and pathology.
|
Download:
|
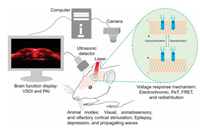
| Fig. 1. Scheme of optical recording of brain functions based on voltage-sensitive dyes. VSDI: voltage sensitive dye imaging; PAI: photoacoustic imaging; PeT: photo-induced electron transfer; FRET: fluorescence resonance energy transfer. Reproduced with permission [22]. Copyright 2016, Elsevier Ltd. | |
At present, the imaging technology based on VSDs provides high temporal and spatial resolution for functional imaging of the in vivo cerebral cortex, which opens a new era for functional imaging of cortical dynamics. It has facilitated the exploration of fundamental mechanisms of cerebral cortex development, function, and plasticity [11]. VSDs are generally believed to bind to the plasma membrane [14], which can exhibit fluorescence or absorbance variations on the membrane surface as the membrane potential changes. Signals are displayed and analyzed by the detection system via converting bioelectric signals into optical signals [15].
The optical properties of VSDs change in microseconds [16], which directly display the membrane potential changes in living systems [17]. Therefore, the optical response recorded by VSDs can reflect the formation and flow of large-scale dynamic neuronal complexes in real time with a high temporal resolution and effectively track the process of voltage waves throughout the neuronal system [18].
Faultless VSDs are very sensitive to changes in transmembrane potential and have almost no pharmacological and phototoxic effects. In addition, the bleaching of dye is required to be as small as necessary [19]. Researchers have synthesized and screened out a large number of dyes with different response sensitivities and speeds in order to select better VSDs. VSDs can be divided into fast-response dyes and slow-response dyes according to their response speed [20]. According to the chemical structure, they can be divided into cyanine dyes with positively charged nitrogen heterocycles (such as IR-780) and nitrogen heterocyclic uncharged merocyanine dyes (such as NK2367), oxonol dyes (such as DiBAC4(3)) and styryl dyes (such as Di-4-ANEPPS). Cyanine and oxonol dyes are generally called slow dyes because of their slow response speed. On the other hand, merocyanine and styryl dyes are considered as fast dyes. In this review, we will focus on introducing the characteristics, response mechanisms, and application examples of slow and fast response dyes.
2.2. Fast VSDsFast VSDs have voltage response kinetics [20], and they are generally amphiphilic molecules that bind to membranes with a response time less than a millisecond potential-dependent fluorescence or absorption changes [21]. Optical recording with fast VSDs can detect lots of neurons action potentials at the same time. Its sub-millisecond resolution helps us have a deep understanding of brain network functions. At present, researchers have synthesized a large number of fast dyes with voltage response capabilities, and they have different structures and response mechanisms [22, 23]. Fast dyes are divided into three categories according to their response mechanism: 1. Electrochromic dyes; 2. Molecular wire-based dyes; 3. Fluorescence resonance energy transfer (FRET)-based dyes (Fig. 2).

|
Download:
|
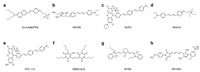
| Fig. 2. Work mechanisms of voltage sensing dyes. (a) Electrochromic dyes respond to voltage through a direct interaction between the chromophore and the electric field. (b) Molecular wire PeT VSDs depend upon the voltage-sensitive electron transfer from an electron-rich donor through a membrane-spanning molecular wire to a fluorescent reporter. (c) FRET-based voltage sensors use lipophilic anions that intercalate into the cellular membrane and distribute between the inner and outer leaflets depending upon the transmembrane potential. Reproduced with permission [23]. Copyright 2011, National Academy of Sciences. (d) Changes in membrane potential cause a redistribution of the mobile anion, which changes the efficiency of energy transfer or quenching. Reproduced with permission [22]. Copyright 2016, Elsevier Ltd. | |
One characteristic of electrochromic dyes is the asymmetry of the chromophore [24], which have aminostyryl pyridine chromophores containing nitrogen atoms, such as Di-4-ANEPPS, RH795, and RH414 (Fig. 3), and it is classified as a hemicyanine dye because of lacking symmetry of regular cyanine dyes [21]. Voltage sensitivity of electrochromic dyes arises from a molecular stark effect (Fig. 2a) [25], which is caused by the charge transfer mechanism. In the electronic ground state, the asymmetry of the chromophore leads to an asymmetric delocalized electron system with the center of a positive charge at the pyridyl group and delocalized electron at the anilino group [22]. When bound to a membrane, the positively charged pyridine ring is located near the extracellular space and the long axis of the molecule is perpendicular to the membrane surface [21]. After photoexcitation, the positive charge center shift from pyridine toward aniline during the absorption process, and it shifts back to pyridine during the emission process. If the charge transfer described above occurs in an external electric field, more or less energy will be required for excitation and released during emission, which depends on whether the work is performed in the same or opposite direction as the external electric field [22]. This energy change will cause a small wavelength shift of the absorption and emission spectrum. Fluorescence signal can be best recorded at the edges of the spectrum, where intensity varies most steeply as a function of wavelength [23]. In general, electrochromic dyes respond to voltage through a direct interaction between the chromophore and the electric field without any movement of the dye molecules. All of these dyes provide rapid absorbance and fluorescence response to the membrane potential. Therefore, electrochromic dyes can record action potentials.
|
Download:
|
| Fig. 3. The structures of fast and slow VSD. (a) Di-4-ANEPPS; (b) RH795; (c) RVF5; (d) RH414; (e) VF2.1.Cl; (f) DiBAC4(3); (g) IR780; (h) RH1691. | |
Voltage-sensitive molecular wire-based dyes have fluorophore, conjugated molecular wire, and aniline donor [20], such as VF2.1.Cl, RVF5 (Fig. 3). For voltage imaging with cellular resolution in vivo, molecular wire-based dyes have the required sensitivity, speed, minimal invasiveness, and compatibility with two photon excitation [17]. This dye changes transmembrane potential through the mechanism of photoinduced electron transfer (PeT), and the rate of PeT competes with the rate of fluorescence. PeT from electron-rich aniline through conjugated molecular wires to fluorescent reporters is controlled by the electric fields passing through the plasma membrane of excitable cells, such as neurons [26]. Under resting or hyperpolarized potentials, the transmembrane electric field promotes the transfer of electrons from the aniline donor to the excited-state fluorophore through molecular wires, resulting in fluorescence quenching (Fig. 2b) [27]. When the membrane is in depolarization during an action potential or the excitatory input integration of connected neurons, the reversal of the electric field hinders the electron transfer, and the decrease of the PeT rate makes the fluorescence increase [23]. This chemically synthesized VSD that uses PeT as a voltage-sensitive trigger has high voltage sensitivity and fast response kinetics [5], which can reach the excitation wavelength ranging from blue to green and into the far red and two-photon infrared region [17].
FRET is a physical dipole-dipole coupling between the excited state of the donor fluorophore and the acceptor chromophore, which relaxes the donor to a non-fluorescent ground state and excites the fluorescence in the acceptor [28]. The efficiency of FRET depends on the properties of the chromophore and the distance between them, which is measured by Förster radius. The size of Förster radius depends on the donor quantum yield and the spectral overlap between the donor emission spectrum and the acceptor absorption spectrum [29]. FRET-based VSDs use hydrophobic anions, which are mainly composed of two fluorescent molecules, a donor, and an acceptor, such as dipicramine-green fluorescent protein (DPA-GFP), DPA-Di-4-ANEPPS [30]. In principle, hydrophobic anions can be used as either the FRET donor or acceptor, but it is most commonly used as the FRET acceptor [31]. FRET-pair voltage sensors with hydrophobic anions could be embedded in the cell membrane, which distributed on the inner or outer leaflet of the membrane in a voltage-dependent manner (Fig. 2c). Taking the hybrid voltage sensor DPA-GFP with membrane targeting GFP as the donor and DPA as the acceptor as an example, the GFP is anchored on the outer surface of the cell. When the membrane potential is resting, the average distance between the DPA distributed in the outer leaflet outside and the GFP the externally bounded is close enough to produce a large amount of quenching [32]. This quenching is then relieved when the DPA anions is redistributed to the inner leaflet upon depolarization. FRET-based hybrid voltage sensors have high sensitivity in neurons and they also have fast kinetics because of the redistribution of hydrophobic anions on the inner and outer leaflet less than a millisecond.
2.3. Slow VSDsSlow VSDs are generally hydrophobic molecules. They are able to penetrate the cell or organelle membrane, and the better the permeability, the faster the dye's time course [21]. The distribution of these permeable dyes on the membrane is controlled by the membrane potential, and the absorption or fluorescence of the dye will change under appropriate conditions. The relationship between the change and the transmembrane potential conforms to the Nernst equation, thus the slow VSDs are also called 'Nernstian' dyes [33]. Compared with fast dyes, slow dyes are based on voltage-dependent accumulation in or redistribution within the membrane, which can achieve up to 80% fluorescence change at 100 mV (Fig. 2d) [22]. However, it takes several seconds to several minutes to reach a new fluorescence signal platform due to slow response. In addition, the time scale of the charged dye molecules passing through the membrane is similar to the potential biological changes, which adds an artificial gated charge or capacitive current and causes prohibitive capacitive load on the membranes [34]. At present, the most commonly used slow dyes are positively charged cyanine dyes and negatively charged oxonine dyes, and their voltage response mechanisms are both on-off mechanisms.
Positively charged cyanine dyes, such as IR-780 (Fig. 3) and PAVSD800-2, redistribute the dye concentration according to the cell membrane potential, which show the functional corresponding to electrophysiological events of biological tissue [35]. They have a tendency of non-fluorescent to form aggregates at high concentrations. Under resting or hyperpolarized potentials, positively charged cyanine dyes are attracted to the cell membrane, and the dye molecules will accumulate at a sufficiently high local concentration to produce non-fluorescent aggregates [36], which dissipates the absorbed light energy in the form of thermal energy. When the membrane is depolarized, the fluorescent aggregates are disassembled and dispersed back into the intracellular/extracellular space to form fluorescent monomers and restore fluorescence emission. Indeed, under the correct circumstances, depolarization can produce > 100-fold increases in cyanine dye fluorescence, resulting in a higher voltage response sensitivity.
Negatively charged oxocyanine dyes, such as RH155, DiBAC4(3), and RH1691 (Fig. 3), move between intracellular and extracellular compartments, and respond to changes in membrane potential with very slow kinetics about seconds to minutes [5]. Moreover, they have a relatively low fluorescence intensity in aqueous solution, and only emit fluorescence when they enter the cell and combine with the protein in the cytoplasm [19]. When the membrane is in hyperpolarization, the negatively charged oxonine dye is distributed outside the cell membrane, and the fluorescence intensity of that is weak. However, the dye penetrates the cell membrane, and binds to the cytoplasmic protein upon membrane depolarization, and the fluorescence intensity significantly increases with a large change in the fluorescence fraction [37].
2.4. Novel voltage sensitive fluorescent probes 2.4.1. QD probesIt is reported that quantum dots (QD) also have voltage-sensitive properties [38]. The strong interaction between QDs and light field leads to large extinction coefficient and efficient emission [39]. QD has higher fluorescence intensity, faster voltage response and better stability under light than fluorescent molecules. QD has been successfully applied in the field of neuroscience because of its unique properties such as light, sound, magnetism, electricity, and heat. For example, QD can sense the single action potential of neurons by means of quantum confinement stark effect [39]. QD has better photostability than molecular or protein voltage sensitive probes.
Up to date, the research on the sensitivity of QD to voltage is still in its infancy, and its toxicity restricts its application in living animals. On one hand, it is necessary to improve the sensitivity of QD. By fusing the voltage sensitive domain with fluorescent protein or chemical covalent fluorescent labeling protein, we can construct a genetic coding voltage sensitive molecular probe to realize high sensitivity and large dynamic range detection. QD theoretical study shows that the generation of action potential can cause the change of 5% fluorescence intensity and 1 nm wavelength of single semiconductor QD, such as ZnS [40, 41]. Moreover, the fluorescence intensity of nerve probe that is composed of two QDs can change 30% under action potential. On the other hand, selective neuronal labeling can be achieved by binding proteins or polypeptides with affinity to neuron type specific membrane receptors, which is conducive to improving the sensitivity and specificity of voltage detection. In addition, it is necessary to overcome the cytotoxicity of QD. Protein coating is also an effective means to solve this problem. Many researchers also tried to use other non-toxic inorganic QDs, such as carbon quantum dots [38].
2.4.2. Other probesThe role of functional nanoparticles in remote control of neural activity is also increasingly prominent. Recently, Chen et al. made use of the thermal effect of superparamagnetic nanoparticles Fe3O4 under alternating magnetic field to open the heat sensitive transient receptor potential cation channel subfamily V member 1 in neurons to achieve the goal of remote control of brain nerve activity [42]. This method is not only harmless to living tissue, but also not limited by the depth of penetration. At present, Liu et al. have developed voltage sensitive probes based on upconversion nanoparticles (UCNPs) [11]. Its unique advantage is that UCNPs can be excited by near-infrared light (980 nm) with high penetration depth in vivo. The results showed that the nanoprobes could sense the changes of cell membrane potential and the variation of luminescence was significantly higher than any other voltage sensitive probes. It can be predicted that the interdisciplinary cooperation between neuroscientists and materials scientists will make a breakthrough in the future.
2.5. Dyes characteristics: specificity, sensitivity, and toxicityThere are as many as 2, 000 VSDs discovered so far [43]. These dyes have different tissue specificities and sensitivity to changes in external electric fields, and may have different staining effects for different research subjects. Therefore, we can screen dyes that are sensitive to transmembrane potential changes and have relatively low pharmacological or phototoxic effects according to experimental design. The sensitivity, toxicity, solubility of different dyes were shown in Table 1. We will make a brief description by comparing two styryl dyes RH795 and Di-4-ANEPPS (Fig. 3).
|
|
Table 1 Characteristics of fast and slow voltage sensitive dyes. |
Fast VSDs RH795 and Di-4-ANEPPS can report membrane potentials with high temporal and spatial resolution, and the response time constants are usually less than 1 millisecond. They can be used to detect membrane potential changes in many different systems and cell types. Di-4-ANEPPS has higher staining intensity, response sensitivity, signal quality, and faster staining speed than RH795 in the staining of motor neurons for stomach-gastric nervous system of a crustacean. Di-4-ANEPPS has longer alkyl chain attached to the chromophore than RH795, which makes Di-4ANEPPS more lipophilic. Moreover, they also show different bleaching characteristics, and the bleaching speed of Di-4-ANEPPS is faster than that of RH795 due to the difference between the conjugated carbon chains of RH795 and Di-4-ANEPPS. Studies have shown that although the signal quality of Di-4-ANEPPS is better, the toxicity and phototoxicity will also be enhanced. And the phototoxicity of RH795 on pyloric motor pattern was weak and developed slowly as well as slow bleaching of the staining, and had little effect on the movement mode. Therefore, Di-4-ANEPPS is more suitable for short-term experiments requiring high signal quality, but RH795 is a better candidate for long-term experiments [44].
3. The application of voltage sensitive dye imaging (VSDI) 3.1. Application of VSDI in visual cortexVision is an important part of feeling. More than 80% of the external information is received, processed and perceived by the visual system. Light stimulation in different positions of the visual field can excite neurons in different positions of the cortex [45]. The spatial distribution of visual stimulation in the cortex is called the retinotopic map on the cortex [46]. Because VSDI can obtain the spatial information of neural electrical activity in visual cortex [47], which cannot be sensed by single cell electrophysiological methods.
Grinvald et al.'s study of monkey primary visual cortex (V1) using VSDI showed that there is not only a corresponding relationship between the spatial distribution of the visual field in layer Ⅳ of V1, but also the horizontal diffusion between functional columns in layer Ⅱ/Ⅲ. The author emphasized that a large amount of input processing by a single cell and its dendritic tree structure are difficult to evaluate with traditional electrophysiological techniques. The optical signal detected by the VSD RH795 mainly reflects the changes in the transmembrane potential of a group of neuronal cells, including the subliminal synaptic potential that affects the extensive arborization of cortical cells [48]. Palagina et al. further found that after retinal injury, neurons in the lesion projection zone will be activated by the propagation of activities originating from the unaffected cortical region, which proves that the horizontal connection of the cortex can compensate for the loss of vertical projection function caused by the injury. These results suggest that the horizontal connections between different cortical functional columns in V1 may be of great significance in visual information processing [49]. Yang et al. reported rapid and accurate mapping of retinal sites in the visual cortex using VSDI in behavior sensitive monkeys. VSDI monitors the population activity of neurons produced by motor stimulation in cortex with high spatial and temporal resolution. The ability to quickly obtain accurate retinal mapping in behavioral monkeys helps to analyze the relationship between temporal and spatial dynamics of population response and perceptual behavior in the visual cortex [47].
In addition, Jancke et al. reported a classic experiment on line motion illusion. The spatiotemporal pattern of visual cortical electrical activity was studied by using VSDI technique (Fig. 4) [50]. VSDI with high spatial and temporal resolution is very sensitive to monitor the changes of membrane potential, especially the synaptic potential of sub threshold and over threshold, which can identify the mechanism of producing illusory motor neurons. These findings demonstrate the effects of temporal and spatial patterns of subthreshold synaptic potentials on cortical processing and perceptual formation. There is a lot of evidence that ongoing activities play an important role in shaping perception and behavior [51-54]. There are few studies on VSDI in conscious animals, and imaging in awake animals is more challenging than that under anesthesia. Most of the animals in the study were anesthetized, however, it is not clear whether spontaneous map like cortical states exist in conscious animals. To solve this problem, David et al. combined VSDI with local field potential and multiunit activity recordings in the main visual cortex of conscious and anesthetized monkeys. The results showed that the number of spontaneous activity patterns was much larger in conscious animals and their dynamics were faster than in anesthetized animals [55].
|
Download:
|
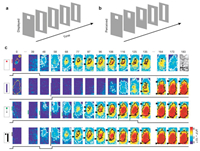
| Fig. 4. VSDI for visual cortical representations of stationary, moving, and illusory moving stimuli. (a) Square presented before a bar stimulus. (b) Subjects report illusory line-drawing. (c) The performance of evoked cortex activity in the four modes of flashing small square, flashing bar, moving small square, and linear-motion paradigm as a function of time. Reproduced with permission [50]. Copyright 2004, Springer Nature. | |
Using sensory input to guide motor output is the most basic operation of the brain. Somatosensory plays an important role in guiding body movement, which can study some simple behaviors based on tactile sensory motor circuit in mammals. There is a strong interaction between the sensory and motor areas of the brain and VSDI can be used to explore the interaction between the sensory and motor cortex.
The whisker barrel cortex of the primary somatosensory cortex (S1) is a typical representative of the functional column theory, and the moustache experiment of rodents is easy to operate. Therefore, the research on somatosensory cortex is mainly focused on the barrel bearded cortex of rodents [56, 57]. Fezerou et al. imaged the voltage-dependent fluorescence changes in the neocortex with RH1691 staining, which observed the dynamic changes of the collecting membrane potential in the epithelial layer at millisecond temporal resolution. The results show that the neural electrical activity generated by stimulating a single beard will spread to the whole barrel cortex and even further to the motor cortex (Fig. 5) [58, 59]. Sato et al. further proved that neuronal excitation can be transmitted to layer Ⅱ/Ⅲ by stimulating layer IV of S1, which not only spreads horizontally in layer Ⅱ/Ⅲ, but also deeply spreads to layer Ⅴ/Ⅵ to cause excitation of insular cortex [60]. In addition, Tang et al. visualized the neural activity induced by thalamic barrel body through the deflection of whiskers in vivo using VSDI with gradient-index rod lens. The results show that this method can be widely used in functional imaging of deep brain structures [61].
|
Download:
|
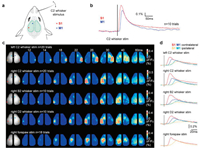
| Fig. 5. Bilateral VSDI of somatosensory cortical responses to tactile stimulation of different whiskers and the forepaw. (a) A brief deflection of a single whisker evokes a depolarizing response in both somatosensory and motor cortex. (b) The craniotomies were stained with VSD RH1691 in a mouse under urethane anesthesia. (c) Images of resting fluorescence of the VSD-stained bilateral craniotomies under stimulation of different whiskers and the forepaw. (d) The time course of response to different stimuli was measured. Reproduced with permission [59]. Copyright 2007, Elsevier Inc. | |
The barrel cortex of the beard is also used to study neural plasticity [62, 63]. The barrel cortex is highly plastic from birth to adulthood, so the barrel cortex of rodents is a convenient model to study the mechanism of cortical plasticity. Wallace et al. observed the pattern distribution of neuronal electrical activity in the barrel cortex of rats after deprivation by depriving the whiskers of groups A, B, and C and retaining the whiskers of groups D and E. VSDI showed that the excitation was significantly enhanced in the contralateral group E, while there was no significant change in the ipsilateral D and E regions. This suggests that the contralateral cortex may be more important than the ipsilateral cortex in the formation of the facial bucket like cortical topology [64].
VSDI can also be used in the study of the repair of the injured somatosensory cortex. Sigler et al. used VSDI to examine the changes of sensory map of forelimb and hindlimb within hours after acute stroke, suggesting that new forelimb regions provide signals that may guide future structural plasticity and subsequent reorganization of somatosensory cortical function over a longer period of time [65]. Brown et al. observed the electrical activity of nerve using VSDI to explore how the front limb (FL) area was remodeled and recovered for adult mice after stroke. The experimental results showed that the newly recovered area occupied a part of the primary motor area on the same side of the injury and the somatosensory area hind limb in charge of the hind limb, and distributed further around to compensate for the damage of FL area [66].
3.3. Application of VSDI in olfactory cortexOdor is detected by olfactory sensory neurons in the nose. Each olfactory neuron can express a kind of odor receptor from different genes, which can recognize and distinguish a large number of different odor molecules [67]. Therefore, the olfactory cortex is more complex than sensory cortex. People tried to find the corresponding relationship between different olfactory stimuli and the excitations of neurons in the olfactory bulb. Freeman et al. found a complex neurodynamic pattern in the olfactory bulb of rabbits when distinguishing odors, but the changes were not significant [68]. Cohen's team used a VSD RH414 and a 464-element photodiode array to measure the spatiotemporal variation of odor induced responses in the olfactory bulb of turtles. They found that inhaling an odor can produce three oscillations in the olfactory bulb [69]. After that, the team carried out further experiments on this phenomenon and found that if another odor was inhaled, the previous two oscillations would be suppressed, while if the new inhaled odor was the same as the first, the third oscillation would be strengthened, and if different, it would be weakened [70]. Recently, Mizoguchi et al. found that the olfactory and gustatory sense of rats have been integrated into the flavor through in vivo optical imaging with VSDs, which is helpful to detect and identify food [71].
4. Application of VSDI in pathological brain 4.1. Epilepsy and VSDIEpilepsy is a kind of paroxysmal and transient brain dysfunction caused by abnormal synchronous discharge of brain neurons, which is a disease caused by many reasons. Temporal lobe epilepsy is the most common type of epilepsy syndrome, which can seriously affect the normal life, work and study of patients, and is one of the most common serious brain diseases [72]. VSDI technology is expected to facilitate the study of the pathogenesis of temporal lobe epilepsy.
At present, we do not have enough understanding of the basic mechanism of the generation and transmission of seizures because of difficultly determining the focus of seizures and often finding multiple locations. Dasheiff et al. used VSDs with paramagnetic or positron emission traces to generate mean membrane polarization maps for analyzing circuits involved in seizures [73]. Sacks et al. demonstrated that slow VSDs have the ability to capture local electrical events, which provides a new tool for exploring brain activity [74]. Derchansky et al. used multi electrode recording and VSDI technology to study the temporal and spatial dynamics of epileptic seizure propagation, which can better visualize the spatial dynamics of epileptic activity. Their findings provide novel insights into the dynamic multifocality of seizures and transmission [75].
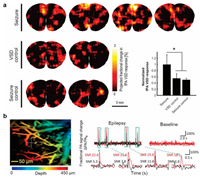
In recent years, PAI is a hybrid method combining high-resolution optical imaging with deep penetration depth of ultrasound imaging where the signal corresponding to neural activity is detected in the form of acoustic transcranial imaging with optical absorbance as an image contrast [76, 77]. PAI is based on the thermal elastic perturbation of the target caused by the optical absorption of pulsed laser, which generates the radio frequency acoustic pressure wave detected by piezoelectric ultrasonic transducer or optical interferometer. This unique imaging technology has been widely used in preclinical and clinical research, including diagnosis of brain glioma [78, 79]. Recently, Wong and Boctor's team proposed a novel mechanism for selective fluorescence quenching of near-infrared cyanine VSD based on the change of membrane potential [36]. It is also reported that near infrared VSD redistribution functional photoacoustic neuroimaging could detect the changes of graded membrane potential in vivo epileptic mice (Fig. 6a) [80]. They further demonstrated that the use of transcranial functional photoacoustic neuroimaging successfully identified focal epileptic foci in the hippocampus of mice evoked by high dose N-methyl-D-aspartate, which was successfully correlated with electrophysiological measurements [81]. Rao et al. reported that the calculated fractional voltage response signal change of DPA dye may show the whole seizure peak envelopes (Fig. 6b) [82]. In a word, PAI based on VSDs opens up a feasible technical path for study of deep brain epilepsy in the future.
|
Download:
|
| Fig. 6. PAI recording of neural activity in epilepsy rodent brain in vivo. (a) In vivo functional PAI and the quantification for seizure, VSD control, and seizure control groups for seizure. Reproduced with permission [80]. Copyright 2019, Frontiers Media S.A. (b) In vivo mouse brain response before and during 4-aminopyridine induced epilepsy and fractional voltage and fractional hemodynamic signal. Reproduced with permission [82]. Copyright 2017, Springer Nature. | |
Depression is a kind of neurosis with depression, thinking retardation, and speech and action reduction and retardation as typical symptoms. The causes of depression include genetic factors, physical factors, abnormal function and metabolism of central nervous medium, and mental factors. In terms of neural mechanism, the current theories mainly include monoamine neurotransmitter theory, stress theory, and neurogenesis theory [83].
According to stress theory, corticotropin releasing hormone (CRH) is considered to play an important role in pathophysiology of major depression. However, how CRH specifically acts on the central nervous system is still unclear. Wolff et al. used VSDI technology to study the effect of CRH on hippocampal slices of mice. By using CRH in hippocampal slices, it can be seen that the transmission of neural activity increases from DG to CA1, but it is not found in the hippocampal slices without CRH. This method enables us to study the propagation of induced neuronal activity through the hippocampal formation with micron spatial and millisecond time resolution. This confirmed that CRH can not only lead to depression through stress pathway, but also has a direct effect on hippocampus, which may be one of the mechanisms of inducing disease [84].
Neurogenesis theory believes that the loss of neurons in the hippocampus can lead to depression [85]. Airan et al. used quantitative VSDI to confirm that DG area of hippocampus was inhibited and excitability of CA1 area was enhanced in depression model rats. Using antidepressant drug fluoxetine can increase the number of new neurons in DG area and improve the excitability, and the pathological pathway of CA1 is inhibited. It shows that the new neurons in DG area have inhibitory effect on depression. This experiment also further elucidates the direct correlation between the intrinsic phenotype of neurophysiological loop and emotional behavior through animal behavior, which is also helpful for the study of emotion [86].
4.3. VSDI and propagating waves in cortexThe brain generates exciting waves all the time in the process of wakefulness, sleep, perception and movement. Propagation wave is a manifestation of neuronal membrane depolarization, and it is a new behavior of neuron group activity, which can affect the activity of single neuron [87]. Due to the fast propagation speed of neuron excitation waves, VSDI technology can accurately describe the spatiotemporal state of these excitation waves.
Xu et al. first discovered that the speed of propagating waves related to vision can be controlled by neural networks through VSDI technology. Their research shows that neurons in two different visual areas may increase their firing probability through propagating waves to promote the exchange of information between these areas within a certain period of time after receiving visual stimuli [88].
Spiral wave is a special form of propagating wave. Prechtl et al. found that the propagating wave generated by visual stimulation had the phenomenon of rotation during VSDI of the optic tectum of tortoise, which first indicated the existence of spiral wave [89]. After that, Huang et al. found that there could be a very strong spiral wave in the rat brain slices after the neural network disinhibited [90]. In subsequent work, they found that spiral waves were very frequent when imaging the cerebral cortex during sleep [91]. The neural network of the cerebral cortex probably has some mechanisms to control the spiral wave. The spiral wave is inhibited in some occasions, and the characteristics of spiral waves are used to perform some functions in other occasions. Further research is needed on the function of excitatory waves.
5. Conclusions and outlookIn this review, we presented the classification and application of VSDs. Optical imaging technology based on VSDs offers many possibilities for brain functional imaging in vivo due to its good spatial and temporal resolution. VSDI could visualize neural activity in somatosensory, visual, and olfactory cortex. In addition, VSDI was widely used to detect abnormal neural activities such as epilepsy, depression, and propagating waves.
Currently, the imaging based on VSD provides the highest spatial and temporal resolution for neocortical functional imaging in the living brain, which paves the way for a new era of cortical dynamic functional imaging. The application of VSDI may provide new insights into the basic principles, development, and feasibility of cortical processing. However, the available signal-to-noise ratio and the photodynamic damage are the limiting factors to obtain better VSDI. Different cortical areas may also require different dyes in the same species. For example, the same dye can provide strong signals in the somatosensory cortex of rats, but the olfactory bulb is well stained, but there is no signal [92]. Therefore, it is very important to make better probes for brain functional imaging. In the future, it should be possible to create improved new dyes and carry out further technological innovations. For example, the genetically engineered probes could express fluorescent substances in specific cells, which could facilitate better brain activity imaging [93-95].
The combination of various technologies is an important direction of VSDI development. Because VSDI cannot record the deep layer of cerebral cortex, the combination of two-photon fluorescence imaging or PAI may be a feasible method with the emergence of high-performance dyes. Moreover, the sensitivity of VSDI to spikes can also be compensated by combining with in vivo two-photon calcium imaging. In addition, the combination of function magnetic resonance imaging, electroencephalogram, patch clamp, and other technologies will have a better role in promoting brain function research fields.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank Mr. Samuel K. Deyekeh for linguistic assistance during the preparation of this manuscript. This work was financially supported by the National Natural Science Foundation of China (Nos. 81922034, 91859113), the Science Fund for Distinguished Young Scholars of Fujian Province (No. 2018J06024).
| [1] |
Y. Wan, Z. Wei, L.L. Looger, et al., Cell 179 (2019) 355-372. DOI:10.1016/j.cell.2019.08.039 |
| [2] |
J.A. Frank, M.J. Antonini, P. Anikeeva, Nat. Biotechnol. 37 (2019) 1013-1023. DOI:10.1038/s41587-019-0198-8 |
| [3] |
L.A. Sieu, A. Bergel, E. Tiran, et al., Nat. Methods 12 (2015) 831-834. DOI:10.1038/nmeth.3506 |
| [4] |
S. Gottschalk, O. Degtyaruk, B. Mc Larney, et al., Nat. Biomed. Eng. 3 (2019) 392-401. DOI:10.1038/s41551-019-0372-9 |
| [5] |
V. Grenier, B.R. Daws, P. Liu, et al., J. Am. Chem. Soc. 141 (2019) 1349-1358. DOI:10.1021/jacs.8b11997 |
| [6] |
Y. Xu, L. Peng, S. Wang, et al., Angew. Chem. Int. Ed. 57 (2018) 3949-3953. DOI:10.1002/anie.201712614 |
| [7] |
F. St-Pierre, J.D. Marshall, Y. Yang, et al., Nat. Neurosci. 17 (2014) 884-889. DOI:10.1038/nn.3709 |
| [8] |
A.L. Efros, J.B. Delehanty, A.L. Huston, et al., Nat. Nanotechnol. 13 (2018) 278-288. DOI:10.1038/s41565-018-0107-1 |
| [9] |
M.L. Chang Liao, T.P. de Boer, H. Mutoh, et al., Circ. Res. 117 (2015) 401-412. DOI:10.1161/CIRCRESAHA.117.306143 |
| [10] |
L.V. Wang, S. Hu, Science 335 (2012) 1458-1462. DOI:10.1126/science.1216210 |
| [11] |
J. Liu, R. Zhang, C. Shang, et al., J. Am. Chem. Soc. 142 (2020) 7858-7867. DOI:10.1021/jacs.0c01025 |
| [12] |
T. Knopfel, C. Song, Nat. Rev. Neurosci. 20 (2019) 719-727. DOI:10.1038/s41583-019-0231-4 |
| [13] |
A. Grinvald, R. Hildesheim, Nat. Rev. Neurosci. 5 (2004) 874-885. DOI:10.1038/nrn1536 |
| [14] |
A.R. Cinelli, J.S. Kauer, Annu. Rev. Neurosci. 15 (1992) 321-351. DOI:10.1146/annurev.ne.15.030192.001541 |
| [15] |
M. Zochowski, M. Wachowiak, C.X. Falk, Biol. Bull. 198 (2000) 1-21. DOI:10.2307/1542798 |
| [16] |
D. Fitzpatrick, Curr. Biol. 10 (2000) R187-R190. DOI:10.1016/S0960-9822(00)00348-1 |
| [17] |
P.E. Deal, P. Liu, S.H. Al-Abdullatif, et al., J. Am. Chem. Soc. 142 (2020) 614-622. DOI:10.1021/jacs.9b12265 |
| [18] |
A. Wlodarczyk, P.F. McMillan, S.A. Greenfield, Chem. Soc. Rev. 35 (2006) 890-898. DOI:10.1039/b517771p |
| [19] |
Y. Momose-Sato, K. Sato, Y. Arai, I. Yazawa, et al., J. Membr. Biol. 172 (1999) 145-157. DOI:10.1007/s002329900592 |
| [20] |
P. Liu, E.W. Miller, Acc. Chem. Res. 53 (2020) 11-19. DOI:10.1021/acs.accounts.9b00514 |
| [21] |
T.J. Ebner, G. Chen, Prog. Neurobiol. 46 (1995) 463-506. |
| [22] |
E.W. Miller, Curr. Opin. Chem. Biol. 33 (2016) 74-80. DOI:10.1016/j.cbpa.2016.06.003 |
| [23] |
E.W. Miller, J.Y. Lin, E.P. Frady, et al., Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 2114-2119. DOI:10.1073/pnas.1120694109 |
| [24] |
B. Kuhn, C.J. Roome, Front. Cell. Neurosci. 13 (2019) 321. |
| [25] |
P. Yan, C.D. Acker, W.L. Zhou, et al., Proc. Natl. Acad. Sci. U. S. A. 109 (2012) 20443-20448. DOI:10.1073/pnas.1214850109 |
| [26] |
V. Grenier, A.S. Walker, E.W. Miller, J. Am. Chem. Soc. 137 (2015) 10894-10897. DOI:10.1021/jacs.5b05538 |
| [27] |
R.U. Kulkarni, D.J. Kramer, N. Pourmandi, et al., Proc. Natl. Acad. Sci. U. S. A. 114 (2017) 2813-2818. DOI:10.1073/pnas.1610791114 |
| [28] |
P.R. Selvin, Nat. Struct. Biol. 7 (2000) 730-734. DOI:10.1038/78948 |
| [29] |
L. Stryer, Ann. Rev. Biochem. 47 (1978) 819-846. DOI:10.1146/annurev.bi.47.070178.004131 |
| [30] |
L. Kaestner, Q. Tian, E. Kaiser, et al., Int. J. Mol. Sci. 16 (2015) 21626-21642. DOI:10.3390/ijms160921626 |
| [31] |
H. Takakusa, K. Kikuchi, Y. Urano, et al., J. Am. Chem. Soc. 124 (2002) 1653-1657. DOI:10.1021/ja011251q |
| [32] |
P. Yan, C.D. Acker, L.M. Loew, ACS Sens. 3 (2018) 2621-2628. DOI:10.1021/acssensors.8b01032 |
| [33] |
T.J. Beeler, R.H. Farmen, A.N. Martonosi, J. Membr. Biol. 62 (1981) 113-137. DOI:10.1007/BF01870205 |
| [34] |
T. Brauner, D.F. Hulser, R.J. Strasser, Biochim. Biophys. Acta 771 (1984) 208-216. DOI:10.1016/0005-2736(84)90535-2 |
| [35] |
J. Kang, D.L. HN, S. Karakus, et al., Sci. Rep. 10 (2020) 6618. DOI:10.1038/s41598-020-63588-2 |
| [36] |
H.K. Zhang, P. Yan, J. Kang, et al., J. Biomed. Opt. 22 (2017) 45006. DOI:10.1117/1.JBO.22.4.045006 |
| [37] |
J.A. Auzmendi, S. Orozco-Suarez, I. Banuelos-Cabrera, et al., Curr. Pharm. Des. 19 (2013) 6732-6738. DOI:10.2174/1381612811319380006 |
| [38] |
S.A. Empedocles, M.G. Bawendi, Science 278 (1997) 2114-2117. DOI:10.1126/science.278.5346.2114 |
| [39] |
D.S. Peterka, H. Takahashi, R. Yuste, Neuron 69 (2011) 9-21. DOI:10.1016/j.neuron.2010.12.010 |
| [40] |
C.E. Rowland, K. Susumu, M.H. Stewart, et al., Nano Lett. 15 (2015) 6848-6854. DOI:10.1021/acs.nanolett.5b02725 |
| [41] |
M. Caglar, R. Pandya, J. Xiao, et al., Nano Lett. 19 (2019) 8539-8549. DOI:10.1021/acs.nanolett.9b03026 |
| [42] |
R. Chen, G. Romero, M.G. Christiansen, et al., Science 347 (2015) 1477-1480. DOI:10.1126/science.1261821 |
| [43] |
K. Kamino, Physiol. Rev. 71 (1991) 53-91. DOI:10.1152/physrev.1991.71.1.53 |
| [44] |
S. Preuss, W. Stein, PLoS One 8 (2013) e75678. DOI:10.1371/journal.pone.0075678 |
| [45] |
R. Kanai, L. Chaieb, A. Antal, et al., Curr. Biol. 18 (2008) 1839-1843. DOI:10.1016/j.cub.2008.10.027 |
| [46] |
S. Schuett, T. Bonhoeffer, M. Hübener, J. Neurosci. 22 (2002) 6549-6559. DOI:10.1523/JNEUROSCI.22-15-06549.2002 |
| [47] |
Z. Yang, D.J. Heeger, E. Seidemann, J. Neurophysiol. 98 (2007) 1002-1014. DOI:10.1152/jn.00417.2007 |
| [48] |
A. Grinvald, E. Lieke, R. Frostig, et al., J. Neurosci. 14 (1994) 2545-2568. DOI:10.1523/JNEUROSCI.14-05-02545.1994 |
| [49] |
G. Palagina, U.T. Eysel, D. Jancke, Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 8743-8747. DOI:10.1073/pnas.0900068106 |
| [50] |
D. Jancke, F. Chavane, S. Naaman, Nature 428 (2004) 423-426. DOI:10.1038/nature02396 |
| [51] |
P. Berkes, G. Orban, M. Lengyel, et al., Science 331 (2011) 83-87. DOI:10.1126/science.1195870 |
| [52] |
E. Zagha, A.E. Casale, R.N. Sachdev, et al., Neuron 79 (2013) 567-578. DOI:10.1016/j.neuron.2013.06.008 |
| [53] |
S. Netser, A. Dutta, Y. Gutfreund, J. Neurophysiol. 111 (2014) 918-929. DOI:10.1152/jn.00527.2013 |
| [54] |
I. Carcea, M.N. Insanally, R.C. Froemke, Nat. Commun. 8 (2017) 14412. DOI:10.1038/ncomms14412 |
| [55] |
D.B. Omer, T. Fekete, Y. Ulchin, et al., Cereb. Cortex 29 (2019) 1291-1304. DOI:10.1093/cercor/bhy099 |
| [56] |
L.A. DeNardo, D.S. Berns, K. DeLoach, et al., Nat. Neurosci. 18 (2015) 1687-1697. DOI:10.1038/nn.4131 |
| [57] |
B.R. Lustig, R.M. Friedman, J.E. Winberry, et al., J. Neurophysiol. 109 (2013) 2382-2392. DOI:10.1152/jn.00430.2012 |
| [58] |
I. Ferezou, S. Bolea, C.C. Petersen, Neuron 50 (2006) 617-629. DOI:10.1016/j.neuron.2006.03.043 |
| [59] |
I. Ferezou, F. Haiss, L.J. Gentet, et al., Neuron 56 (2007) 907-923. DOI:10.1016/j.neuron.2007.10.007 |
| [60] |
H. Sato, Y. Shimanuki, M. Saito, et al., J. Neurosci. 28 (2008) 3076-3089. DOI:10.1523/JNEUROSCI.0172-08.2008 |
| [61] |
Q. Tang, V. Tsytsarev, C.P. Liang, et al., Sci. Rep. 5 (2015) 17325. DOI:10.1038/srep17325 |
| [62] |
S. An, J.W. Yang, H. Sun, et al., J. Neurosci. 32 (2012) 9511-9516. DOI:10.1523/JNEUROSCI.1212-12.2012 |
| [63] |
C.C. Petersen, B. Sakmann, J. Neurosci. 21 (2001) 8435-8446. DOI:10.1523/JNEUROSCI.21-21-08435.2001 |
| [64] |
D.J. Wallace, B. Sakmann, Cereb. Cortex 18 (2008) 1361-1373. DOI:10.1093/cercor/bhm168 |
| [65] |
A. Sigler, M.H. Mohajerani, T.H. Murphy, Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 11759-11764. DOI:10.1073/pnas.0812695106 |
| [66] |
C.E. Brown, K. Aminoltejari, H. Erb, et al., J. Neurosci. 29 (2009) 1719-1734. DOI:10.1523/JNEUROSCI.4249-08.2009 |
| [67] |
L. Buck, R. Axel, Cell 65 (1991) 175-187. DOI:10.1016/0092-8674(91)90418-X |
| [68] |
W.J. Freeman, Electroencephalogr. Clin. Neurophysiol. 44 (1978) 586-605. DOI:10.1016/0013-4694(78)90126-8 |
| [69] |
Y.W. Lam, L.B. Cohen, M. Wachowiak, et al., J. Neurosci. 20 (2000) 749-762. DOI:10.1523/JNEUROSCI.20-02-00749.2000 |
| [70] |
M.R. Zochowski, L.B. Cohen, J. Neurophysiol. 94 (2005) 2667-2675. DOI:10.1152/jn.00328.2005 |
| [71] |
N. Mizoguchi, K. Muramoto, M. Kobayashi, Pflugers Arch. 472 (2020) 721-732. DOI:10.1007/s00424-020-02399-w |
| [72] |
R.D. Thijs, R. Surges, T.J. O'Brien, et al., Lancet 393 (2019) 689-701. DOI:10.1016/S0140-6736(18)32596-0 |
| [73] |
R.M. Dasheiff, D.S. Sacks, Seizure 1 (1992) 79-87. DOI:10.1016/1059-1311(92)90004-K |
| [74] |
D.S. Sacks, R.M. Dasheiff, Brain Res. 595 (1992) 79-86. DOI:10.1016/0006-8993(92)91455-N |
| [75] |
M. Derchansky, D. Rokni, J.T. Rick, R. Wennberg, et al., Neurobiol. Dis. 23 (2006) 312-328. DOI:10.1016/j.nbd.2006.03.014 |
| [76] |
X. Wang, Y. Pang, G. Ku, et al., Nat. Biotechnol. 21 (2003) 803-806. DOI:10.1038/nbt839 |
| [77] |
Q. Yu, S.S. Hunag, Z.Y. Wu, et al., J. Nucl. Med. 61 (2020) 1079-1085. DOI:10.2967/jnumed.119.233155 |
| [78] |
Y. Liu, Y. Yang, M. Sun, et al., Chem. Sci. 8 (2017) 2710-2716. DOI:10.1039/C6SC04798J |
| [79] |
Y. Liu, H. Liu, H. Yan, et al., Adv. Sci. 6 (2019) 1801615. DOI:10.1002/advs.201801615 |
| [80] |
J. Kang, H.K. Zhang, S.D. Kadam, et al., Front. Neurosci. 13 (2019) 579. DOI:10.3389/fnins.2019.00579 |
| [81] |
J. Kang, S.D. Kadam, J.S. Elmore, et al., J. Neural Eng. 17 (2020) 025001. DOI:10.1088/1741-2552/ab78ca |
| [82] |
B. Rao, R. Zhang, L. Li, et al., Sci. Rep. 7 (2017) 2560. DOI:10.1038/s41598-017-02458-w |
| [83] |
S. Lee, J. Jeong, Y. Kwak, et al., Mol. Brain 3 (2010) 8. DOI:10.1186/1756-6606-3-8 |
| [84] |
G. von Wolff, C. Avrabos, J. Stepan, et al., J. Psychiatr. Res. 45 (2011) 256-261. DOI:10.1016/j.jpsychires.2010.06.007 |
| [85] |
A. Sahay, R. Hen, Nat. Neurosci. 10 (2007) 1110-1115. DOI:10.1038/nn1969 |
| [86] |
R.D. Airan, L.A. Meltzer, M. Roy, et al., Science 317 (2007) 819-823. DOI:10.1126/science.1144400 |
| [87] |
J.Y. Wu, H. Xiaoying, Z. Chuan, Neuroscientist 14 (2008) 487-502. DOI:10.1177/1073858408317066 |
| [88] |
W. Xu, X. Huang, K. Takagaki, et al., Neuron 55 (2007) 119-129. DOI:10.1016/j.neuron.2007.06.016 |
| [89] |
J.C. Prechtl, L.B. Cohen, B. Pesaran, et al., Proc. Natl. Acad. Sci. U. S. A. 94 (1997) 7621-7626. DOI:10.1073/pnas.94.14.7621 |
| [90] |
X. Huang, W.C. Troy, Q. Yang, et al., J. Neurosci. 24 (2004) 9897-9902. DOI:10.1523/JNEUROSCI.2705-04.2004 |
| [91] |
X. Huang, W. Xu, J. Liang, et al., Neuron 68 (2010) 978-990. DOI:10.1016/j.neuron.2010.11.007 |
| [92] |
H. Spors, Neuron 34 (2002) 301-315. DOI:10.1016/S0896-6273(02)00644-X |
| [93] |
A.S. Abdelfattah, T. Kawashima, A. Singh, et al., Science 365 (2019) 699-704. DOI:10.1126/science.aav6416 |
| [94] |
Y. Adam, J.J. Kim, S. Lou, et al., Nature 569 (2019) 413-417. DOI:10.1038/s41586-019-1166-7 |
| [95] |
V. Villette, M. Chavarha, I.K. Dimov, et al., Cell 179 (2019) 1590-1608. DOI:10.1016/j.cell.2019.11.004 |
 2021, Vol. 32
2021, Vol. 32 


