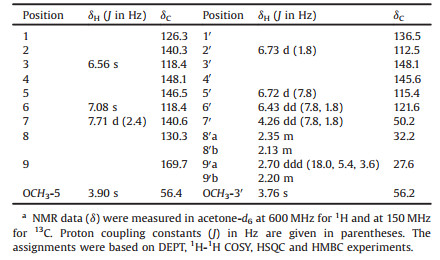
The dried whole roots of Angelica sinensis (Oliv.) Diels (Umbelliferae) is a common traditional Chinese medicine, named "danggui", having medicinal functions of tonifying blood, invigorating blood circulation and relieving pain [1]. Different parts of "danggui" are considered in traditional Chinese medicine to have different functions and applications [1]. The whole root is also used as a tonic medicinal food to cooking with meat or making soup in China and as food/dietary supplement in Europe and North America [2, 3]. This plant is mainly cultivated in Gansu province of China not only to satisfy demands of various applications but also to support local economy. Pharmacological studies on crude extracts and purified constituents of danggui revealed various activities, including anti-inflammation, anti-spasticity, antioxidation, anti-fibrosis and neuroprotection, as well as effects against cardio- and cerebrovascular diseases [1-3]. Meanwhile, around 100 chemical constituents have been isolated from the extracts, mainly including structural types of phthalide, phenylpropanoid, lignan, coumarin, alkyne, terpene, sterol, alkaloid, fatty acid and polysaccharide [2-10]. The main constituents, Z-ligustilide, ferulic acid and polysaccharides, are considered as the active constituents. However, contents of the active constituents were varied significantly by processing and decocting [2-4]. Especially, specific investigation on the root head "guitou" is rare, and the chemical studies were carried out predominantly on the EtOH or MeOH extract of the whole root, which differs classical utilization by decocting with water [5-12]. Thus, an aqueous extract of "guitou" was investigated as part of our program to explore the chemical diversity of traditional Chinese medicines, focusing on the minor constituents [13-23]. From the extract, a pair of neolignan enantiomers with an unprecedented carbon skeleton, (+)-/(−)-angelignanine [(+)-/(−)-1] (Fig. 1), has been isolated (see Supporting information) and structurally determined. Herein, described are details.
|
Download:
|
| Fig. 1. Structures of (+)-1 and (−)-1. | |
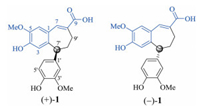
The racemate 1 was isolated as a white amorphous powder. The infrared (IR) spectrum of 1 showed characteristic absorption bands for aromatic ring (1611 and 1514 cm−1), conjugated carbonyl (1673 cm−1) and hydroxyl (3348 cm−1) functionalities. Its molecular formula C20H20O6 with 11 degrees of unsaturation was determined by negative mode high resolution electrospray ionization mass spectroscopy [(–)-HR-ESIMS] at m/z 355.1182 [M-H]− (calcd. for C20H19O6, 355.1187), together with the nuclear magnetic resonance (NMR) spectroscopic data (Table 1). The 1H NMR spectrum of 1 in acetone-d6 exhibited signals due to a conjugated trisubstituted double bond at δH 7.71 (d, J =2.4Hz, H-7), an ortho-meta-para-trisubstituted phenyl at δH 7.08 (s, H-6) and 6.56 (s, H-3), a meta-para-disubstituted phenyl at δH 6.73 (d, J = 1.8Hz, H-2′), 6.72 (d, J = 7.8Hz, H-5′) and 6.43 (dd, J = 7.8 and 1.8Hz, H-6′), two aromatic methoxy groups at δH 3.90 (s, 5-OCH3) and 3.76 (s, 3′-OCH3), and a sp3 hybrid methine at δH 4.26 (dd, J = 7.8 and 1.8Hz, H-7′). Additionally, in the 1H NMR spectrum the signals attributable to vicinal coupling protons of two sp3 hybrid aliphatic methylenes were observable between δH 2.70 and 2.13. The 13C NMR and distortionless enhancement by polarization transfer (DEPT) spectra of 1 showed 20 carbon signals (Table 1) corresponding to the aforementioned structural units and a conjugated carboxylic carbon (δC 169.7, C-9). When compared with those of compounds previously reported from A. sinensis [2-10], the aforementioned spectroscopic data revealed that1 was an abnormal noelignan possessing an unusual skeleton. Subsequently, the structure was further deduced by two dimension (2D) NMR spectroscopic data analysis (Fig. 2).
|
|
Table 1 NMR spectroscopic data for compound 1. |
|
Download:
|
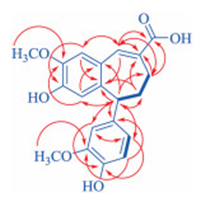
| Fig. 2. The 1H-1H COSY (thick lines) and main two- and three-bond HMBC correlations (red arrows, from 1H to 13C) of 1. | |
Analysis of correlation peaks in the heteronuclear single quantum coherence (HSQC) spectrum led to assignment of the proton-bearing carbon and corresponding proton signals in the NMR spectra of 1 (Table 1). In the heteronuclear multiple bond correlation (HMBC) spectrum, the heteronuclear long-range correlation peaks of H-3/C-1 and C-5; H-6/ C-2, C-4 and C-7; H-7/C-2, C-6, C-8 and C-9; and 5-OCH3/C-5, along with their chemical shifts, indicated the presence of a 2, 8-disubstituted 4-hydroxy-5-methoxyphenyl-propenoic acid moiety in 1. Meanwhile, the presence of a 7′, 9′-disubstituted 4′-hydroxy-3′-methoxyphenylpropane moiety in 1 was deducible from the HMBC correlation peaks of H-2′/C-4′, C-6′ and C-7′; H-5′/C-1′ and C-3′; H-6′/C-2′, C-4′ and C-7′; and H-7′/C-1′, C-2′, C-6′, C-8′ and C-9′; and 3′-OCH3/C-3′, combined with the chemical shifts of these hydrogen and carbon resonances as well as the vicinal coupling cross-peaks of H-5′/H-6′ and H-7′/H2-8′/H2-9′ in the 1H–1H coupling correlation spectroscopy (COSY) spectrum (Fig. 2) and the coupling constant J5′, 6′ (Table 1). In addition, the HMBC correlations of H-3/C-7′ and H-7′/C-1, C-2 and C-3 established a 2, 7′-linkage of the two structural moieties, while a 8, 9′-linkage was constructed by the HMBC correlations of H-7/C-9′, H2-8′/C-8, and H2-9′/C-7, C-8 and C-9. Accordingly, the planar structure of 1 was elucidated as a novel 2, 7′-cyclo-8, 9′-neolignan, of which the carbon skeleton has never been reported in natural and synthetic products.
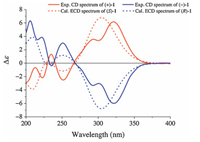
Using high performance liquid chromatography (HPLC) equipped with a semi-preparative chiral column (Chiralpak IG, n-hexane-EtOH, 3:1), separation of 1 afforded (+)-1 {[α] null +101.8 (c 0.11, MeOH)} and (−)-1 {[α] null –96.4 (c 0.14, MeOH)}. The measured CD curves of (+)- and (−)-1 were mirrored each other (Fig. 3), but the NMR spectroscopic data were identical to those of 1 prior to separation, proving that the two compounds were a pair of enantiomers. The CD spectra of (+)- and (−)-1 displayed typical exciton coupling Cotton effects at λmax 251.0±0.5 and 293.0±0.5 nm, respectively, arising from coupling between π→π* transitions of the two substituted phenyl chromophores. Using the CD exciton chirality method [24], the positive CD exciton chirality predicts the 7′S configuration for (+)-1, while the negative exciton chirality of suggests the 7′R configuration for (−)-1 (Figs. S4 and S5 in Supporting information). The assignment was further supported by comparison of the experimental CD spectra with that calculated by quantum mechanical time dependent density functional theory (TDDFT) [25]. The theoretically calculated electronic CD spectra of 7′S- and 7′R-1 were in good agreement with the experimental CD spectra of (+)- and (−)-1 (Fig. 3), respectively. Therefore, the structures of (+)- and (−)-1 were unambiguously determined and trivially designated as (+)- and (−)-angelignanine, respectively. Based on international union of pure and applied chemistry (IUPAC) recommendation on nomenclature of neolignans [26], the two enantiomers are systematical named (+)-(7′S)- and (−)-(7′R)-4, 4′-dihydroxy-3′, 5-dimethoxy-2, 7′-cyclo-8, 9′-neolign-7-en-9-oic acid, respectively.
|
Download:
|
| Fig. 3. The overlaid experimental CD (full lines) and calculated ECD (dash lines) spectra of (+)-1 (red) and (−)-1 (blue). | |
According to the unique structural architecture of 2, 7′-cyclo-8, 9′-noelignan skeleton and substituents in 1, the plausible biosynthetic pathway is proposed in Scheme 1. The biosynthetic precursors are traced to be ferulic acid (2) and coniferyl alcohol (3), which are biosynthesized through shikimate pathway and abundantly co-occur in this plant [4]. Intermolecular free radical coupling and intramolecular radical cyclization reactions are proposed to involve in biosynthesis of 1. Briefly enzyme-assisted oxidation of 2 and dihydroxylation of 3 give free radicals i and ii, respectively. An intermolecular coupling of the free radicals generates an intermediate iii, which would be enzymatically oxidized to give a double bond-rearranged radical iv then undergoes cyclization and captures a hydrogen radical via v to produce 1. Alternatively, 1 may be formed from sequential intermolecular nucleophilic addition and intramolecular free radical cyclization (Scheme S1 in Supporting information). A nucleophilic addition of ferulic acid with a dehydrate product of coniferyl alcohol to yield 8-coniferylferulic acid, which undergoes oxidation-assisted intramolecular free radical cyclization to give 1. Since 1 was isolated as the racemate, formation of the sole chiral center at C-7′ may be non-enzymatic or enzyme-uncontrollable in the plausible pathways.

|
Download:
|
| Scheme 1. The plausible biosynthetic pathway of 1. | |
Because compound 1 was undetectable in a fresh crude extract by HPLC/HR-ESIMS analysis (possibly due to low content), to exclude fortuitous formation of 1 during the isolation procedure, 2 and 3 were refluxed in H2O or methanol with or without silica gel (the main solvents and absorbent used in the isolation procedure) for 8 h. HPLC/HR-ESIMS analysis demonstrated that 1 was also undetectable in the reaction mixtures. The simulation is not enough but partially supportive to conclude that 1 is a natural product.
According to the clinical application of "guitou", the enantiomers were tested for their hypnotic effect in mice. As compared with vehicle control group, at a dosage of 10.0 mg/kg (i.g.) both the enantiomers significantly increased the rate of sleep onset in mice treated with sub-hypnotic dose of sodium pentobarbital (Table S2 in Supporting information), and prolonged the duration of loss of righting reflex in mice treated with hypnotic dose of sodium pentobarbital [27].
In conclusion, (+)-/(−)-angelignanine [(+)-/(−)-1] was isolated as the minor constituent from the aqueous extract of "guitou". The enantiomers have the undescribed 2, 7′-cyclo-8, 9′-neolignan carbon skeleton biogenetically proposed from coupling between ferulic acid and coniferyl alcohol. The structural novelty not only enriches the diversity of neolignans and the chemical constituents of "danggui", but also challenges synthetic and biosynthetic chemists to obtain enough amount of the sample for further biological evaluation.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsFinancial support from the National Natural Sciences Foundation of China (No. 81630094), CAMS Innovation Fund for Medical Science (No. 2017-I2M-3-010) and The Drug Innovation Major Project (No. 2018ZX09711001-001) is acknowledged.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.067.
| [1] |
Jiangsu New Medical College, Dictionary of Traditional Chinese Medicine. Shanghai: Shanghai Science and Technology Publishing House, 1997: pp. 876-879.
|
| [2] |
I.L.I. Hook, J. Ethnopharm. 152 (2014) 1-13. DOI:10.1016/j.jep.2013.12.018 |
| [3] |
W.L. Wei, R. Zeng, C.M. Gu, Y. Qu, L.F. Huang, J. Ethnopharm. 190 (2016) 116-141. DOI:10.1016/j.jep.2016.05.023 |
| [4] |
J.P. Ma, Z.B. Guo, L. Jin, Y.D. Li, Chin. J. Nat. Med. 13 (2015) 241-249. |
| [5] |
L.B. Zhang, J.L. Lv, J.W. Liu, J. Nat. Prod. 79 (2016) 1857-1861. DOI:10.1021/acs.jnatprod.6b00080 |
| [6] |
W. Gong, Y. Zhou, X. Li, et al., Molecules 21 (2016) 549. DOI:10.3390/molecules21050549 |
| [7] |
L. Zhang, J. Lv, Chem. Nat. Comp. 54 (2018) 13-17. DOI:10.1007/s10600-018-2248-8 |
| [8] |
J.L. Lv, L.B. Zhang, L.M. Gao, Fitoterapia 129 (2018) 102-107. DOI:10.1016/j.fitote.2018.06.016 |
| [9] |
J. Zou, G.D. Chen, H. Zhao, et al., Org. Lett. 20 (2018) 884-887. DOI:10.1021/acs.orglett.8b00017 |
| [10] |
J. Zou, G.D. Chen, H. Zhao, et al., Chem. Commun. 55 (2019) 6221-6224. DOI:10.1039/C9CC02681A |
| [11] |
B. Sheng, Y. Vo, P. Lan, et al., Org. Lett. 21 (2019) 6295-6299. DOI:10.1021/acs.orglett.9b02172 |
| [12] |
K. Duric, Y. Liu, S.N. Chen, et al., J. Nat. Prod. 82 (2019) 2400-2408. DOI:10.1021/acs.jnatprod.8b00962 |
| [13] |
L.J. Meng, Q.L. Guo, C.G. Zhu, et al., Chin. Chem. Lett. 29 (2018) 119-122. DOI:10.1016/j.cclet.2017.05.019 |
| [14] |
L. Meng, Q. Guo, M. Chen, et al., Chin. Chem. Lett. 29 (2018) 1257-1260. DOI:10.1016/j.cclet.2017.12.001 |
| [15] |
Q. Guo, H. Xia, X. Meng, et al., Acta Pharm. Sin. B 8 (2018) 409-419. DOI:10.1016/j.apsb.2018.03.009 |
| [16] |
Q. Guo, C. Xu, M. Chen, et al., Acta Pharm. Sin. B 8 (2018) 933-943. DOI:10.1016/j.apsb.2018.08.005 |
| [17] |
Q. Guo, H. Xia, G. Shi, et al., Org. Lett. 20 (2018) 816-819. DOI:10.1021/acs.orglett.7b03956 |
| [18] |
Y. Wu, S. Shao, Q. Guo, et al., Org. Lett. 21 (2019) 6850-6854. DOI:10.1021/acs.orglett.9b02479 |
| [19] |
J. Cai, Q.L. Guo, R.F. Li, et al., Acta Pharm. Sin. 54 (2019) 1075-1081. |
| [20] |
Q. Guo, D. Li, C. Xu, et al., Acta Pharm. Sin. B 10 (2020) 895-902. DOI:10.1016/j.apsb.2019.09.001 |
| [21] |
C. Xu, Y. Xin, M. Chen, et al., Eur. J. Med. Chem. 189 (2020) 112071. DOI:10.1016/j.ejmech.2020.112071 |
| [22] |
Q. Guo, H. Xia, Y. Wu, et al., Acta Pharm. Sin. B 10 (2020) 1954-1965. DOI:10.1016/j.apsb.2020.01.013 |
| [23] |
H. Liu, Y. Wu, Q. Guo, et al., Chin. Chem. Lett. 32 (2021) 33-36. DOI:10.1016/j.cclet.2020.09.062 |
| [24] |
K. Nakanishi, N. Berova, The exciton chirality method, in: K. Nakanishi, N. Berova, R.W. Woody (Eds. ), Circular Dichroism Principles and Applications, Wiley-VCH, New York, 1994, pp. 361-398.
|
| [25] |
X.C. Li, D. Ferreira, Y.Q. Ding, Curr. Org. Chem. 14 (2010) 1678-1697. DOI:10.2174/138527210792927717 |
| [26] |
G.P. Moss, Pure Appl. Chem. 72 (2000) 1493-1523. DOI:10.1351/pac200072081493 |
| [27] |
Y. Zhang, M. Li, R.X. Kang, et al., Pharmacol. Biochem. Behav. 102 (2012) 450-457. DOI:10.1016/j.pbb.2012.06.002 |
 2021, Vol. 32
2021, Vol. 32