b Xiangya School of Public Health, Central South University, Changsha 410078, China;
C Key Laboratory of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210023, China
The concept of "green chemistry" was proposed in the 1990 s to solve environmental problems caused by chemical pollution and resource depletion, and was then defined as the "design of chemical products and processes to reduce or eliminate the use and generation of hazardous substances". In 1998, P.T. Anastas and J.C. Waner published the 12 Principles of green chemistry (Table 1). Today, the 12 Principles of green chemistry, have been widely accepted and used as guidelines for the design of green and sustainable chemical processes [1].
|
|
Table 1 The 12 Principles of green chemistry. |
Of all the principles of green chemistry, "Prevention" is the most fundamental and critical one because "It is better to prevent waste than to treat or clean up waste after it has been created". However, in organic synthesis, the vast majority of organic synthesis reactions are carried out in petroleum-based solvents. These petroleum-based solvents are not only harmful, but also used in large quantities. Otherwise, the conversion rate of reactions will be affected, resulting in low atomic efficiency. These solvents are difficult to recycle after being used, which consume a lot of fossil resources and cause serious environmental pollution. Moreover, these synthetic reactions often afford mixed target products with unnecessary starting materials and by-products, which require tedious separation and purification steps to be removed. These traditional separation and purification processes are laborious, further consuming a lot of petroleum-based solvents, and pushing up the production cost. Chemical wastes contribute heavily to environmental pollutions, and waste disposal has become a serious economic, environmental and social responsibility. Even in developed countries with advanced technologies, each year it costs about 1 billion USD to eliminate the chemical waste that is being generated by economic activities and left over from history. In addition to economic factors, it also has an impact on the ecosystem as well as the health of workers, surrounding communities, consumers and the public. Waste prevention is the cornerstone of green chemistry, and it can be achieved by rational choice of raw materials, reaction pathways, solvent systems and separation methods to reduce waste generation production costs.
In the past few years, our team has been committed to developing green and sustainable synthetic methodologies, building valuable compounds from easily available starting materials, and has gained some interesting results. Herein we summarize some of our observations in clean production of value-added molecules, which employed eco-friendly reaction solvents and applied clean separation methods in (nearly) quantitative organic synthesis.
2. Collect products by filtrationFiltration is a routine experimental operation, which utilizes a filter medium to separate the solid matter and liquid from a multiphase mixture. The complex porous structure of the filter medium makes the liquid pass through, forming the filtrate. The solid particles that cannot pass through are called oversize.
2.1. Quinolin-2(1H)-onesWater is a critical reaction medium for various chemical and biological processes. It has unique physical and chemical properties, such as hydrophobicity, polarity and hydrogen bonding effect, which endow the reactions "in water" or "on water" with unique selectivity and reactivity. This cannot be obtained by using common organic solvents. Therefore, from the perspective of "green and sustainable chemistry", it is an ideal, economical, effective and safe solvent for organic chemistry. In the past few decades, much effort has been made in the utilization of water as the solvent and a number of aqueous synthetic reactions have been well-established [2]. For reactions in aqueous solutions, a key problem is that the addition of petroleum-based solvent is necessary to extract products for separation, thus greatly compromise the green advantages of aqueous synthetic reactions. Therefore, one of the ultimate goals of synthetic chemistry is to develop novel aqueous reactions in which the pure products can be obtained and collected without using any petroleum-based solvent.
Quinolin-2(1H)-one and its derivatives represent an important class of structural features which widely exist in synthetic pharmaceuticals, natural products and advanced functional materials [3]. In 2017, our group developed a practical method to prepare quinolin-2(1H)-ones form easily available quinoline N-oxides in the presence of methylsufonyl chloride (MsCl) as the promoter in water (Scheme 1) [4]. The investigation of reaction mechanism revealed that the quinolineN-oxides was firstly activated with MsCl, then water attacked the α-position of activated quinoline N-oxides to generated the desired quinolin-2(1H)-ones along with the side product MsOH. Importantly, the quinolin-2(1H)-ones were formed in quantitative yields and crystallized during the reaction so that they could be easily collected through simple filtration (Fig. 1). The side product MsOH was also simply recycled via liquid-liquid separation. After isolation of these compounds, the reaction medium (water) could be reused for five times without efficiency loss. A comparison of green chemistry merits showed that our methodology is markedly greener than Zhang [5] and Yu's methods [6] with an Eco-scale value of 89.5 and E-factor of 1.6.

|
Download:
|
| Scheme 1. Clean production of quinolin-2(1H)-ones by filtration. | |

|
Download:
|
| Fig. 1. Process for the clean production of quinolin-2(1H)-ones: (a) before reaction; (b) during addition of MsCl; (c) phase separation at the end of the reaction; (d) isolated product. | |
Sulfonylated N-heterocycles are highly valuable compounds in synthetic chemistry, pharmaceutical chemistry and materials science [7]. Conventional methods for synthesizing these compounds are the transition metal-catalyzed cross-coupling reactions of halogenated N-heterocycles with sodium sulfinates in alkaline conditions [8]. A problem that accompanies conventional methods is that the sodium sulfinates are often not commercially available, therefore they have to be prepared via the corresponding sulfonyl chlorides, which require pernicious alkaline reaction conditions [9]. Moreover, the reaction conditions of cross-coupling reactions usually include the use of harmful organic solvents and toxic heavy metal salts such as palladium salt.
The essence of a cascade reaction is to manipulate multiple chemical reactions in the same reaction system without isolation of intermediates. The products are often afforded in higher yields with separation and purification procedures skipped. Since it has the virtue of high atom economy, step economy and reaction efficiency, and is applicable to the construction of a variety of complex molecules from simple and readily available starting materials. The performance of cascade reactions may be a valuable strategy to solve the above problems.
In 2018, our group developed an aqueous one-pot cascade reaction for the constructing sulfonylated N-heterocycles from readily available sulfonyl chlorides and halogenated N-heterocycles in the presence of Na2SO3 as the reductant (Scheme 2) [10]. In this cascade reaction, the in situ generated proton (derived from water) activated the halogenated N-heterocycles. The sulfonyl chlorides were reduced by Na2SO3 to produce a nucleophilic sulfonyl anion, which directly attacked the N-heterocycles, followed by deprotonation to afford the sulfonylated N-heterocycles. The sulfonylated N-heterocycles crystallized in the reaction process. In large-scale synthesis, highly pure sulfonylated products could be easily obtained by filtration and washing with eco-friendly alcohol. In summary, our protocol uses a cascade reaction to avoid the generation of chemical waste from the derivatization of sulfonyl chlorides and takes advantage of the solubility difference to separate the target sulfonylated N-heterocycles from the reaction mixture. In this means, this method provides a better choice for the green industrial preparation of sulfonylated N-heterocyclic compounds than the traditional cross-coupling ways.

|
Download:
|
| Scheme 2. Clean production of sulfonylated N-heterocycles by filtration. | |
2.3. Quinolin-2-yl substituted ureas
In many biologically active molecules, synthetic drugs and functional materials there exists urea-containing structures. Because of their inherent value, it is significant and urgent to establish novel and practical methods to effectively integrate urea skeletons into valuable synthetic scaffolds, and great progress has been made in recent years.
In 2019, our research group developed an aqueous synthetic protocol for quinolin-2-yl substituted ureas from easily available quinoline N-oxides and cheap carbodiimides in the presence of CuSO4·5H2O as the catalyst under base-, organic solvent-free and mild conditions (Scheme 3) [11]. This reaction is proposed to involve a nonaromatic oxadiazolidinimine intermediate, which is generated by the [3+2] cycloaddition of quinoline N-oxide and carbodiimide. 100% Atom economy is achieved through the transfer of oxygen and hydrogen atoms from quinoline N-oxide to quinoline-2-yl substituted urea products. The water can be recycled and reused up to five times without any loss in its activity. During the reaction, the target product could be eco-friendly collected in highly pure through filtration and washing with alcohol. It is expected that the merits of extremely mild conditions, 100% atom-economy of transformation, and high functional group compatibility will make the established protocol highly attractive in the field of synthetic chemistry and for the pharmaceutical industry.
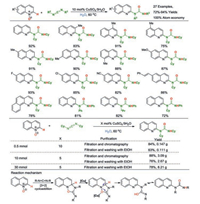
|
Download:
|
| Scheme 3. Clean production of quinolin-2-yl substituted ureas by filtration. | |
2.4. 2, 5-Furandicarboxylic acid
2, 5-Furandicarboxylic acid (FDCA) is identified as one of the twelve pivotal value-added chemicals and has attracted special attention in recent decades [12]. A recently developed strategy for their preparation is to oxidize 5-hydroxymethylfurfural (HMF) with high pressure molecular oxygen in the presence of certain catalyst to catalyze the oxidation reaction. These oxidation reactions use transition metal salts and/or a large amount of inorganic bases to promote the oxidations, producing a lot of chemical wastes, and also making the tedious separation and purification process inevitable.
Peroxides are a common class of mild oxidants and have good oxidation ability, which is suitable for HMF oxidation. It is well known that ether compounds can be converted into peroxides under aerobic conditions [13]. In 2019, our group reported a green and practical protocol for the oxidation of HMF to FDCA with 1, 2-diethoxyethylane as the solvent (Scheme 4) [14]. In the absence of any metal catalyst, additives and external free-radical initiator, 1, 2-diethoxyethylane firstly reacted with molecular oxygen to produce a peroxide, which served as an oxidant for the oxidation of HMF to FDCA. The aerobic oxidation of HMF might undergo two possible pathways. In pathway A, the reaction of 1, 2-diethoxyethylane and molecular oxygen at heating conditions generated the peroxide, which oxidized HMF to furan-2, 5-dicarbaldehyde (DFF). Subsequently, DFF underwent radical autoxidation to deliver the deep oxidative product FDCA. In pathway B, HMF was first converted into 5-hydroxymethyl-2-furancarboxylic acid (HMFCA) via radical autoxidation in the presence of hot molecular oxygen. Next, the in situ generated peroxide oxidized HMFCA to form 5-formylfuran-2-carboxylic acid (FFCA), which on further oxidation resulted in the formation of the finally oxidative product FDCA. Then the HMFCA was oxidized into 5-formylfuran-2-carboxylic acid (FFCA), which on further oxidation afford the finally oxidative product FDCA. With the oxidation reaction going on, FDCA crystallized directly from the solution to form a solid-liquid mixture, which was facilely filtered after to afford the product (Fig. 2).
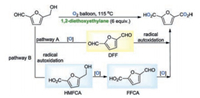
|
Download:
|
| Scheme 4. Clean production of 2, 5-furandicarboxylic acid by filtration. | |

|
Download:
|
| Fig. 2. Process for clean production of FDCA: (a) before reaction; (b) during the reaction process; (c) after reaction; (d) pure product. | |
Precipitation is the process of producing a separable solid phase from the liquid phase, or an insoluble substance from a supersaturated solution. When a precipitation process takes place in a liquid solution, the solids formed are called precipitates.
3.1. 5-Organylselanyl uracilsOrganoselenium compounds are a kind of multifunctional organic synthetic reagents with remarkable biological activities. They can be used as both drugs and synthetic intermediates [15]. Due to their characteristics of hydrogen bond acceptor and electron donor, organoselenium compounds can significantly improve the biological activity of natural substrates. Among all kinds of organoselenium compounds, 5-organoselenium uracil is a very important compound and has been applications in the area of anticancer drugs and antiviral drugs.
Diorganyl diselenides are among the most synthetically versatile and widely used selenylation reagents for the preparation of various selenylated products. However, most of the selenylation reactions suffer from low atom economy (< 50%) because of the other organyl selenide byproducts generated simultaneously with the desired product.
Recently, our group established a practical method for synthesizing 5-organylselanyl uracils with excellent atom economy [16]. By using KI as the catalyst and electrolyte, and dimethyl sulfoxide as the sole solvent, various 5-organylselanyl uracils were produced in excellent yields under 10 mA constant current with a platinum plate as the anode, a graphite rod as the cathode at room temperature (Scheme 5). The dual effect from KI significantly simplifies the electrochemical reaction system and reduces the generation of chemical waste by using electricity as the sole oxidant, producing hydrogen as the only by-product. This reaction started with the formation of reactive selenium cation intermediate, which underwent the electrophilic addition of uracil to give the iminium ion intermediate. The protonation of iminium ion intermediate delivered the 5-organylselanyl uracil. The pure selenylated products can be easily obtained via water precipitation and filtration, thus avoiding environmentally unfriendly isolation and purification procedures (Fig. 3). Importantly, the KI/DMSO system could be conveniently re-used, and its activity did not show any significant decrease after five consecutive runs. In contrast to the previously reported method [17], this process presented 2.7-fold increase in atom economy and 2.1-fold increase in Eco-scale value.

|
Download:
|
| Scheme 5. Clean production of 5-organylselanyl uracils by precipitation. | |

|
Download:
|
| Fig. 3. Process for clean production of 6-amino-5-(phenylselanyl)pyrimidine-2, 4(1H, 3H)-dione: (A) before reaction; (B) after reaction; (C) phase after added water; (D) dry product. | |
Biomass-derived solvents have great economic potential and are considered as green alternatives to petroleum based solvents [18]. Among these biomass-derived solvents, an attractive example is lactic acid, which has attracted more and more attention as a green chemical material and intermediate. Besides chemical methods, biological fermentation of carbohydrates such as monosaccharides and disaccharides is also an effective approach to prepare lactic acid. Structurally, lactic acid contains the α-hydroxy acid scaffold with a neighboring hydroxyl group by the carboxyl group [19]. Z-3-Selenocyanatoacrylate is an important selenium containing compound, which is widely used in organic synthesis and pharmaceutical chemistry.
In 2019, our team reported a practical and sustainable method to prepare Z-3-selenocyanatoacrylates with lactic acid as the reaction media and reusable catalyst (Scheme 6) [20]. The reaction mechanism includes initial formation of hydrogen bond between the oxygen atom of the alkyl ester group and the hydrogen atom of lactic acid carboxyl group, thus activating the alkyne substrate. Similarly, the nitrogen atom of SeCN may form a second hydrogen bond with the hydrogen atom of hydroxyl group in lactic acid. These hydrogen bonds between the two molecules facilitate the regioselective and stereoselective selenidation reaction to proceed. The heat and mass transfer problems were well solved by taking advantage of ultrasound irradiation. In the gram-scale cases, the pure Z-3-selenocyanatoacrylates products could be simply collected by liquid-liquid separation without the employment of any petroleum-based solvents (Fig. 4). For five consecutive reaction runs, the cheap lactic acid could be reused, and the catalytic activity showed no significant decrease. Compared with these previously reported protocols, this strategy has greater industrial application potential.
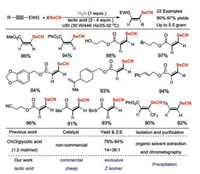
|
Download:
|
| Scheme 6. Clean preparation of Z-3-selenocyanatoacrylates by precipitation. | |

|
Download:
|
| Fig. 4. Process for clean preparation of (Z)-methyl 3-selenocyanatoacrylate. (a) Before reaction; (b) after reaction; (c) add water to the reaction mixture. | |
Liquid-liquid extraction is a method to separate a mixture by using the different solubility of components in two different immiscible liquids (usually a nonpolar organic solvent with polar water), i.e., by using the different solubility or partition coefficient of substances in two mutually immiscible (or slightly soluble) solvents, the solute can be separated from one solvent by internal transfer to another solvent, usually from the aqueous phase to the organic phase.
Petroleum-based solvents are usually harmful, but are heavily used in most chemical synthetic processes, accounting for around nine-tenths of the total reaction mass. In this regard, solvent-free synthetic reactions are ideal because they are in conformity with two of the 12 Principles of green chemistry: "prevent waste" and "safer solvents and auxiliaries" [21].
Polysubstituted pyrroles are widely found in natural products, small molecule drugs and organic functional materials [22]. In 2020, our research group reported a practical method for the synthesis of polysubstituted pyrroles under oxidant-, transition metal- and solvent-free conditions (Scheme 7) [23]. By using eco-friendly molecular iodine as the catalyst [24], the multicomponent reaction [25] of alkynes with equal equiv. of TMSCN and N, N-disubstituted formamides delivered the desired polysubstituted pyrroles in good to excellent yields (Scheme 7). This reaction was proposed to involve the [3+2] cycloaddition of nonaromatic oxadiazolidinimine intermediate and phenylethylene followed by dehydrogenation delivered the dihydropyrrolecarbonitrile intermediate, which underwent oxidative dehydrogenation and aromatization to give the desired polysubstituted pyrrole product. For large-scale synthesis, only simple liquid-liquid extraction is needed to obtain high purity target products without time-consuming and laborious chromatographic purification. In terms of green chemistry parameters, our developed method increased the EcoScale value by 2.1 fold, molar efficiency by 7.5-fold, atom economy by 6-fold, and process mass intensity by 85.7-fold when compared with Wang's method [26].
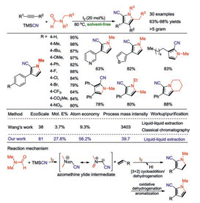
|
Download:
|
| Scheme 7. Clean production of polysubstituted pyrroles by liquid-liquid extraction. | |
5. Collect sulfones by recrystallization
Recrystallization is a technique used to purify impure chemicals or to separate mixed substances from each other by dissolving impurities and compounds in a suitable solvent and subsequent recrystallizing from solution.
Molecular oxygen is an abundant and easily available, environmentally friendly oxidant. The direct oxidation of organic compounds with O2 as the sole oxidant is one of the most ideal methods for synthesizing oxygenated chemicals. However, the utilization of O2 is challenging due to the triplet-ground-state of O2, which imposes large kinetic barriers to oxygen reduction and leads to poorly selective transformation, considering the election disparity between the four-electron molecular oxygen reduction and two-electron substrate oxidation. The vast majority of oxidation reactions with O2 have to rely on metal catalysts and/or eco-unfriendly initiators, which usually generate undesired metal salts and by-products. In addition, the traditional oxidation reactions generally require a large excess amount of volatile petroleum-based solvent as a reaction medium, which not only leads to a potentially flammable and possibly explosive mixture in the presence of highly energetic oxidants but also causes environmental and resource problem.
Sulfones and sulfoxides are significant structural motifs existing in plenty of bioactive natural products, synthetic pharmaceuticals and pesticides. They are also acting as valuable intermediates and versatile building blocks in organic synthesis [27].
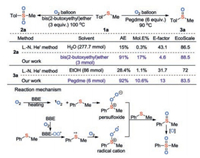
Recently, our group developed a solvent-controlled selective oxidation of sulfides with O2 [28]. Sulfone compounds can be efficiently formed in the presence of 3 equiv. of bis(2-methoxyethyl)ether as the solvent, whereas sulfoxide compounds could be generated in high yields with 6 equiv. of pegdme as the solvent (Scheme 8). The dual-function of ether solvents largely simplified the oxidation system, thus avoiding the need of external catalysts and additives. The plausible mechanism was that the sulfide was oxidized into persulfoxide intermediate through energy-transfer-process or radical cation intermediate through electron-transfer-process. These intermediates reacted with another molecule of sulfide to deliver the sulfoxide product, which can be further oxidized into the sulfone product. In the gram-scale oxidation of sulfides, the pure oxidative products could be isolated through simple recrystallization. The results of calculating the atom economy, molar efficiency, E-factor and EcoScale value show this process is far more eco-friendly than the previously reported methods for selective oxidation of sulfides [29]. For synthesizing sulfone, this protocol presented a 6-fold increase in atom economy, 56.7-fold increase in mole efficiency, 9.4-fold reduce in E-factor. For preparing sulfoxide, this method has a 3.2-fold increase in atom economy, 9.6-fold increase in mole efficiency, 2.4-fold reduce in E-factor and 16% increase in EcoScale value.
|
Download:
|
| Scheme 8. Clean production of sulfones and sulfoxides by recrystallization. | |
6. Conclusion
Synthetic chemistry has made a great contribution to the progress of human society over the past two centuries. Despite significant achievements that have been made, traditional synthetic strategies usually cause some problems to the environment. Under this circumstance, synthetic chemistry is driven to develop novel synthetic methods in order to meet the standards of the 12 Principles of green chemistry, with the first being that it is better to prevent waste rather than to treat or clean up waste after it is formed. To our delight, a series of environmentally friendly synthetic processes have been well-established during the past years. In this perspective article, we have summarized the recent works of our group, which are in conformity with the first Principle of green chemistry.
It has been demonstrated by our group that it is feasible to synthesize and separate quinolin-2(1H)-ones, sulfonylated N-heterocycles, quinolin-2-yl substituted ureas and 5-furandicarboxylic acid cleanly and efficiently by filtration without any petroleum-based solvent. With the assistance of water, precipitation was also applied as a clean separation method for preparing 5-organylselanyl uracils and Z-selenocyanatoacrylates. Polysubstituted pyrroles separated by liquid-liquid extraction and sulfones separated by recrystallization are also recommendable clean isolation examples.
With the increasing research interest in green and sustainable chemistry, the development of clean production for structurally diverse organic molecules is highly desirable. We hope that this article will inspire more researchers who are interested in this area to open new horizon in clean organic synthesis.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsWe are grateful for financial support from the Scientific Research Fund of Hunan Provincial Education Department (No. 19C0783) and Hunan Provincial Natural Science Foundation of China (No. 2019JJ20008).
| [1] |
(a) P. Anastas, N. Eghbali, Chem. Soc. Rev. 39(2010) 301-312; (b) H.C. Erythropel, J.B. Zimmerman, T.M. de Winter, et al., Green Chem. 20(2018) 1929-1961; (c) Y. Kim, C.J. Li, Green Synth. Catal. 1(2020) 1-11; (d) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30(2019) 2132-2138; (e) L.Y. Xie, S. Peng, L.H. Yang, et al., Green Chem. 23(2021) 374-378. |
| [2] |
(a) D.K. Romney, F.H. Arnold, B.H. Lipshutz, C.J. Li, J. Org. Chem. 83(2018) 7319-7322; (b) T. Kitanosono, K. Masuda, P. Xu, S. Kobayashi, Chem. Rev. 118(2018) 679-746; (c) X.Y. Yuan, F.L. Zeng, H.L. Zhu, et al., Org. Chem. Front. 7(2020) 1884-1889; (d) K. Sun, Q.Y. Lv, X. Chen, L. Qu, B. Yu, Green Chem. 23(2021) 232-248; (e) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30(2019) 2287-2290; (f) Q. Sun, L. Liu, Y. Yang, Z. Zha, Z. Wang, Chin. Chem. Lett. 30(2019) 1379-1382. |
| [3] |
(a) T. Tashima, H. Murata, H. Kodama, Biorg. Med. Chem. 22(2014) 3720-3731; (b) P. Montuschi, G. Ciabattoni, J. Med. Chem. 58(2015) 4131-4164. |
| [4] |
L.Y. Xie, Y. Duan, L.H. Lu, et al., ACSSustainableChem.Eng. 5 (2017) 10407-10412. |
| [5] |
B. Ying, J. Xu, X. Zhu, C. Shen, P. Zhang, ChemCatChem 8 (2016) 2604-2608. DOI:10.1002/cctc.201600555 |
| [6] |
D. Wang, J. Zhao, Y. Wang, et al., Asian J. Org. Chem. 5 (2016) 1442-1446. DOI:10.1002/ajoc.201600430 |
| [7] |
(a) Y. Wan, N. Dai, Z. Tang, H. Fang, Eur. J. Med. Chem. 146(2018) 471-482; (b) X. Mi, Y. Kong, J. Zhang, C. Pi, X. Cui, Chin. Chem. Lett. 30(2019) 2295-2298; (c) D.Q. Dong, L.X. Li, G.H. Li, et al., Chin. J. Catal. 40(2019) 1494-1498; (d) D.Q. Dong, W.J. Chen, D.M. Chen, et al., Chin. J. Org. Chem. 39(2019) 3190-3198; (e) Y. Feng, Z. Zhang, Q. Fu, et al., Chin. Chem. Lett. 31(2020) 58-60; (f) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31(2020) 67-70; (g) C.M. Huang, J. Li, S.Y. Wang, S.J. Ji, Chin. Chem. Lett. 31(2020) 1923-1926. |
| [8] |
(a) S. Cacchi, G. Fabrizi, A. Goggiamani, L.M. Parisi, R. Bernini, J. Org. Chem. 69(2004) 5608-5614; (b) W. Zhu, D. Ma, J. Org. Chem. 70(2005) 2696-2700. |
| [9] |
B. Du, P. Qian, Y. Wang, et al., Org. Lett. 18 (2016) 4144-4147. DOI:10.1021/acs.orglett.6b02289 |
| [10] |
L.Y. Xie, S. Peng, J.X. Tan, et al., ACSSustainableChem.Eng. 6 (2018) 16976-16981. |
| [11] |
L.Y. Xie, S. Peng, F. Liu, et al., ACS Sustainable Chem. Eng. 7 (2019) 7193-7199. DOI:10.1021/acssuschemeng.9b00200 |
| [12] |
A.A. Rosatella, S.P. Simeonov, R.F.M. Frade, C.A.M. Afonso, Green Chem. 13 (2011) 754-793. DOI:10.1039/c0gc00401d |
| [13] |
K.J. Liu, J.H. Deng, T.Y. Zeng, et al., Chin. Chem. Lett. 31 (2020) 1868-1872. DOI:10.1016/j.cclet.2020.01.036 |
| [14] |
K.J. Liu, T.Y. Zeng, J.L. Zeng, et al., Chin. Chem. Lett. 30 (2019) 2304-2308. DOI:10.1016/j.cclet.2019.10.031 |
| [15] |
(a) W. Xiao, J. Wu, Chin. Chem. Lett. 31(2020) 3083-3094; (b) J.Y. Chen, H.Y. Wu, Q.W. Gui, et al., Chin. J. Catal. (2021), doi: http://dx.doi.org/10.1016/S1872-2067(20)63750-0. |
| [16] |
J.Y. Chen, C.T. Zhong, Q.W. Gui, et al., Chin. Chem. Lett. 32 (2021) 475-479. DOI:10.1016/j.cclet.2020.09.034 |
| [17] |
X.D. Li, Y.T. Gao, Y.J. Sun, et al., Org. Lett. 21 (2019) 6643-6647. DOI:10.1021/acs.orglett.9b02183 |
| [18] |
J. Chen, L. Wu, J. Wu, Chin. Chem. Lett. 31 (2020) 2993-2995. DOI:10.1016/j.cclet.2020.06.034 |
| [19] |
Y. Gu, F. Jerome, Chem. Soc. Rev. 42 (2013) 9550-9570. DOI:10.1039/c3cs60241a |
| [20] |
L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30 (2019) 1237-1240. DOI:10.1016/j.cclet.2019.04.033 |
| [21] |
(a) M.B. Gawande, V.D.B. Bonifácio, R. Luque, P.S. Branco, R.S. Varma, ChemSusChem 7(2014) 24-44; (b) D.J.C. Constable, C. Jimenez-Gonzalez, R.K. Henderson, Org. Process Res. Dev. 11(2007) 133-137. |
| [22] |
(a) H. Fan, J. Peng, M.T. Hamann, J.F. Hu, Chem. Rev. 108(2008) 264-287; (b) I.S. Young, P.D. Thornton, A. Thompson, Nat. Prod. Rep. 27(2010) 1801-1839; (c) R. Khajuria, S. Dham, K.K. Kapoor, RSC Adv. 6(2016) 37039-37066. |
| [23] |
(a) Q.W. Gui, X. He, W. Wang, et al., Green Chem. 22(2020) 118-122; (b) Q.W. Gui, F. Teng, S.N. Ying, et al., Chin. Chem. Lett. 31(2020) 3241-3244. |
| [24] |
Z. Shi, L. Wang, X. Cui, Chin. J. Org. Chem. 39 (2019) 1596-1612. DOI:10.6023/cjoc201902001 |
| [25] |
(a) Z. Shen, C. Pi, X. Cui, Y. Wu, Chin. Chem. Lett. 30(2019) 1374-1378; (b) Y. He, C. Pi, Y. Wu, X. Cui, Chin. Chem. Lett. 31(2020) 396-400; (c) Z. Yang, C. Pi, X. Cui, Y. Wu, Org. Chem. Front. 6(2019) 2897-2901; (d) Z. Gan, G. Li, X. Yang, et al., Sci. China Chem. 63(2020) 1652; (e) X.Y. Li, Y. Liu, X.L. Chen, et al., Green Chem. 22(2020) 4445-4449; (f) G.H. Li, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31(2020) 3255-3258; (g) K. Zhou, J. Chen, J. Wu, Chin. Chem. Lett. 31(2020) 2996-2998. |
| [26] |
X.Q. Mou, Z.L. Xu, L. Xu, et al., Org. Lett. 18 (2016) 4032-4035. DOI:10.1021/acs.orglett.6b01883 |
| [27] |
K.J. Liu, Z. Wang, L.H. Lu, et al., Green Chem. 23 (2021) 496-500. DOI:10.1039/D0GC02663H |
| [28] |
K.J. Liu, J.H. Deng, J. Yang, et al., Green Chem. 22 (2020) 433-438. DOI:10.1039/C9GC03713F |
| [29] |
(a) B. Yu, A.H. Liu, L.N. He, et al., Green Chem. 14(2012) 957-962; (b) L. Tang, K. Du, B. Yu, L. He, Chin. Chem. Lett. 31(2020) 2991-2992. |
 2021, Vol. 32
2021, Vol. 32 


