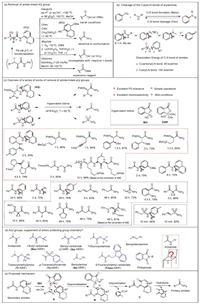
8-Aminoquinoline (AQ) has been widely used as a bidentate auxiliary in various metal-catalyzed C-H functionalization reactions[1]. However, like many directing groups, it is non-trivial to be removed. Generally, harsh conditions were employed for its removal to release free carboxylic acid or primary amide via hydrolysis, ammonolysis, or alcoholysis mechanism (Fig. 1a) [[2]]. Compared to AQ, N-aryl groups are more difficult to remove [3], and ceric ammonium nitrate (CAN) mediated oxidative N-dearylation only works for p-aminoanisole-containing substrates [2c, 2h, 4].
|
Download:
|
| Fig. 1. Regiospecific cleavage of aryl CN bonds in N-aryl amides (color online). | |
Existing C(aryl)-N bond cleavage reactions of aromatic amines are limited to a specific set of amino substituted aromatic amines, including arylhydrazines and aniline derivatives (Fig. 1b) [5]. Regiospecific cleavage of the inert C(aryl)-N bonds of N-aryl amides is a formidable challenge and has been largely unexplored because the C(carbonyl)-N bonds are more reactive, and are generally broken prior to C(aryl)-N bonds in N-aryl amides [6], owing to the lower bond dissociation energy of the former (95 kcal/mol) compared to that of the latter (104 kcal/mol) (Fig. 1b) [7]. On the other hand, literatures precedents on the C(aryl)-N bonds cleavage reaction of N-aryl amides primarily focused on functionalization of aryl moiety [8], with the amide group being treated as a leaving group whose fate are rarely studied [9].
Recently, Zhang's group from Henan Normal University collaborating with Chen's group of Nankai University developed a series of hypervalent iodine(V) reagent promoted regiospecific cleavage of inert aryl C-N bonds in N-aryl amides (Fig. 1c) [10]. They also demonstrated a more economical protocol using a catalytic amount of 2-iodobenzoic acid and oxone co-oxidant. With this protocol, a variety of primary amides were obtained in excellent yields and chiral integrity from the chemoselective N-aryl bond cleavage of N-aryl amides and sulfonamides. It is worth noting that some primary amides cannot be accessed by conventional methods, highlighting the advantages and practical value of this strategy. Especially, the reactions worked well with a variety of chiral α-amino acid substrates bearing multi-functionalized side chains. The reactions showed remarkable chemoselectivity and functional group tolerance, and allowed for easy removal of N-aryl groups of amides under mild conditions without affecting any other active functional groups. In this sense, it bestows these aryl groups with the characteristics of amino protecting groups (Fig. 1d). Mechanistically, the organoiodine(V) reagent-mediated removal of aryl group most likely starts with a substitution reaction between amide starting materials and the hypervalent iodine(V) reagent, such as IBX, to form iodoimidate A [11]. Intramolecular nucleophilic attack of the oxo group on the iodine center of A onto the ortho-carbon of aryl group triggers the dearomatization of aniline moiety to form intermediate B [11, 12]. Deprotonation and cleavage of exocyclic O-I bond of B give o-iminoquinone species C and 2-iodobenzoic acid. Hydrolysis of C gives the primary amides and quinolinediones. The metal-free, chemoselective N-aryl deprotection procedure allows removal of sort of aryl groups under mild conditions to give primary amides in high efficiency without affecting other C-N bonds or reactive function group. Significantly, chiral substrates also gave primary amides in excellent isolated yield and with excellent chiral fidelity. This feature renders the aryl group with the characteristics of a N-protection group that would be a supplement to the currently used ones. We anticipate that this feature may be used widespread in organic synthesis, biochemistry, asymmetric catalysis and material chemistry, where protection of amino groups is necessary.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
| [1] |
(a) X.L. Han, P.P. Lin, Q. Li, Chin. Chem. Lett. 30 (2019) 1495-1502; (b) H. Xia, Y. An, X. Zeng, J. Wu, Chem. Commun. 53 (2017) 12548-12551; (c) Z.Z. Zhang, Y.Q. Han, B.B. Zhan, S. Wang, B.F. Shi, Angew. Chem. Int. Ed. 56 (2017) 13145-13149; (d) X. Lu, B. Xiao, R. Shang, L. Liu, Chin. Chem. Lett. 27 (2016) 305-311. |
| [2] |
(a) H. Wang, Z. Bai, T. Jiao, et al., J. Am. Chem. Soc. 140 (2018) 3542-3546; (b) T. Deguchi, H.L. Xin, H. Morimoto, T. Ohshima, ACS Catal. 7 (2017) 3157-3161; (c) M. Berger, R. Chauhan, C.A.B. Rodrigues, N. Maulide, Chem. Eur. J. 22 (2016) 16805-16808; (d) T. Ly Dieu, O. Daugulis, Angew. Chem. Int. Ed. 51 (2012) 5188-5191; (e) D. Shabashov, O. Daugulis, J. Am. Chem. Soc. 132 (2010) 3965-3972; (f) Y. Feng, G. Chen, Angew. Chem. Int. Ed. 49 (2010) 958-961; (g) G. He, S.Y. Zhang, W.A. Nack, Q. Li, G. Chen, Angew. Chem. Int. Ed. 52 (2013) 11124-11128; (h) D.R. Kronenthal, C.Y. Han, M.K. Taylor, J. Org. Chem. 47 (1982) 2765-2768. |
| [3] |
S.E. Wang, L. Wang, Q. He, R. Fan, Angew. Chem. Int. Ed. 54 (2015) 13655-13658. DOI:10.1002/anie.201508161 |
| [4] |
(a) P. Guo, J.H. Huang, Q.C. Huang, X.H. Qian, Chin. Chem. Lett. 24 (2013) 957-961; (b) A.T. Hewson, D.A. Sharpe, A.H. Wadsworth, Synth. Commun. 19 (1989) 2095-2099. |
| [5] |
(a) T. Taniguchi, M. Imoto, M. Takeda, et al., Tetrahedron 72 (2016) 4132-4140; (b) T. Koreeda, T. Kochi, F. Kakiuchi, Organometallics 32 (2013) 682-690; (c) T. Koreeda, T. Kochi, F. Kakiuchi, J. Am. Chem. Soc. 131 (2009) 7238-7239; (d) S. Ueno, N. Chatani, F. Kakiuchi, J. Am. Chem. Soc. 129 (2007) 6098-6099; (e) U. Azzena, M. Cattari, G. Melloni, L. Pisano, Synthesis 2003 (2003) 2811-2814. |
| [6] |
L.I. Robins, E.J. Fogle, J.F. Marlier, BBA-Proteins Proteom. 1854 (2015) 1756-1767. DOI:10.1016/j.bbapap.2014.12.016 |
| [7] |
S.J. Blanksby, G.B. Ellison, Acc. Chem. Res. 36 (2003) 255-263. DOI:10.1021/ar020230d |
| [8] |
M. Tobisu, K. Nakamura, N. Chatani, J. Am. Chem. Soc. 136 (2014) 5587-5590. DOI:10.1021/ja501649a |
| [9] |
K.J. Geng, Z.L. Fan, A. Zhang, Org. Chem. Front. 3 (2016) 349-353. DOI:10.1039/C5QO00387C |
| [10] |
(a) M. Song, Z. Zhang, D. Zheng, et al., Chin. J. Org. Chem. 40 (2020) 2433-2441; (b) Z. Zhang, X. Li, M. Song, et al., J. Org. Chem. 84 (2019) 12792-12799; (c) Z. Zhang, D. Zheng, Y. Wan, et al., J. Org. Chem. 83 (2018) 1369-1376. |
| [11] |
K.C. Nicolaou, K. Sugita, P.S. Baran, Y.L. Zhong, J. Am. Chem. Soc. 124 (2002) 2221-2232. DOI:10.1021/ja012125p |
| [12] |
K.C. Nicolaou, P.S. Baran, Y.L. Zhong, K. Sugita, J. Am. Chem. Soc. 124 (2002) 2212-2220. DOI:10.1021/ja012124x |
 2021, Vol. 32
2021, Vol. 32 

