b Sino-German Centre for Water and Health Research, Sichuan University, Chengdu 610065, China
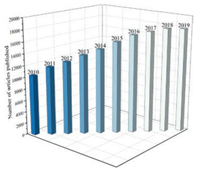
With the rapid development of society, finding and utilizing green renewable resources is an urgent task to achieve sustainable development in the face of increasingly depleted natural resources and environmental pollution problems. Carbon-based materials have stimulated an immense impetus in promoting the development of society due to their abundance on the earth and environmental friendliness and other merits. Carbon nanotubes (CNTs) are one of the carbon-based materials, discovered firstly by Sumio Iijima in the early 1990 s [1]. In recent years, CNTs have become a research hotspot in the field of materials and focused on many researchers. As shown in Fig. 1, a number of CNTs articles have been published and increased every year according to search engine of web of science and key word of carbon nanotubes.
|
Download:
|
| Fig. 1. Number of publications per year on carbon nanotubes materials from 2010 to 2019 on dated 30 July 2020. Search engine: web of science; key word: carbon nanotubes. | |
Usually, CNTs are one-dimensional nanomaterials with nanometer diameter and micron axial length [2]. According to the different number of layers, CNTs can divide into single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). SWCNTs are composed of a single graphite sheet curled into a seamless cylindrical tube, and MWCNTs are simply composed of multiple concentric SWCNTs [2]. The unique structure of CNTs makes them have unusual physical and chemical properties, including large specific surface area, superior thermal, mechanical and electric properties [2]. Thus, CNTs have been used widely in various applications, e.g., electromagnetic shielding, sensors, catalysis, energy, environment and other fields [3-9].
Recently, organic pollutants are frequently detected in the water environment and become an urgent problem worldwide [10]. Thus, it is of great significance to develop effective treatment technology to remove organic pollutants in the water environment. In the past few years, the application of the CNTs for organic pollutant elimination from wastewater has been widely studied. For example, Peng et al. first reported in 2003 that CNTs were promising adsorbents to remove 1, 2-dichlorobenzene from water [11]. To date, CNTs-based materials have been used to adsorb organic pollutants from wastewater based on their porous structure and large specific surface area [12]. To broaden the application range and improve the performance of CNTs, a variety of approaches have been used to prepare different types of CNTs-based materials. Besides, advanced oxidation processes (AOPs) have been regarded as a promising technology for the organic pollutant removal. CNTs-based materials are also widely used in AOPs, which involve different oxidation systems, such as O3, Fenton, persulfates (PMS/PDS) and Fe(Ⅵ)/Mn(Ⅶ). For example, in 2008, Liu et al. first found that CNTs-based material was effective in the catalytic ozonation of oxalic acid [13]. Nowadays, more and more researchers begin to study the application of CNTs-based materials in the field of AOPs.
Generally, these oxidation systems can produce reactive oxygen species (ROS) to degrade organic pollutants effectively. For instance, O3 (E0=2.07 V) can degrade organic pollutants by direct oxidation of ozone molecules or indirect oxidation of hydroxyl radicals (·OH) produced. Hydrogen peroxide (H2O2, E0=1.8 V) can react with the catalyst to ·OH (E0=2.8 V) [14]. PMS (E0=1.82 V) and PDS (E0=2.01 V) are strong oxidants, which can be activated by various methods (i.e., ultraviolet (UV) [15], heat [16], transition metals [17], microwave [18], ultrasound (US) [19] and carbon-based materials [20]). Ferrate (Fe(Ⅵ), E0=2.2 V, Hg/Hg2SO4/K2SO4 as reference electrode in acid condition) and permanganate (Mn(Ⅶ), E0=0.558 V–1.692 V versus NHE) can oxidize organic pollutants quickly under a wide operating pH range through a nonradical mechanism [21, 22]. The unique properties of CNTs-based materials make them widely applied in AOPs for removing organic pollutants from wastewater. Recently, a growing number of publications on this topic indicate that CNTs-based materials will play an important role in organic pollutant elimination.
Accordingly, the primary focus of this review is to undertake an in-depth review of CNTs-based materials, currently available for organic pollutant removal in water treatment field. Firstly, we summarize the research of CNTs-based materials in the adsorption field of organic pollutants. Besides, particular emphasis is placed on the application of CNTs-based materials in AOPs (e.g., H2O2, PMS/PS, O3 and Fe(Ⅵ)/Mn(Ⅶ). Special attention has been focused on reaction mechanisms of organic pollutant degradation in catalytic oxidation systems of CNTs-based materials. Finally, the current challenges of CNTs-based materials current in the AOPs field are pointed out and future research directions are proposed.
2. Preparation of CNTsCNTs can be synthesized using three main methods, such as chemical vapor deposition, arc discharge and laser deposition methods [23]. Compared with other methods, the low-temperature chemical vapor deposition technique is economical, feasible and mass production [23, 24]. Generally, carbon sources, including methane, acetylene, benzene, xylene, and carbon monoxide ethylene, etc., are utilized to prepare CNTs. MWCNTs are more easily produced from most hydrocarbons by low-temperature chemical vapor deposition, while SWCNTs are only generated by high-temperature chemical vapor deposition technology (900–1200 ℃) from selective hydrocarbons (i.e., methane and carbon monoxide) [24]. Arc discharge method uses high temperature (> 3000 ℃) to evaporate carbon atoms into plasma, which is necessary to form MWCNTs [23]. The most common method for the preparation of MWCNTs is to use two graphite electrodes (usually water-cooled electrodes) in a chamber filled with helium at sub-atmospheric pressure. SWCNTs are prepared by arc discharge reaction in hydrogen or argon atmosphere by using composite anode and adding catalyst such as Co, Y, Ni, Fe. But for MWCNTs, the existence of catalyst is not mandatory [23]. Besides, the principles of the laser ablation method are similar to the arc discharge. But the difference is that the laser ablation method involves graphite pellet containing catalyst materials (usually Co or Ni) and evaporating in an electric furnace heated at 1200 ℃ [25].
3. Organic pollutant removalWith the development of society, the pollution of organic compound in water is an urgent problem to be solved [26]. The common organic compounds in water include dye wastewater, petroleum wastewater, antibiotics, drugs, etc., which have brought a lot of adverse effects on human health and social development [27, 28]. The most commonly used method for removing organic wastewater is biological method. However, some refractory organic pollutants contained in wastewater are difficult to be effectively removed by biological method due to their limitations [17]. Therefore, some other methods have been developed to remove refractory organic pollutants from wastewater such as adsorption and AOPs [29, 30]. Generally speaking, these methods involve the use of environment-friendly materials, such as filter materials, membrane separation materials, adsorption materials and catalytic materials. Recently, CNTs-based materials have been widely concerned by many researchers in organic wastewater treatment. They can be employed as the adsorbents to remove effectively organic pollutants from wastewater due to their unique physical and chemical properties. Besides, CNTs-based materials can also be applied in AOPs to degrade organic pollutants. In a word, the CNTs-based materials may have potential application prospects in water treatment.
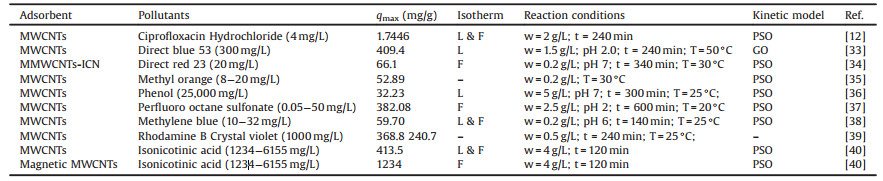
3.1. CNTs-adsorption systemsCNTs, with unique nano-sized structure, relatively large specific surface areas and strong hydrophobicity, have been widely used to absorb various organic pollutants, such as methylene blue [31], crystal violet [32], direct blue 53 [33] and direct red 23 [34] and are presented in Table 1 [12, 33-40]. According to Table 1, the Langmuir and Freundlich isotherms are the most common adsorption models in CNTs-based materials adsorption processes.
|
|
Table 1 Previous researches in the application of CNTs based adsorption systems for exclusion of organic pollutants in wastewater (qmax = maximum adsorption capacity (mg/g); L=Langmuir; F=Freundlich; PFO =pseudo first order; PSO = pseudo second order; GO=General-order; IPD=intra-particle diffusion). |
The Langmuir isotherm model is denoted as follows (Eq. 1) [41]:
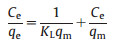
|
(1) |
Freundlich isotherm model equation is described as follows (Eq. 2) [41]:
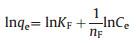
|
(2) |
where qe indicates mass of surfactant adsorbed at equilibrium (mg/g) for organic pollutants, Ce respects equilibrium concentration of surfactant (mg/L), KL is adsorption equilibrium constant related to energy of adsorption (L/mg), KF refers to Freundlich parameter (mg/g), nF is Freundlich parameter related to surface heterogeneity.
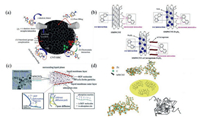
The two main kinetic models namely the pseudo-first order and pseudo-second order are applied in adsorption processes. CNTs mixed with an organic binder could be extruded into strips with a diameter of 3 mm and a length of 20−30 cm [42]. The strips could effectively remove different organic matters from wastewater. Moreover, the strips exhibited excellent recycling capability [42]. Besides, CNTs could also be prepared into CNTs hybrid polymer beads by the phase inversion method, which could effectively remove p-chlorophenol [43]. Xu et al. reported that a core-shell structural CNT-based composite adsorption material could be synthesized to absorb 2-naphthol from water by a fixed-bed column system [44]. The results showed that CNT-based composite adsorption material had excellent adsorption properties for 2-naphthol removal [44]. In addition, an adsorbent of sludge biochar modified by CNT composite (CNT-SBC) could be synthesized to remove low concentrations of sulfamethoxazole (SMX) from water. The existence of CNTs significantly improved CNT-SBC adsorption capacity. Classically, the interaction mechanisms of organic pollutants with CNTs surface involve physisorption and chemisorption, physisorption including valence forces, hydrogen bonding and pore filling, and chemisorption including hydrophobic effects, electrostatic interactions, π-π interaction and functional groups complexation [45, 46]. As shown in Fig. 2a, in CNT-SBC adsorption system, the adsorption mechanism of the SMX onto CNT-SBC mainly included pore filling in the physisorption process and functional groups complexation and π-π interaction in the chemisorption process [46].
|
Download:
|
| Fig. 2. (a) Possible adsorption mechanisms during the adsorption of sulfamethoxazole on CNT/SBC surfaces. Reproduced with permission [46]. Copyright 2020, Elsevier. (b) The possible interactions between adsorbents and diquat dibromide. Reproduced with permission [49]. Copyright 2018, Elsevier. (c) Possible adsorption mechanisms of BZF adsorption on MFe2O4/CNTs. Reproduced with permission [50]. Copyright 2017, the Royal Society of Chemistry. (d) Schematic illustration of the MWCNT modified MIL-53(Fe). Reproduced with permission [48]. Copyright 2018, Elsevier. | |
Moreover, to better separate the adsorption material from the solution, magnetic CNTs-based materials were usually synthesized by loading magnetic materials on CNTs, such as Fe3O4 [40], Fe3C [34, 47], γ-Fe2O3 [47] and iron metal-organic framework (MIL-53(Fe)) [48]. The modified magnetic CNTs-based materials had not only excellent adsorption performance but also were easily to be separated from aqueous solution by magnetism. Duman et al. studied the structural and surface properties of non-magnetic oxidized MWCNT (OMWCNT), magnetic OMWCNT-Fe3O4 and OMWCNT-k-carrageenan-Fe3O4 composites, and their adsorptive properties for the removal of diquat dibromide were determined [49]. The results of this study showed that non-magnetic OMWCNT have the highest adsorption capacity for diquat dibromide compared to magnetic OMWCNT-Fe3O4 and OMWCNT-k-carrageenan-Fe3O4 composites. As shown in Fig. 2b, π-π interaction and electrostatic interaction were the main interaction mechanism between composites and diquat dibromide [49]. The π-electron density, pore volume and specific surface area of these composites played a very important role in diquat dibromide adsorption [49]. Wu et al. synthesized magnetic ferrite modified CNTs (MFe2O4/CNTs, M: Mn or Co), which could be employed as adsorbents to remove organic pollutants from wastewater [50]. The possible adsorption mechanism was present in Fig. 2c [50]. More specifically, adsorption mechanisms mainly included surface diffusion, pore diffusion and adsorption reaction. Although the introduced ferrite on MFe2O4/CNTs did not play a major role in the bezafibrate (BZF) adsorption, MFe2O4/CNTs could be easy to be separated magnetically and regenerated [50]. Additionally, MWCNT loaded iron metal-organic framework nanocomposite (MWCNT/MIL-53(Fe)) was successfully synthesized to adsorb tetracycline antibiotics from aqueous solutions (Fig. 2d) [48]. MWCNT/MIL-53(Fe) showed excellent adsorption properties and reusability for the adsorption of tetracycline antibiotics, indicating the potential application of the adsorbent in tetracycline antibiotic removal from aqueous solutions. These studies showed that CNTs-based adsorption materials are potentially applicable for the efficient removal of organic pollutants from wastewaters.
Some other modification methods are also used to improve the adsorption properties of CNTs. CNTs were oxidized to ox-CNTs and further modified by esterification of ox-CNTs with pentaerythritol (PER) to ox-MWCNT-PER, which facilitated organic dyes removal from wastewater effectively [51]. Doping heteroatoms in CNTs is an effective approach to enhance the electronic polarizability of CNTs surface, which may be favorable for adsorptive interactions of organic contaminants [52]. Yi et al. successfully synthesized nitrogen-doped CNTs (N-CNTs) to adsorb the bisphenol A, tylosin, and tetracycline [52]. Compared with the non-doped CNTs, N-CNTs exhibited much stronger adsorption capacity, which attributed to their higher electron-exhaustion and more evenly uniform π-electron-acceptor sites [52]. Besides, some researchers studied the adsorption of the binary system. The synergistic and antagonistic adsorption effects could be observed in the simultaneous removal of crystal violet and rhodamine B [39]. There are many pollutants in the actual wastewater. Thus, the interaction between different organic pollutants should also be further studied in multicomponent systems. The above researches show that CNTs can be used as efficient adsorbents to remove organic pollutants in aqueous solution, and modified CNTs-based materials may have wider application prospects.
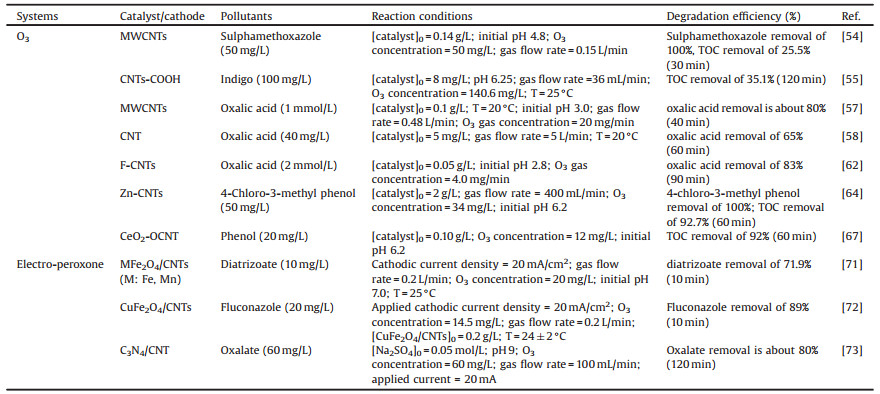
3.2. CNTs-ozone systemsThe studies found that CNTs-based materials are effective catalyst for catalytic ozonation of organic pollutants. As reported, the CNTs-based materials catalytic ozonation process have been successfully used to remove the organic pollutants from wastewater, such as oxalic acid [53], sulphamethoxazole [54] and indigo [55] in the aqueous solution and presented in Table 2. Furthermore, the mineralization efficiency and effluent toxicity by MWCNTs catalytic ozonation process were also assessed in the literature, the results showed a higher mineralization efficiency and a lower toxicity of MWCNTs/O3 system compared with non-catalytic ozonation process [56].
|
|
Table 2 Previous researches in the application of CNTs based O3 systems for exclusion of organic pollutants in wastewater. |
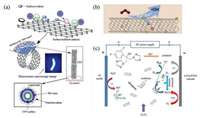
It is well known that there are many functional groups on the surface of CNTs, which would affect catalytic activity in the ozonation process. Liu et al. found that ozonation pretreatment could affect surface functional groups of MWCNT, leading to the change of catalytic activity [57]. Zhang et al. studied that CNTs catalyzed ozonation to form ROS and the mechanisms mainly involved two pathways: (ⅰ) Interphase "HO· zone" could be generated in CNTs surface by directly attacking the active sites of CNTs surface with O3 and (ⅱ) O3 could transform into O2·− under an efficient initiator of CNTs (Fig. 3a) [58]. The graphitic structure of CNTs may be promising to optimize the density of surface reactive sites responsible for the local concentration of HO·.
|
Download:
|
| Fig. 3. (a) A schematic diagram describing the formation of the interphase "HO zone" via CNT-mediated catalytic ozonation. Reproduced with permission [58]. Copyright 2019, Elsevier. (b) Possible mechanism of organic pollutant elimination in the CeO2-OCNT/O3 system. Reproduced with permission [67]. Copyright 2019, Elsevier. (c) Possible mechanism of fluconazole elimination in CuFe2O4/CNTs catalytic electro-peroxone system. Reproduced with permission [72]. Copyright 2019, Elsevier. | |
Although CNTs are considered to have great potential in heterogeneous catalytic ozonation, their performance still needs to be optimized. Some methods have been developed to modify the surface properties and structure of CNTs. Soares et al. reported that ball-milling could change CNTs' structure and morphology, leading to larger specific surface area and smaller particle size [59]. The modified CNTs are in favor of the catalytic oxidation process. Besides, non-metallic heteroatoms (e.g., N, B, S, and F) doped carbon materials have been widely studied due to better electronic structure and electrochemical properties [60, 61]. Fluorine-doped CNTs (F-CNTs) have been synthesized to catalyze ozonation for the degradation of oxalic acid and the result showed a higher degradation rate of oxalic acid [62]. The high electronegativity nature of F and positive charge density of C atoms adjacent to F atoms had positive effects for F-CNTs in the catalytic ozonation process, which was favourable to the generation of ROS for the degradation of oxalic acid [62]. Some other non-metallic heteroatoms CNTs may have similar catalytic properties. Therefore, the catalytic ozonation performance by doping carbon materials with different non-metallic heteroatoms is worth further comparing in the future.
Recently, metal catalysts have exhibited excellent catalytic activity for catalytic ozonation [63]. However, most metals catalysts may lead to secondary pollution due to the serious leaching of metal ions. Thus, some researchers have focused on loading transition metals catalysts on an environment-friendly metal-free material to reduce metal ions leaching significantly. CNTs are an ideal choice, which shows not only excellent catalytic ability but also has unique properties of load material. As reported, Zn-CNTs composite, Fe3O4-MWCNTs, MnOx/MWCNT and Pt/CNTs were synthesized to enhance degradation and mineralization of organic pollutants [13, 64-66]. Wang et al. prepared a highly dispersed cerium oxide on oxidized carbon nanotubes (CeO2-OCNT) for catalytic ozonation of phenol, the result shows that as-synthesized CeO2-OCNT significantly accelerated the mineralization of organic pollutants during catalytic ozonation process [67]. Specifically, CeO2-OCNT/O3 system showed 96% TOC removal, while TOC removals were only 33% and 47% by OCNT/O3 and CeO2/O3 systems, respectively [67]. The result demonstrates that there was a synergic effect between OCNT and CeO2 for catalytic ozonation. As seen in Fig. 3b, the abundant π-π electrons on OCNT surfaces facilitated the reduction of Ce(Ⅳ) from Ce(Ⅲ) by electron transfer, promoting the production of HO· for the degradation of organic pollutants [67]. In addition, some other metal oxides could also be loaded on CNTs to enhance catalytic performance and the enhancement may contribute to the generation of ROS (e.g., H2O2 and HO·) by catalytic ozonation process. For instance, in situ generation of H2O2 played a significant role in catalytic ozonation for the degradation of 4-chloro-3-methyl phenol in Zn-CNTs/O3 system [64], HO· was the main active species for the degradation of organic pollutants in Fe3O4/MWCNTs/O3 and MnOx/MWCNT/O3 systems [65, 66].
CNTs-based materials catalytic ozonation system can be combined with other technologies as a couple system, whose oxidation capacity is much stronger due to the synergistic effect. Mahmoodi et al. reported that MWCNTs could be used as a heterogeneous catalyst for the degradation of dye by the photocatalytic ozonation process [68]. Orge et al. studied the degradation of oxamic acid (OMA) by combining synthesis of TiO2-CNTs, UV and O3, which presented the highest OMA removal rate than other control systems [69]. In other sides, CNTs could be used as an anode in electrochemical filtration (ECF). ECF process combined O3 showed the synergistic effect for phenol oxidation and completed nearly mineralization of phenol and degradation products [70]. Moreover, the specific energy consumption and the operational cost of the hybrid O3-ECF process were lower compared to the O3 process, which would be a promising technology in wastewater treatment applications [70]. Besides, CNTs-based materials were also used as the cathode electrode in O3-electrolysis process. For example, CNTs-polytetrafluoroethylene (CNTs-PTFE) and ferrite modified CNTs-PTFE (MFe2O4/CNTs, M: Fe, Mn) were prepared into the cathode electrode [71, 72], the mixture of g-C3N4/CNT-polyvinylidene fluoride (PVDF) spread on the two-sided graphite as the cathode electrode [73]. These electrodes played an important role for catalytic ozonation process in O3-electrolysis systems. According to the study reported by Wu et al., CNTs-PTFE and Pt plate were used as cathode and anode, respectively, and the result suggested that electro-O3 process was efficient for the degradation of fluconazole [72]. Additionally, CuFe2O4/CNTs could be synthesized as adsorbents/catalysts in O3-electrolysis system, and the introduce of CuFe2O4/CNTs significantly improved the degradation of fluconazole [72]. As shown in Fig. 3c, the synthetic CuFe2O4/CNTs could accelerate the decomposition of ozone and H2O2 produced in situ by electrochemical process to produce HO·, leading to the high degradation efficiency of fluconazole [72]. In a word, CNTs-based materials are promising catalysts and electrode materials in ozone and ozone coupling systems. The above researches show that CNTs-based materials have a positive effect on the degradation of organic pollutants and they are a partner with great potential for catalytic ozonation systems.
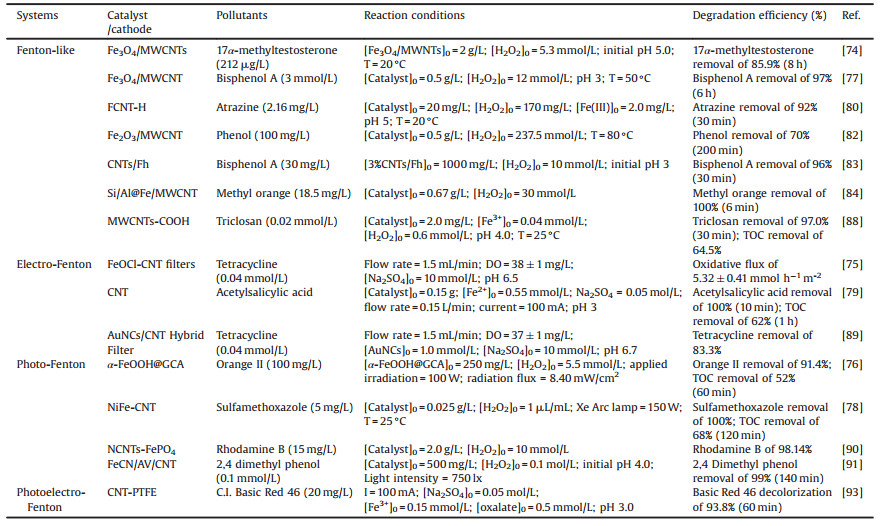
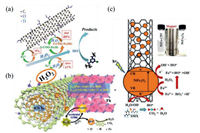
3.3. CNTs-Fenton-like systemsRecently, CNTs-based materials have been widely used to remove organic pollutants by Fenton-like systems due to their excellent performances. CNTs/Fenton-like systems used for the degradation of various organic pollutants (e.g., 17α-methyltestosterone [74], tetracycline [75], Orange Ⅱ [76], bisphenol A [77], SMX [78], acetylsalicylic acid [79] and atrazine [80]) were investigated. Normally, the study shows that functionalized MWCNTs (FCNT-H) could effectively improve the removal rate of atrazine in the Fe(Ⅲ)-mediated Fenton-like system [80]. H2O2 could effectively reduce the surface-bound Fe(Ⅲ) to achieve a fast Fe(Ⅲ)/Fe(Ⅱ) cycling. As shown in Fig. 4a, 11% of Fe(Ⅱ) was formed via classical reactions in aqueous solution (Eqs. 3-7 [81]. Besides, 9% of Fe(Ⅱ) was formedvia the direct reduction of Fe(Ⅲ) by carbonyl groups and quinone groups on the surface of FCNT-H. Above all, the faster reduction of FCNT-H-Fe(Ⅲ) complexes by H2O2 accounted 80% of the generated Fe(Ⅱ) [80]. The research suggests that FCNT-H could be well utilized to Fe(Ⅲ)/Fe(Ⅱ) cycling, not only to the effective complexation of Fe(Ⅲ) [80].
|
Download:
|
| Fig. 4. (a) Fe(Ⅲ)/Fe(Ⅱ) cycle mechanism in Fe(Ⅲ)/H2O2/FCNT-H. Reproduced with permission from [80]. Copyright 2018, Elsevier. (b) Possible heterogeneous Fenton catalytic mechanism in the CNTs/Fh system. Reproduced with permission [83]. Copyright 2020, Elsevier. (c) The possible mechanism of SMX degradation by NiFe-CNT composite in photo-Fenton system. Reproduced with permission [78]. Copyright 2019, Elsevier. | |
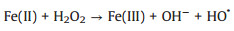
|
(3) |
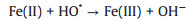
|
(4) |

|
(5) |
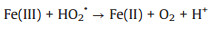
|
(6) |
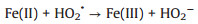
|
(7) |
On the other side, some loaded CNTs-based materials catalyst have been obtained, such as metal (e.g., Fe3O4/CNTs, Fe2O3/CNTs, CNTs/ferrihydrite (CNTs/Fh)) [74, 77, 82, 83] or composite metal (e.g., Si/Al@Fe-CNTs, ZnPOM@APIB-CNTs) [84, 85], which could enhance degradation of organic pollutants in Fenton-like systems. In the heterogeneous Fenton reaction processes, the generation of Fe(Ⅲ) back to Fe(Ⅱ) is the rate-limiting step [86]. Zhu et al. synthesized heterogeneous Fenton catalysts by combining CNTs with ferrihydrite (CNTs/Fh) [83]. As shown Fig. 4b, CNTs/Fh could effectively enhance Fe(Ⅲ)/Fe(Ⅱ) cycling from both dynamic and thermodynamic aspects in the heterogeneous Fenton reaction system, which could accelerate the decomposition of H2O2 to produce HO· [83]. On the one hand, the electron conductivity of CNTs/Fh composites formed after combining CNTs was stronger than that of ferrihydrite, significantly accelerating the electron transfer from H2O2 to Fh in dynamic aspect [83]. On the other hand, the redox potential of Fe(Ⅲ)/Fe(Ⅱ) significantly decreased from thermodynamic aspect when CNTs were coupled with ferrihydrite [83]. Besides, Yang et al. synthesized two kinds of CNTs with different structures, i.e., loading Fe2O3 nanoparticles on the outer surface of CNT (Fe2O3/FCNT-L), Fe2O3 nanoparticles were anchored inside the CNT (Fe2O3/FCNT-H), and the mechanisms of these two kinds of materials in Fenton-like systems have been discussed in detail [87]. In Fe2O3/FCNT-L/H2O2 system, HO· was the main active species [87]. However, in Fe2O3/FCNT-H/H2O2 system, CNTs could provide the confined space at a nanoscalenano confinement and 1O2 was the reactive intermediate. Remarkably, Fe2O3/FCNT-H/H2O2 exhibited remarkably higher degradation kinetics of methylene blue (22.5 times faster) compared with Fe2O3/FCNT-L/H2O2. Moreover, Fe2O3/FCNT-H exhibited higher pH stability over a wide pH range (pH 5.0–9.0), which broadened the application of the Fenton-like system under alkaline conditions [87]. Besides, low concentration MWCNTs-COOH showed excellent catalytic performance for the degradation of disinfectants by Fe3+/H2O2 system [88]. The nanoconfined Fenton reaction may also be the direction of future research.
In addition, there are some studies on the CNTs/Fenton-like coupling process involving electron, UV irradiation, photoelectron for degradation of organic pollutants and presented in Table 3. Except for the systems with external addition of H2O2, CNTs can be used as electrode material for the in situ generation of H2O2 in the electro-Fenton process owing to their excellent electrical conductivity. CNT filter functionalized with Au nanoclusters (AuNCs) was designed to catalyze antibiotic tetracycline degradation by electro-Fenton process [89]. Yang et al. prepared the CNT-gas diffusion electrode (CNT-GDE) as a cathode by a rolling method to degrade acetylsalicylic acid in the electro-Fenton system [79]. Compared to some other cathode materials, CNT-GDE was used as a cathode material to improve the yield of H2O2 generation rate and current efficiency, which exhibited a great application potential in the treatment of organic pollutants [79]. The porous CNTs with FeOCl could be used as a functionalized filter in the electro-Fenton process to efficiently oxidize tetracycline [75]. FeOCl significantly promoted the cycling of Fe(Ⅲ)/Fe(Ⅱ) for the generation of HO·, which had synergistic effects on tetracycline oxidation [75]. In addition, the flow-through design, limited pore size could effectively improve the reaction activity [75].
|
|
Table 3 Previous researches in the application of CNTs based H2O2 systems for exclusion of organic pollutants in wastewater. |
Nawaz et al. synthesized a highly photoactive and magnetically recyclable MWCNTs incorporated NiFe2O4 (NiFe-CNT) composite, which could degrade SMX efficiently by photo-Fenton process [78]. The possible mechanism of the photo-Fenton system was presented in Fig. 4c. H2O2 could react with Fe3+ and Fe2+ to generate HO·. Then, ultraviolet-A illuminated NiFe-CNT to generate electron/hole (e−/h+) pair on the NiFe-CNT surface. The photogenerated electron eCB− can react directly with H2O2 to produce HO· and the photogenerated holes hVB+ can react with H2O or OH− to generate HO·. The generation of HO· and h+ were the main ROS for the removal of SMX. Furthermore, nitrogen-doped carbon nanotubes-FePO4 (NCNTs-FePO4) [90], Z-scheme Fe doped graphitic carbon nitride coupled Ag3VO4 compounded with CNTs (FeCN/AV/CNT) [91] and hierarchical graphene oxide-CNTs-α-FeOOH decorated composite aerogel (α-FeOOH@GCA) [76] could be also synthesized to use as a photocatalyst for the degradation of organic pollutants in photo-Fenton process [76]. In these systems, involving HO·, h+, 1O2 and O2·− would be mainly ROS for the degradation of organic pollutants, i.e., HO· was mainly ROS in NCNTs-FePO4/photo-Fenton and FeCN/AV/CNT/photo-Fenton systems, and HO·, 1O2 and O2·− played an important role in α-FeOOH@GCA/photo-Fenton system. Furthermore, photoelectron-Fenton processes were studied and the synergistic effect could be strengthened due to the interaction of the various process, leading to more ROS production. CNTs could be stabilized on carbon paper to prepare the cathode, which exhibited an excellent effect on the degradation of C.I. Direct Red 23 in the photoelectron-Fenton system [92]. Carbon nanotube-polytetrafluoroethylene (CNT-PTFE) could also be used as a cathode in the electro-Fenton process by combining oxalate photocatalytic for the decolorization of C.I. Basic Red 46 [93]. CoSP/CNTs were used as novel air-diffusion cathodes because of their excellent electrocatalytic properties (i.e., large surface area, chemical stability and electroactivity), which realized high mineralization efficiency of herbicide bentazon by photoelectro-Fenton process [94]. In general, the H2O2 could be generated by the reduction of O2 on CNTs cathode, and then the generated H2O2 decomposed through UV irradiation to produce HO·, which showed high degradation efficiency of organic pollutants. The above is the analysis of the CNTs-based materials applied in Fenton-like processes and they have a positive effect on the degradation of organic pollutants. The mechanisms by which CNTs/Fenton-like and several common CNTs/Fenton-like coupling systems degrade pollutants have been analyzed and summarized in recent years.
3.4. CNTs-persulfates systemsRecently, AOPs based peroxydisulfate (PDS) and peroxymonosulfate (PMS) have attracted considerable attention due to their potential application in situ chemical oxidation (ISCO) and water/wastewater decontamination [95-97]. SO4·− can be generated by cleaving of peroxide bond in PDS and PMS (persulfates). Compared with HO·, SO4·− has many superiorities such as longer half-life, higher oxidation potential and wider suitable pH range, etc. [95]. Therefore, how to activate persulfates with lower cost and less secondary contamination is a hot research field of environmental researchers. Metal-based catalysts can catalyse persulfates effectively for SO4·− generation thus have been investigated extensively in recent years [98, 99]. However, the trade-off between enhancement of persulfates activation efficiency and accompanying secondary contamination problem has to be carefully evaluated due to the potential risk of metal leaching. Interestingly, CNTs as metal-free carbonaceous materials have proved excellent persulfates activators with no need for energy input and no problem of metal leaching, which initiate a new research direction [100].
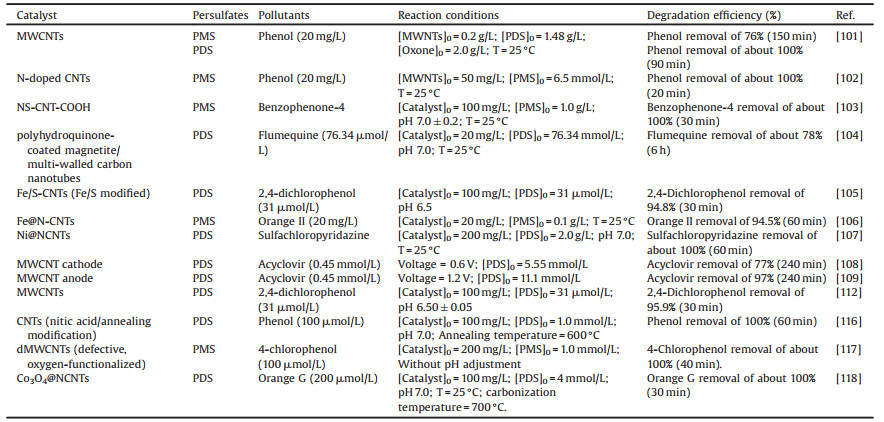
Sun et al. reported that pristine MWCNTs were able to catalyze persulfates effectively to generate sulfate radicals for the degradation of phenol [101]. It was suggested that sp2 carbon and oxygen-containing groups as active sites on MWCNTs played an essential role in persulfates activation. In order to enhance the activation efficiency of CNTs, heteroatom doping have been proposed and proved as a promising strategy for the degradation of organic pollutants enhancement in persulfates based AOPs. Sun et al. used nitrogen modified CNTs for persulfates activation [101]. After surface nitrogen modification, the ability of MWCNTs to activate PMS was improved but the activation of PDS was inhibited. It was suggested that pyridinic and pyrrolic N dopants were highly active sites for PMS activation but not effective regarded to the PS activation. However, the stability and reusability of N-CNTs were not satisfactory. Soon after, N-doped carbon nanotubes (NoCNTs) were synthesized and used for PMS activation [102]. It was found that NoCNTs presented an extraordinary catalytic performance for the degradation of phenol by PMS activation. Interestingly, incorporation of N atom into SWCNTs enabled NoCNTs to present excellent stability due to the presence of nonradical processes. That was, graphitic N played a key role in this process. N and sulfur (S) co-doped multi-walled CNTs also had excellent PMS activation performance [103]. It was proved that the activity level of N and S-doped CNT-COOH (NS-CNT-COOH) exhibited approximately five-fold higher than N and S-doped CNT and N-doped CNT-COOH for the degradation of benzophenone-4 (BP-4) by PMS activation. After that, various methods that improve the catalytic activity of CNTs, for example, load and modification have applied for the degradation of various organic pollutants by persulfates activation [104-107]. Table 4 summarized a variety of CNTs-based materials combined with persulfates systems for enhanced the degradation of organic pollutants. Interestingly, in most situations the activation efficiency of CNTs-based materials shows superior efficacy than that of pristine CNTs [108, 109]. However, how to guarantee that the benefits obtained by introduction of metal outweigh the potential metal leaching risk of this approach should be carefully assessed.
|
|
Table 4 Previous researches in the application of CNTs based persulfates systems for exclusion of organic pollutants in wastewater. |
Identification of ROS in AOPs is very critical because it is related to the organic pollutant degradation characteristic and the oxidation/disinfection by-products generation. At first, it was suggested that SO4·− and HO· were the main ROS in CNT/persulfates systems [101, 110]. But in Lee et al.'s study, it was found that the CNT/persulfates systems selectively oxidized organic pollutants, which mean nonradical oxidation played a significant role in this process [111]. Use of linear sweep voltammetry with CNTs as electrode further explore nonradical mechanism. As shown Fig. 5a, they proposed that the adsorption of persulfates to the surface of CNTs and the formation of reactive complexes were the prerequisite to the degradation of organic pollutants.

|
Download:
|
| Fig. 5. (a) Selective oxidation of various organic pollutants and proposed nonradical mechanism in CNTs/persulfates system. Reproduced with permission [111]. Copyright 2014, Elsevier. (b) Proposed nonradical mechanism in CNTs/PS system. Reproduced with permission [113]. Copyright 2019, Elsevier. (c) Electron-transfer mechanism in CNT/PDS system. Reproduced with permission [120]. Copyright 2019, American Chemical Society. | |
Their subsequent study further comprehensively investigated the CNTs/PMS system, where mediated electron transfer mechanism was suggested a plausible nonradical mechanism. Cheng et al. proposed a different nonradical mechanism in CNTs/PDS [112]. They suggested 1O2 was generated by this non-photochemical system and played an important role in the degradation of 2, 4-dichlorophenol. Subsequently, as shown in Fig. 5b, they further proved that carbonyl and CNTs defects were the main active sites for direct electron transfer oxidation and 1O2 generation, respectively [113]. 1O2 generally considered as the important ROS were frequently reported in some CNTs-based materials/persulfates systems [114-118]. However, there are some researchers questioned the 1O2 oxidation in nonradical mechanism for the degradation of organic pollutants from the reactivity of 1O2 and the defects of 1O2 identification methods [119]. Meanwhile, recent studies have reinvestigated the CNTs/PDS system in detail. The electron transfer regime was also suggested the reaction mechanism rather than 1O2 oxygenation process [20, 120]. To be more specific, PDS was catalyzed by CNTs to form activated PDS nearby CNTs surface (CNT-PDS*) with high redox potential (Fig. 5c) [120]. Then, co-adsorbed organic pollutants were degraded by transfer of electron from organic pollutants to CNT-PDS*. The study is of significance to exploring the elimination of organic pollutants with activated persulfate. More importantly, the nonradical system could be used for selective removal of target organic pollutants in complicated water matrix. Based on the above discussion, CNTs-based materials become an activator to promote the degradation of organic pollutants in persulfates activation systems. The possible mechanisms have been studied continuously by the researchers. A unified and detailed reaction mechanism may require further efforts by researchers in future.
3.5. CNTs-ferrate/permanganate systemsCNTs have the reductive properties due to their reduction groups, which can accelerate ferrate/permanganate (Fe(Ⅵ)/Mn(Ⅶ)) activation for the degradation of organic pollutants. Sun et al. found that CNTs could activate Fe(Ⅵ) to accelerate the degradation of bromophenols [121]. The reduction groups on CNTs surface could react with Fe(Ⅵ) to generate high-valent metal-oxo intermediates (Fe(Ⅴ)/Fe(Ⅳ)), which showed 2–5 orders higher reactivity compare to Fe(Ⅵ). On the other hand, by-products produced from the reaction process could be absorbed by CNTs' surface and thereby reducing the potential risks of by-products in the effluent. Besides, Zhao et al. discovered that CNTs also could significantly accelerate the degradation of phenols by Mn(Ⅶ). In CNTs/Mn(Ⅶ) system, the reducing ability of CNTs could reduce Mn(Ⅶ) to form MnO2, which could catalyze Mn(Ⅶ) oxidation of phenols. Compared to CNTs, Tian et al. reported that biochar could enhance Mn(Ⅶ) to form high-valent intermediate manganese species due to the reduction property of biochar, which resulted in significant improvement of SMX removal [122]. CNTs and biochar are rich in the reductive functional group, high-valent intermediate manganese species could also play an essential role in the degradation of organic pollutants by CNTs/Mn(Ⅶ) system. The intermediate manganese species (i.e., Mn(Ⅲ), Mn(Ⅴ) and Mn(Ⅵ)) may also be involved in CNTs/Mn(Ⅶ) system and their contribution to the degradation of pollutants deserves further study. In a word, introducing CNTs for the activation of Fe(Ⅵ)/Mn(Ⅶ) may have great application potential in wastewater treatment.
4. Conclusions and perspectivesIn this article, we provide a review of recent developments in CNTs-based materials for the application of organic pollutant removal. CNTs with special nano-sized structure, relatively large specific surface areas and electrical conductivity characteristics could be rationally designed to different types of CNTs-based materials for organic pollutant removal in adsorption process and AOPs. We summarized the adsorption performances of CNTs-based materials as the adsorbent for the organic pollutants and comprehensive analysis of the adsorption mechanism is conducted. The CNTs/O3 and CNTs/Fenton-like systems were introduced to remove various organic pollutants and the generation of ROS play a dominant effect for organic pollutant removal. Interestingly, a nonradical oxidation mechanism in CNTs/persulfates systems was proposed through continuous research, which was different from the traditional PDS activation system. It is noteworthy that CNTs could also be used to reduce Fe(Ⅵ)/Mn(Ⅶ) to form the intermediate metal-oxo species because of their reductive functional groups, accelerating the organic pollutant removal.
Despite advances, the application of CNTs-based materials in organic pollutant removal still requires further in-depth investigation. Therefore, the following aspects might be paid more attention in future research.
(1) The poor separability limits CNTs practical application and the release of CNTs in the wastewater treatment process causes secondary pollution. Modification of CNTs with magnetic metal nanomaterials may be an effective way to overcome the faultiness. However, the stability of CNTs-based materials should be intensified to avoid the leaching of metals into solution. Besides, the design of CNTs filter could also be a promising research direction to improve stability.
(2) At present, most of the CNTs-based materials studies are only used to deal with single organic pollutants. However, the composition of practical wastewater is often complex, usually contains multiple components, i.e., metal ions, anions, humic acid and organic solvents. Hence, attentive research is required to evaluate the practical application potential of CNTs-based materials in wastewater treatment.
The ultimate objective of CNTs-based materials in wastewater treatment is industrialization. In practical applications, more attention should be paid to economic and operational feasibility to meet commercial requirements.
In conclusion, this review not only summarizes in-depth insights into the characteristics and mechanisms of CNTs-based materials applied in wastewater treatment, but also provides reference and guidance for the development of more environment-friendly, efficient and stable CNTs-based materials.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe authors would like to acknowledge the financial support from the National Natural Science Foundation of China (No. 51878423), Fundamental Research Funds for the Central Universities (No. 2018SCUH0071), Excellent Youth Foundation of Sichuan Scientific Committee (No. 2019JDJQ0005).
| [1] |
S. Iijima, Nature 354 (1991) 56-58. DOI:10.1038/354056a0 |
| [2] |
E.T. Thostenson, Z. Ren, T.W. Chou, Compos. Sci. Technol. 61 (2001) 1899-1912. DOI:10.1016/S0266-3538(01)00094-X |
| [3] |
L.Q. Zhang, B. Yang, J. Teng, et al., J. Mater. Chem. C: Mater. Opt. Electron. Devices 5 (2017) 3130-3138. DOI:10.1039/C6TC05516H |
| [4] |
J. Wang, M. Musameh, Y. Lin, JACS 125 (2003) 2408-2409. DOI:10.1021/ja028951v |
| [5] |
C. Huang, T. Ouyang, Y. Zou, N. Li, Z.Q. Liu, J. Mater. Chem. A: Mater. Energy Sustain. 6 (2018) 7420-7427. DOI:10.1039/C7TA11364A |
| [6] |
T. Ouyang, Y.Q. Ye, C.Y. Wu, K. Xiao, Z.Q. Liu, Angew. Chem. Int. Ed. 58 (2019) 4923-4928. DOI:10.1002/anie.201814262 |
| [7] |
J.Y. Wang, W.T. Liu, X.P. Li, T. Ouyang, Z.Q. Liu, Chem. Commun. (Camb.) 56 (2020) 1489-1492. DOI:10.1039/C9CC09303F |
| [8] |
J.Y. Wang, T. Ouyang, N. Li, T. Ma, Z.Q. Liu, Sci. Bull. (Beijing) 63 (2018) 1130-1140. DOI:10.1016/j.scib.2018.07.008 |
| [9] |
G. An, W. Ma, Z. Sun, et al., Carbon 45 (2007) 1795-1801. DOI:10.1016/j.carbon.2007.04.034 |
| [10] |
T. Qiang, Y. Xia, J. Zhao, J. Leather Sci. Eng. 1 (2019) 1. DOI:10.1186/s42825-019-0004-x |
| [11] |
X. Peng, Y. Li, Z. Luan, et al., Chem. Phys. Lett. 376 (2003) 154-158. DOI:10.1016/S0009-2614(03)00960-6 |
| [12] |
A. Avcı, I.Inci, N. Baylan, J. Mol. Struct. 1206(2020) 127711.
|
| [13] |
Z.Q. Liu, J. Ma, Y.-H. Cui, Carbon 46 (2008) 890-897. DOI:10.1016/j.carbon.2008.02.018 |
| [14] |
C. Wang, H. Wang, Y. Cao, J. Clean. Prod. 253 (2020) 119921. DOI:10.1016/j.jclepro.2019.119921 |
| [15] |
P. Xie, J. Ma, W. Liu, et al., Water Res. 69 (2015) 223-233. DOI:10.1016/j.watres.2014.11.029 |
| [16] |
C. Tan, N. Gao, Y. Deng, N. An, J. Deng, Chem. Eng. J. 203 (2012) 294-300. DOI:10.1016/j.cej.2012.07.005 |
| [17] |
H. Zhang, Q. Ji, L. Lai, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 1129-1132. DOI:10.1016/j.cclet.2019.01.025 |
| [18] |
Y.C. Lee, S.L. Lo, P.T. Chiueh, D.G. Chang, Water Res. 43 (2009) 2811-2816. DOI:10.1016/j.watres.2009.03.052 |
| [19] |
X. Lu, J. Zhao, Q. Wang, et al., Water Res. 165 (2019) 114969. DOI:10.1016/j.watres.2019.114969 |
| [20] |
W. Ren, L. Xiong, G. Nie, et al., Environ. Sci. Technol. 54 (2020) 1267-1275. DOI:10.1021/acs.est.9b06208 |
| [21] |
X. Xu, J. Chen, S. Wang, et al., Water Res. 138 (2018) 293-300. DOI:10.1016/j.watres.2018.03.057 |
| [22] |
S. Wang, Y. Hu, J. Wang, Sci. Total Environ. 687 (2019) 1028-1033. DOI:10.1016/j.scitotenv.2019.06.189 |
| [23] |
N. Anzar, R. Hasan, M. Tyagi, N. Yadav, J. Narang, Sensor. Int. 1 (2020) 100003. DOI:10.1016/j.sintl.2020.100003 |
| [24] |
J. Prášek, J. Drbohlavova, J. Chomoucka, et al., J. Mater. Chem. 21 (2011) 15872-15884. DOI:10.1039/c1jm12254a |
| [25] |
T. Ikegami, F. Nakanishi, M. Uchiyama, K. Ebihara, Thin Solid Films 457 (2004) 7-11. DOI:10.1016/j.tsf.2003.12.033 |
| [26] |
J. Li, Y. Li, Z. Xiong, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 2139-2146. DOI:10.1016/j.cclet.2019.04.057 |
| [27] |
Y. Gao, Y. Li, L. Zhang, et al., J. Colloid Interface Sci. 368 (2012) 540-546. DOI:10.1016/j.jcis.2011.11.015 |
| [28] |
Y. Yao, F. Xu, M. Chen, Z. Xu, Z. Zhu, Bioresour. Technol. 101 (2010) 3040-3046. DOI:10.1016/j.biortech.2009.12.042 |
| [29] |
I. Ali, V.K. Gupta, Nat. Protoc. 1 (2006) 2661-2667. DOI:10.1038/nprot.2006.370 |
| [30] |
M.A. Oturan, J.J. Aaron, Crit. Rev. Environ. Sci. Technol. 44 (2014) 2577-2641. DOI:10.1080/10643389.2013.829765 |
| [31] |
B. Maazinejad, O. Mohammadnia, G.A.M. Ali, et al., J. Mol. Liq. 298 (2020) 112001. DOI:10.1016/j.molliq.2019.112001 |
| [32] |
I.A. Lawal, M.M. Lawal, M.A. Azeez, P. Ndungu, J. Mol. Liq. 288 (2019) 110895. DOI:10.1016/j.molliq.2019.110895 |
| [33] |
L.D. Prola, F.M. Machado, C.P. Bergmann, et al., J. Environ. Manage. 130 (2013) 166-175. DOI:10.1016/j.jenvman.2013.09.003 |
| [34] |
W. Konicki, I. Pełech, E. Mijowska, I. Jasińska, Chem. Eng. J. 210 (2012) 87-95. DOI:10.1016/j.cej.2012.08.025 |
| [35] |
D. Zhao, W. Zhang, C. Chen, X. Wang, Procedia Environ. Sci. 18 (2013) 890-895. DOI:10.1016/j.proenv.2013.04.120 |
| [36] |
N.T. Abdel-Ghani, G.A. El-Chaghaby, F.S. Helal, J. Adv. Res. 6 (2015) 405-415. DOI:10.1016/j.jare.2014.06.001 |
| [37] |
X. Li, H. Zhao, X. Quan, et al., J. Hazard. Mater. 186 (2011) 407-415. DOI:10.1016/j.jhazmat.2010.11.012 |
| [38] |
S. Wang, C.W. Ng, W. Wang, Q. Li, Z. Hao, Chem. Eng. J. 197 (2012) 34-40. DOI:10.1016/j.cej.2012.05.008 |
| [39] |
Z. Li, L. Sellaoui, D. Franco, et al., Chem. Eng. J. 389 (2020) 124467. DOI:10.1016/j.cej.2020.124467 |
| [40] |
D. Bhatia, D. Datta, A. Joshi, S. Gupta, Y. Gote, J. Mol. Liq. 276 (2019) 163-169. DOI:10.1016/j.molliq.2018.11.127 |
| [41] |
A.A. Siyal, M.R. Shamsuddin, N.E. Rabat, et al., J. Clean. Prod. 229 (2019) 232-243. DOI:10.1016/j.jclepro.2019.04.384 |
| [42] |
Z. Yin, H. Chen Duoni, et al., Carbon 132 (2018) 329-334. DOI:10.1016/j.carbon.2018.02.074 |
| [43] |
L. Xu, Z. Wang, S. Ye, X. Sui, Chem. Eng. Res. Des. 123 (2017) 76-83. DOI:10.1016/j.cherd.2017.04.034 |
| [44] |
L. Xu, S. Wang, J. Zhou, H. Deng, R.L. Frost, Chem. Eng. J. 335 (2018) 450-457. DOI:10.1016/j.cej.2017.10.176 |
| [45] |
K. Yang, B. Xing, Chem. Rev. 110 (2010) 5989-6008. DOI:10.1021/cr100059s |
| [46] |
Y. Ma, L. Yang, L. Wu, et al., Sci. Total Environ. 718 (2020) 137299. DOI:10.1016/j.scitotenv.2020.137299 |
| [47] |
J.L. Gong, B. Wang, G.M. Zeng, et al., J. Hazard. Mater. 164 (2009) 1517-1522. DOI:10.1016/j.jhazmat.2008.09.072 |
| [48] |
W. Xiong, G. Zeng, Z. Yang, et al., Sci. Total Environ. 627 (2018) 235-244. DOI:10.1016/j.scitotenv.2018.01.249 |
| [49] |
O. Duman, C. Ozcan, T. Gurkan Polat, S. Tunc, Environ. Pollut. 244 (2019) 723-732. DOI:10.1016/j.envpol.2018.10.071 |
| [50] |
D. Wu, J. Yao, G. Lu, et al., RSC Adv. 7 (2017) 39594-39603. DOI:10.1039/C7RA07260K |
| [51] |
J.Y. Yang, X.Y. Jiang, F.-P. Jiao, J.G. Yu, Appl. Surf. Sci. 436 (2018) 198-206. DOI:10.1016/j.apsusc.2017.12.029 |
| [52] |
L. Yi, L. Zuo, C. Wei, et al., Sci. Total Environ. 719 (2020) 137389. DOI:10.1016/j.scitotenv.2020.137389 |
| [53] |
Z.Q. Liu, J. Ma, Y.H. Cui, L. Zhao, B.P. Zhang, Sep. Purif. Technol. 78 (2011) 147-153. DOI:10.1016/j.seppur.2011.01.034 |
| [54] |
A.G. Gonçalves, J.J.M. Órfão, M.F.R. Pereira, J. Hazard. Mater. 239-240 (2012) 167-174. DOI:10.1016/j.jhazmat.2012.08.057 |
| [55] |
R. Qu, B. Xu, L. Meng, L. Wang, Z. Wang, Water Res. 68 (2015) 316-327. DOI:10.1016/j.watres.2014.10.017 |
| [56] |
X. Fan, J. Restivo, J.J.M. Órfão, M.F.R. Pereira, A.A. Lapkin, Chem. Eng. J. 241 (2014) 66-76. DOI:10.1016/j.cej.2013.12.023 |
| [57] |
Z.Q. Liu, J. Ma, Y.H. Cui, B.P. Zhang, Appl. Catal. B 92 (2009) 301-306. DOI:10.1016/j.apcatb.2009.08.007 |
| [58] |
S. Zhang, X. Quan, J.F. Zheng, D. Wang, Water Res. 122 (2017) 86-95. DOI:10.1016/j.watres.2017.05.063 |
| [59] |
O.S.G.P. Soares, A.G. Gonçalves, J.J. Delgado, J.J.M. Órfão, M.F.R. Pereira, Catal. Today 249 (2015) 199-203. DOI:10.1016/j.cattod.2014.11.016 |
| [60] |
K. Gong, F. Du, Z. Xia, M. Durstock, L. Dai, Science 323 (2009) 760. DOI:10.1126/science.1168049 |
| [61] |
S. Pearton, Nanoscale 2 (2010) 1057. DOI:10.1039/c005273f |
| [62] |
J. Wang, S. Chen, X. Quan, H. Yu, Chemosphere 190 (2018) 135-143. DOI:10.1016/j.chemosphere.2017.09.119 |
| [63] |
Z. Xu, M. Xie, Y. Ben, et al., J. Hazard. Mater. 365 (2019) 146-154. DOI:10.1016/j.jhazmat.2018.11.006 |
| [64] |
Y. Liu, A. Zhou, Y. Liu, J. Wang, Chemosphere 191 (2018) 54-63. DOI:10.1016/j.chemosphere.2017.10.025 |
| [65] |
Z.Y. Bai, Q. Yang, J.L. Wang, Int. J. Environ. Sci. Technol. (Tehran) 13 (2015) 483-492. |
| [66] |
M. Sui, S. Xing, L. Sheng, S. Huang, H. Guo, J. Hazard. Mater. 227- 228 (2012) 227-236. |
| [67] |
J. Wang, X. Quan, S. Chen, H. Yu, G. Liu, J. Hazard. Mater. 368 (2019) 621-629. DOI:10.1016/j.jhazmat.2019.01.095 |
| [68] |
N.M. Mahmoodi, J. Mol. Catal. A: Chem. 366 (2013) 254-260. DOI:10.1016/j.molcata.2012.10.002 |
| [69] |
C.A. Orge, O.S.G.P. Soares, J.L. Faria, M.F.R. Pereira, J. Environ. Chem. Eng. 5 (2017) 5599-5607. DOI:10.1016/j.jece.2017.10.030 |
| [70] |
B.M. Souza-Chaves, M. Dezotti, C.D. Vecitis, J. Hazard. Mater. 382(2020) 121085.
|
| [71] |
D. Wu, G. Lu, R. Zhang, et al., Electrochim. Acta 236 (2017) 297-306. DOI:10.1016/j.electacta.2017.03.196 |
| [72] |
D. Wu, G. Lu, J. Yao, et al., Chem. Eng. J. 370 (2019) 409-419. DOI:10.1016/j.cej.2019.03.192 |
| [73] |
Z. Guo, H. Cao, Y. Wang, et al., Chemosphere 201 (2018) 206-213. DOI:10.1016/j.chemosphere.2018.02.176 |
| [74] |
X. Hu, B. Liu, Y. Deng, et al., Appl. Catal. B 107 (2011) 274-283. DOI:10.1016/j.apcatb.2011.07.025 |
| [75] |
Z. Li, C. Shen, Y. Liu, et al., Appl. Catal. B 260 (2020) 118204. DOI:10.1016/j.apcatb.2019.118204 |
| [76] |
Y. Liu, X. Liu, Y. Zhao, D.D. Dionysiou, Appl. Catal. B 213 (2017) 74-86. DOI:10.1016/j.apcatb.2017.05.019 |
| [77] |
V. Cleveland, J.P. Bingham, E. Kan, Sep. Purif. Technol. 133 (2014) 388-395. DOI:10.1016/j.seppur.2014.06.061 |
| [78] |
M. Nawaz, A. Shahzad, K. Tahir, et al., Chem. Eng. J. 382 (2020) 123053. DOI:10.1016/j.cej.2019.123053 |
| [79] |
H. Yang, M. Zhou, W. Yang, G. Ren, L. Ma, Chemosphere 206 (2018) 439-446. DOI:10.1016/j.chemosphere.2018.05.027 |
| [80] |
Z. Yang, A. Yu, C. Shan, G. Gao, B. Pan, Water Res. 137 (2018) 37-46. DOI:10.1016/j.watres.2018.03.006 |
| [81] |
J. De Laat, H. Gallard, Environ. Sci. Technol. 33 (1999) 2726-2732. DOI:10.1021/es981171v |
| [82] |
Q. Liao, J. Sun, L. Gao, Colloids Surf. A: Physicochem. Eng. Asp. 345 (2009) 95-100. DOI:10.1016/j.colsurfa.2009.04.037 |
| [83] |
R. Zhu, Y. Zhu, H. Xian, et al., Appl. Catal. B 270 (2020) 118891. DOI:10.1016/j.apcatb.2020.118891 |
| [84] |
M. Arshadi, M.K. Abdolmaleki, F. Mousavinia, et al., Chem. Eng. Res. Des. 112 (2016) 113-121. DOI:10.1016/j.cherd.2016.05.028 |
| [85] |
R. Hajian, Z. Alghour, Chin. Chem. Lett. 28 (2017) 971-975. DOI:10.1016/j.cclet.2016.12.003 |
| [86] |
P. Zhou, W. Ren, G. Nie, et al., Angew. Chem. Int. Ed. 59 (2020) 16517-16526. DOI:10.1002/anie.202007046 |
| [87] |
Z. Yang, J. Qian, A. Yu, B. Pan, Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 6659-6664. DOI:10.1073/pnas.1819382116 |
| [88] |
J. Peng, J. Xue, J. Li, et al., Chem. Eng. J. 321 (2017) 325-334. DOI:10.1016/j.cej.2017.03.133 |
| [89] |
F. Liu, Y. Liu, Q. Yao, et al., Environ. Sci. Technol. 54 (2020) 5913-5921. DOI:10.1021/acs.est.0c00427 |
| [90] |
L. Wei, Y. Zhang, S. Chen, et al., J Environ. Sci. (China) 76 (2019) 188-198. DOI:10.1016/j.jes.2018.04.024 |
| [91] |
P. Raizada, A. Aslam Parwaz Khan, P. Singh, Sep. Purif. Technol. 247(2020) 116957.
|
| [92] |
A. Khataee, H. Marandizadeh, B. Vahid, M. Zarei, S.W. Joo, Chem. Eng. Process. Proc. Intens. 73 (2013) 103-110. DOI:10.1016/j.cep.2013.07.007 |
| [93] |
A.R. Khataee, M. Zarei, L. Moradkhannejhad, Desalination 258 (2010) 112-119. DOI:10.1016/j.desal.2010.03.028 |
| [94] |
F. Alcaide, G. Álvarez, D.R.V. Guelfi, E. Brillas, I. Sirés, Chem. Eng. J. 379(2020) 122417.
|
| [95] |
F. Ghanbari, M. Moradi, Chem. Eng. J. 310 (2017) 41-62. DOI:10.1016/j.cej.2016.10.064 |
| [96] |
J. Peng, X. Lu, X. Jiang, et al., Chem. Eng. J. 354 (2018) 740-752. DOI:10.1016/j.cej.2018.08.038 |
| [97] |
J. Peng, H. Zhou, W. Liu, et al., Chem. Eng. J. 397 (2020) 125387. DOI:10.1016/j.cej.2020.125387 |
| [98] |
H. Liu, T.A. Bruton, F.M. Doyle, D.L. Sedlak, Environ. Sci. Technol. 48 (2014) 10330-10336. DOI:10.1021/es502056d |
| [99] |
A. Jawad, K. Zhan, H. Wang, et al., Environ. Sci. Technol. 54 (2020) 2476-2488. DOI:10.1021/acs.est.9b04696 |
| [100] |
X. Duan, H. Sun, S. Wang, Acc. Chem. Res. 51 (2018) 678-687. DOI:10.1021/acs.accounts.7b00535 |
| [101] |
H. Sun, C. Kwan, A. Suvorova, et al., Appl. Catal. B 154- 155 (2014) 134-141. |
| [102] |
X. Duan, H. Sun, Y. Wang, J. Kang, S. Wang, ACS Catal. 5 (2014) 553-559. |
| [103] |
H. Liu, P. Sun, M. Feng, et al., Appl. Catal. B 187 (2016) 1-10. DOI:10.1016/j.apcatb.2016.01.036 |
| [104] |
M. Feng, R. Qu, X. Zhang, et al., Water Res. 85 (2015) 1-10. DOI:10.1016/j.watres.2015.08.011 |
| [105] |
X. Cheng, H. Guo, Y. Zhang, et al., J. Colloid Interface Sci. 469 (2016) 277-286. DOI:10.1016/j.jcis.2016.01.067 |
| [106] |
Y. Yao, H. Chen, J. Qin, et al., Water Res. 101 (2016) 281-291. DOI:10.1016/j.watres.2016.05.065 |
| [107] |
J. Kang, X. Duan, C. Wang, et al., Chem. Eng. J. 332 (2018) 398-408. DOI:10.1016/j.cej.2017.09.102 |
| [108] |
C. Nie, Z. Ao, X. Duan, et al., Chemosphere 206 (2018) 432-438. DOI:10.1016/j.chemosphere.2018.04.173 |
| [109] |
C. Nie, Z. Dai, H. Meng, et al., Water Res. 166 (2019) 115043. DOI:10.1016/j.watres.2019.115043 |
| [110] |
X. Duan, H. Sun, J. Kang, et al., ACS Catal. 5 (2015) 4629-4636. DOI:10.1021/acscatal.5b00774 |
| [111] |
H. Lee, H.J. Lee, J. Jeong, et al., Chem. Eng. J. 266 (2015) 28-33. DOI:10.1016/j.cej.2014.12.065 |
| [112] |
X. Cheng, H. Guo, Y. Zhang, X. Wu, Y. Liu, Water Res. 113 (2017) 80-88. DOI:10.1016/j.watres.2017.02.016 |
| [113] |
X. Cheng, H. Guo, Y. Zhang, G.V. Korshin, B. Yang, Water Res. 157 (2019) 406-414. DOI:10.1016/j.watres.2019.03.096 |
| [114] |
C. Guan, J. Jiang, C. Luo, et al., Chem. Eng. J. 337 (2018) 40-50. DOI:10.1016/j.cej.2017.12.083 |
| [115] |
W. Ma, N. Wang, Y. Fan, et al., Chem. Eng. J. 336 (2018) 721-731. DOI:10.1016/j.cej.2017.11.164 |
| [116] |
W. Yang, Z. Jiang, X. Hu, et al., Chemosphere 220 (2019) 514-522. DOI:10.1016/j.chemosphere.2018.12.136 |
| [117] |
S. Adil, W.S. Kim, T.H. Kim, et al., J. Hazard. Mater. 396 (2020) 122757. DOI:10.1016/j.jhazmat.2020.122757 |
| [118] |
Z. Jiang, J. Zhao, C. Li, et al., Carbon 158 (2020) 172-183. DOI:10.1016/j.carbon.2019.11.066 |
| [119] |
J. Lee, U. von Gunten, J.H. Kim, Environ. Sci. Technol. 54 (2020) 3064-3081. DOI:10.1021/acs.est.9b07082 |
| [120] |
W. Ren, L. Xiong, X. Yuan, et al., Environ. Sci. Technol. 53 (2019) 14595-14603. DOI:10.1021/acs.est.9b05475 |
| [121] |
S. Sun, J. Jiang, L. Qiu, et al., Water Res. 156 (2019) 1-8. DOI:10.1016/j.watres.2019.02.057 |
| [122] |
S.Q. Tian, L. Wang, Y.L. Liu, et al., Environ. Sci. Technol. 53 (2019) 5282-5291. DOI:10.1021/acs.est.9b00180 |
 2021, Vol. 32
2021, Vol. 32