Phosphate anions are essential nutrients necessary for maintaining cell viability and biological responses, widely distributing in biological environment [1]. Unremitting balance of phosphorus is indispensable for the maintenance of biological cell vital movement [2, 3]. Different types of intracellular signaling pathways take phosphate anions to carry out critical cellular reactions [4]. In addition, phosphate anions are generally associated with the growth of cancer cells. Marco et al. found that increased phosphate in cells induced apoptosis of EAhy926 and GM-7373 endothelial cells, which made endothelial integrity damaged [5]. Sapio et al. reported that phosphate inhibited the proliferation of triple-negative breast cancer cells MDA-MB-231 by slowing down the cell cycle process [6]. This indicates that the phosphate anions have potential for tumor therapy. However, as a charged inorganic ion, there is difficult for phosphate anions to traverse the cell membrane and locate into cancer cell [7]. Then the endocytosis of nanomaterials gives us exciting inspiration. Recently, our team has synthesized a kind of nanocarrier based on poly(D, L-lactic-co-glycolic acid) for the transmembrane transport of chloride anion (Cl−) and sodium cation (Na+) [8].
For the recent few years, black phosphorus nanosheets (BPs) has been suggested for electron transmission [9], photoelectric conversion [10], biomedicine [11, 12] and other fields because its excellent electronic and optical properties, including high carrier mobility, layer-dependent adjustable band gaps, wide absorption range and anisotropy etc. [13, 14]. In addition, BPs process distinct chemical properties which can degrade in the environments of water and oxygen, and phosphate anions are produced [15, 16]. Yu's group has reported this special property of the degradation of BPs in cancer cells in chemotherapy [17]. Nevertheless, the further evolution of BPs in the field of chemotherapy remains to be excavated.
Artesunate (ART) is a derivative of artemisinin, which is semi-synthesized with the sesquiterpene lactone extracted from the stems and leaves of inflorescence Artemisia annua [18]. The endoperoxide groups of artesunate structure prone to react with Fe(Ⅱ) provided from heme to generate reactive oxygen species (ROS) [19-21]. We envisioned that the collaboration between ART and BPs make the degradation rate and degree of BPs be promoted and intensified in cancer cells based on the ART peculiarity. In order to overcome the early undesirable degradation and activation of BPs and ART during the circulation, we employed a simple strategy of polydopamine (PDA) modification. Dopamine (DA) is a kind of catechol derivative, which can self-polymerize at weak alkaline and the film of PDA formed on the surface of substrates [22]. Therefore, the PDA coating could seclude BPs and ART with surrounding environment. In addition, the reactive quinones framework of PDA affords anchoring points for further surface chemical functionalization via Schiff base reactions and Michael addition [23, 24].
Folate (FA) and hyaluronic acid (HA) as well-known targeted therapeutic ligand are widely applied in various species of nanocarriers [25, 26]. FA is a vital vitamin for cell survival and plays high affinity with the folate receptor, a polypeptide overexpressed in cancer cells [27, 28]. HA is a natural linear polysaccharide with monosaccharide residues range from 500 to 50, 000 [29]. Activation of HA receptor causes CD44 overexpress on cancer cells, which draw a distinction with the healthy cells. The CD44 displays unique ability to internalize HA and supports HA as a targeted therapeutic ligand [30]. Hence, considering the PDA coating and demand for eximious tumor-targeting, FA and HA modified with amine were adopted in the carriers.
In this study, we proposed a reactive convey strategy via the synergy of ART and BPs as phosphate anions carriers, in which the targeting ligands FA and HA were appended for the effective cancer-targeting capacity (Fig. 1). The constructed carrier could be captured into cancer cells, then PDA coating gradually decomposed and the ART was released to react with BPs in the acidic tumor microenvironment, following with the death of cancer cells. Various cellular experiments were utilized to explore the lethal effect on cancer cells.

|
Download:
|
| Fig. 1. Schematic illustration of the construction and the mechanism of BPs-based cancer-targeting phosphate anions BPs/ART@PDA-FA/HA. | |
The synthesis of BPs/ART@PDA-FA/HA was schematically illustrated in Fig. 1. Thin-layer BPs were prepared by a modified mechanical exfoliation method and the final product of BPs/ART@PDA-FA/HA was acquired by three further procedures. ART was loaded via physical adsorption with loading content of 63.2% (Fig. S1 in Supporting information), measured by UV–vis spectrophotometer. The detailed procedure is demonstrated in Supporting information. TEM images (Fig. 2) show that the average lateral size of bare BPs and functionalized one were approximately 200−300 nm. The bare BPs is thinner than the modified. The successful encapsulation of PDA and conjugation of FA and HA on BPs was demonstrated by Fourier transform infrared (FT-IR) spectroscopy, shown in Fig. 2C. The spectra exhibited characteristic peaks of BPs at 534 cm−1 caused by the formation of PO43− [31]. Several absorption signals changed after the modification of PDA. Firstly, the weakened peak at 534 cm−1 indicated that coating of PDA may inhibit the oxidation of BPs in the air. Secondly, the presence of the absorption band of benzene skeleton vibrations at 1400 cm−1 and the N-H bond stretching vibration supplied evidence of the encapsulation of PDA. The spectra of the BP/ART@PDA-FA and BP/ART@PDA-FA/HA express characteristic absorption signals at approximately 1520 cm−1 and 1010 cm−1, attributed to the secondary amide vibration mode. These findings indicate the conjugation of FA and HA on BPs/ART@PDA. To further characterized the chemical properties of three materials, Raman scattering spectrum was utilized (Fig. 2D). Three representative peaks were observed in the spectrum of BPs, which was corresponded with the out-of-plane vibration peaks Ag1 at 363 cm−1 and in-plane vibration peaks B2g and Ag2 at 437 cm−1, 462 cm−1, severally [32]. It can be seen that the modified materials BPs/ART@PDA and BPs/ART@PDA-FA/HA show the same characteristic peaks of BPs, indicating that the modification process made no change in the structure of black phosphorus. The particle size of the samples was analyzed by the dynamic light scattering (Fig. S2 in Supporting information). Figs. S2a-c display the particle size of BPs, BPs/ART@PDA and BPs/ART@PDA-FA/HA in water, respectively. The average size of the raw BPs was approximately 180 nm in the aqueous solution. The size of BPs/ART@PDA and BPs/ART@PDA-FA/HA increased after the PDA encapsulation and modification of targeted groups to 300 nm approximately.

|
Download:
|
| Fig. 2. Characterization of the carriers. TEM images of (A) bare BPs (scale bar: 100 nm), (B) BPs/ART@PDA-FA/HA (scale bar: 200 nm). (C) FT-IR spectra of bare BPs, BPs/ART@PDA, BPs/ART@PDA-FA and BPs/ART @PDA-FA/HA. (D) Raman scattering spectrum of BPs, BPs/ART@PDA and BPs/ART@PDA-FA/HA. (E) Degradation of BPs@PDA (BPs: 100 μg) at the conditions of pH 5.0, 6.8 and 7.4 (50 mmol/L, Hepes buffer). (F) Degradation of BPs (100 μg) in water, anaerobic water and H2O2 solution ([H2O2] = 1.0 μmol/L). | |
The self-polymerization of dopamine needed to be carried out at pH 8.5, and PDA [33]. Therefore, the degradation experiment was guided to determine phosphate anions release in Hepes buffer solution with pH of 7.4 and 5.0 for 48 h, respectively. About 15 μmol/L phosphate anions were detected from BPs@PDA in Hepes buffer of pH 5.0 at 6 h, and the concentration gradually increased with time (Fig. 2E). However, only a few phosphate anions were detected in the pH 7.4 buffer. The result may be ascribed the PDA coating was sensitive to acidic environment [11, 34]. The PDA coating would detach from nanoparticles partially, and resulted the exposure of the BPs to degrade and produced phosphate anions. As the pH of the tumor's acidic microenvironment is 6.8, the release of phosphate is detected in Hepes buffer at pH 6.8. The results show that BPs@PDA can gradually decompose and produce phosphate anions in cancer cells.
In order to verify the mechanism that ART promotes the production of phosphate anions from BPs/ART@PDA, the degradation of BPs in aqueous solution, aqueous solution deoxygenated by N2 bubbles and 1.0 μmol/L H2O2 solution was detected, respectively, and the results are shown in Fig. 2F. The results showed that BPs are gradually degraded with different degree. The lowest phosphate anions level was detected in aqueous solution deoxygenated by N2 bubbles, while the highest was detected in 1.0 μmol/L H2O2 solution, which attributed to more reactive oxygen species in the latter one. The degradation of the BPs, as well as the phosphate anions concentration increased with the increase of reactive oxygen species in the solution. We can speculate that the added ART in carriers could accelerate the degradation of BPs in cancer cells. There was no basical difference for the degradation of BPs between in the aqueous solution and the N2 deoxidized aqueous solution, which meant the dissolved oxygen in the natural aqueous solution cannot speed the degradation of BP.
In the light of previous studies [35], the way BPs@PDA enter cells is to reach the external environment of cancer cells through circulation and interact with the cell plasma membrane surface. Then enter the cell through endocytosis and transfer to the lysosome by the endosome [36]. In order to verify the targeting effect of FA and HA, FITC was first loaded onto the surface of BPs to prepare the nanocarriers BPs/FITC@PDA and BPs/FITC@PDA-FA/HA for the observation of targeted cellular uptake with fluorescence microscope. As shown in Fig. S3 (Supporting information), the green fluorescence intensity of Hela cells incubated with nanocarriers showed that BPs/FITC@PDA and BPs/FITC@PDA-FA/HA have passed into cells at 6 h. The fluorescence intensity of the cells increased along with prolonged times. The fluorescence intensity of the cells treated with the carrier BPs/FITC@PDA-FA/HA was much higher, indicating that the targeted groups of FA and HA made the carrier to enter cells more quickly and efficiently. Simultaneously, HUVEC cells used as non-cancer cell model to verify the targeting function of BPs/FITC@PDA-FA/HA. As shown in Fig. S4 (Supporting information), there is no fluorescence emission of FITC was detected, which further proved the cancer cell targeting effect of FA/HA.
Intracellular ROS was measured using the probe DCFH-DA (Fig. S5 in Supporting information). It is found that the fluorescence intensity of BPs/ART@PDA-FA/HA group is apparently higher than of the control and of the BPs@PDA-FA/HA group. The semi quantitative fluorescence intensity of images was calculated and analyzed using Image-J software, the same conclusion was obtained clearly.
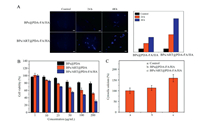
A phosphate anions probe N1 was used to record its concentration change of phosphate anions (The detail is shown in Supporting information). The import of BPs/ART@PDA-FA/HA made the intracellular phosphate anions concentration increase after 24 h and 48 h to emit brighter blue fluorescence (Fig. 3A). However, the BPs@PDA-FA/HA group only emitted a weak blue fluorescence after 48 h. In other words, the import of BPs/ART@PDA-FA/HA caused more rapid increase of the intracellular phosphate concentration. The level of intracellular phosphate anions increases following the enhancive concentration of BPs/ART@PDA-FA/HA (Fig. S6 in Supporting information). It indicates that ROS generated from ART accelerates the degradation of BPs in Hela cells, causing the concentration of phosphate anions to increase significantly in a short time.
|
Download:
|
| Fig. 3. (A) Intracellular phosphate anions evaluation using a phosphate fluorescent probe N1 as treated with BPs/FITC@PDA and BPs/FITC@PDA-FA/HA for 24 h and 48 h. Scale bar: 50 μm. The inset in right shows the semi quantitative fluorescence intensity of images. (B) Cytotoxicity of different carriers on Hela cells at 24 h.n = 6. (C) Assessment of cytosolic calcium level for Hela cells at 24 h.n = 6. | |
The cytotoxicity of the carriers against HeLa cells is evaluated using the MTT assay. Fig. 3B displays cell viabilities of Hela cells after incubation for 24 h with the different concentrations of BPs@PDA, BPs/ART@PDA and BPs/ART@PDA-FA/HA. At the concentration of 25 μg/mL, the cell viabilities of which incubated with BPs@PDA was about 98%, showing good biocompatibility. However, the cell viabilities of BPs/ART@PDA and BPs/ART@PDA-FA/HA group decreased to 80% and 69%. When the concentration of carriers reached 200 μg/mL, the cell viabilities of BPs/ART@PDA-FA/HA group decreased to 28%, which indicates that the carriers BPs/ART@PDA-FA/HA has a better effect on inhibiting proliferation of cancer cells. Furthermore, the concentration of the uploaded ART is only 1.3 μmol/L at the largest concentration of BPs/ART@PDA-FA/HA (200 μg/mL). ART is basically non-toxicity to cells at this concentration [37], indicating that the inhibit proliferation effect of the nanocarrier BPs/ART@PDA-FA/HA on Hela cells is caused by only acute elevation of phosphate anions caused by the degradation of BPs.
Cell death is usually inextricable from the level of cytosolic calcium ions and the cytosolic calcium level is increased with cell death [38, 39]. To verify the inhibit proliferation of Hela cells by the nanocarriers, we detected the level of cytosolic calcium with commercial calcium probe Fura 2-AM (Fig. 3C). When incubated with two kinds of nanocarriers after 24 h, the concentration of intracelluar calcium ions increased in Hela cells. Compared to control group, the addition of nanocarrier BPs/ART@PDA-FA/HA makes the cytosolic calcium level increase to 158%, which may be one of the reasons of cell death.
Apoptosis and necrosis are two kind of programmed cell death with different characteristics in morphology and molecular biology, while they both wind up with cell death. Cell death pathways are studied using Hoechst 33342 and Propidium Iodide (PI) staining. Hoechst 33342 can enter normal cells and apoptotic cells and combine with DNA, displaying blue fluorescence. And the fluorescence of apoptotic cells after staining is significantly enhanced than normal cells, because the nuclear chromatin shrinks in apoptotic cells. PI cannot penetrate the cell membrane and stain normal cells. While for necrotic cells, the integrity of the cell membrane is destroyed. PI can enter the cell and stain the necrotic cells to produce red fluorescence. Thus, apoptotic cells exhibit weak red fluorescence and strong blue fluorescence, while necrotic cells exhibit strong red fluorescence emission and strong blue fluorescence emission. As illustrated in Fig. 4, the control group displays weak blue fluorescence (Hoechst 33342) and red fluorescence (PI). And the stronger blue fluorescence and the similar red fluorescence as control group came out in BPs@PDA-FA/HA treated cells, which is manifested as apoptosis. However, the BPs/ART@PDA-FA/HA group shows strongest blue fluorescence and red fluorescence compared to previous groups, indicating the process of necrosis. It could be concluded that increase of phosphate anions level released from carriers in cells will induce apoptosis and necrosis. The semi quantitative fluorescence intensity of images was showed Fig. S7 (Supporting information). Combining the preceding experimental results, the following explanation is concluded. When the carrier BPs@PDA-FA/HA enters Hela cells, BPs will slowly degrade and produce a small amount of phosphate anions due to the higher ROS in cancer cells, and the increase of phosphate anions induces apoptosis, gradually. In BPs/ART@PDA-FA/HA group, the addition of ART makes the amount of ROS in cancer cells higher. At the same time, the degradation rate of BPs will become faster, which increases the concentration of phosphate anions and results in the acute elevation of phosphate anions. The cells marched to necrosis, when the phosphate anions concentration rises to the limit of the cell ion balance.

|
Download:
|
| Fig. 4. Apoptosis and necrosis mediated cell death caused by phosphate imbalance in Hela cells after incubated with BPs/ART@PDA and BPs/ART@PDA-FA/HA for 24 h and 48 h. Scale bar: 50 μm. | |
In summary, we successfully designed artesunate loaded tumor microenvironment responsive BPs/ART@PDA-FA/HA as a novel reactive cancer-targeted phosphate anions carrier. The carrier structure, degradation process, the cytotoxicity, cellular uptake and the mechanism of phosphate anions transport in cells were systematically detected. The integrated studies suggested that the PDA coating was able to partially decompose and shed in Hela cells after the carrier BPs/ART@PDA-FA/HA entered, exposing the inside ART and BPs to the responsive microenvironment. And the follow acute elevation of phosphate anions induced the Hela cells necrosis. Overall, the novel reactive phosphate anions carrier BPs/ART@PDA-FA/HA exhibited favorable inhibitory ability against the proliferation of Hela cells, which might possess potential applications as anticancer agents.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis research was financially supported by the National Natural Science Foundation of China (No. 21575055) and the Fundamental Research Funds for the Central Universities (No. 561220006).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.09.052.
| [1] |
M.S. Razzaque, Arch. Biochem. Biophys. 561 (2014) 154-158. DOI:10.1016/j.abb.2014.06.031 |
| [2] |
M.S. Razzaque, Nat. Rev. Endocrinol. 5 (2009) 611-619. DOI:10.1038/nrendo.2009.196 |
| [3] |
Y. Lin, K.E. McKinnon, S.W. Ha, G.R. Beck Jr., Mol. Carcinogen. 54 (2015) 926-934. DOI:10.1002/mc.22153 |
| [4] |
P. Liu, H. Cheng, T.M. Roberts, J.J. Zhao, Nat. Rev. Drug Discov. 8 (2009) 627-644. DOI:10.1038/nrd2926 |
| [5] |
G.S.D. Marco, M. Hausberg, U. Hillebrand, et al., Am. J. Physiol. Renal Physiol. 294 (2008) F1381-F1387. DOI:10.1152/ajprenal.00003.2008 |
| [6] |
L.S. Sapio, L. Sorvillo, M. Illiano, E. Chiosi, A. Spina, S. Naviglio, Molecules 20 (2015) 15910-15928. DOI:10.3390/molecules200915910 |
| [7] |
C.F. Dick, A.L.A. Dos-Santos, J.R. Meyer-Fernandes, BBA-Gen. Subjects 1840 (2014) 2123-2127. DOI:10.1016/j.bbagen.2014.03.014 |
| [8] |
C. Ma, Y.D. Zhang, Z.J. Jiao, et al., Chin. Chem. Lett. 31 (2019) 1635-1639. |
| [9] |
Y. Zhang, L. Wang, H. Xu, et al., Adv. Funct. Mater. 30 (2020) 1909372. DOI:10.1002/adfm.201909372 |
| [10] |
L. Wang, Y. Hu, F. Qi, et al., ACS Appl. Mater. Inter. 12 (2020) 8157-8167. DOI:10.1021/acsami.9b19408 |
| [11] |
X.W. Zeng, M.M. Luo, G. Liu, et al., Adv. Sci. 5 (2018) 1800510. DOI:10.1002/advs.201800510 |
| [12] |
B.Q. Chen, R.K. Kankala, Y. Zhang, et al., Chem. Eng. J. 390 (2020) 124312. DOI:10.1016/j.cej.2020.124312 |
| [13] |
X. Zhang, H.M. Xie, Z.D. Liu, et al., Angew. Chem. Int. Ed. 54 (2015) 3653-3657. DOI:10.1002/anie.201409400 |
| [14] |
X.F. Li, R. Grassi, S.C. Li, et al., Nano Lett. 18 (2018) 26-31. DOI:10.1021/acs.nanolett.7b02278 |
| [15] |
Q.H. Zhou, Q. Chen, Y.L. Tong, J.L. Wang, Angew. Chem. Int. Ed. 55 (2016) 11437-11441. DOI:10.1002/anie.201605168 |
| [16] |
J.O. Island, G.A. Steele, H.S.J. van der Zant, Castellanos-Gomez, 2D Mater. 2 (2015) 011002. DOI:10.1088/2053-1583/2/1/011002 |
| [17] |
W.H. Zhou, T. Pan, H.D. Cui, et al., Angew. Chem. Int. Ed. 58 (2019) 769-774. DOI:10.1002/anie.201810878 |
| [18] |
S.R. Zhang, H. Yuan, Y. Guo, et al., Chem. Commun. 54 (2018) 11717-11720. |
| [19] |
C. Sun, Y. Cao, P. Zhu, B. Zhou, Sci. Rep.-UK 7 (2017) 45665. DOI:10.1038/srep45665 |
| [20] |
X. Zhang, Q. Ba, Z.N. Gu, et al., Chem. Eur. J. 21 (2015) 17415-17421. DOI:10.1002/chem.201502543 |
| [21] |
J.G. Wang, C.J. Zhang, W.N. Chia, et al., Nat. Commun. 6 (2015) 10111. DOI:10.1038/ncomms10111 |
| [22] |
D.D. Bi, L. Zhao, R.Q. Yu, et al., Drug Deliv. 25 (2018) 564-575. DOI:10.1080/10717544.2018.1440447 |
| [23] |
N. Li, T.T. Li, C. Hu, et al., ACS Appl. Mater. Inter. 8 (2016) 15013-15023. DOI:10.1021/acsami.5b02037 |
| [24] |
Y.L. Liu, K.L. Ai, L.H. Lu, Chem. Rev. 114 (2014) 5057-5115. DOI:10.1021/cr400407a |
| [25] |
X.H. Liang, Y. Sun, L.S. Liu, et al., Chin. Chem. Lett. 23 (2012) 1133-1136. DOI:10.1016/j.cclet.2012.08.006 |
| [26] |
X.Q. Quan, L. Kang, X.Z. Yin, et al., Chin. Chem. Lett. 26 (2015) 695-699. DOI:10.1016/j.cclet.2015.04.024 |
| [27] |
S. Haller, J. Reber, S. Brandt, et al., Nucl. Med. Biol. 42 (2015) 770-779. DOI:10.1016/j.nucmedbio.2015.06.006 |
| [28] |
K. Cohen, R. Emmanuel, E. Kisin-Finfer, D. Shabat, D. Peer, ACS Nano 8 (2014) 2183-2195. DOI:10.1021/nn500205b |
| [29] |
A.K. Yadav, P. Mishra, S. Jain, et al., J. Drug Target. 16 (2008) 464-478. DOI:10.1080/10611860802095494 |
| [30] |
M. Culty, H.A. Nguyen, C.B. Underhill, J. Cell Biol. 116 (1992) 1055-1062. DOI:10.1083/jcb.116.4.1055 |
| [31] |
B.W. Yang, J.H. Yin, Y. Chen, et al., Adv. Mater. 30 (2018) 1705611. DOI:10.1002/adma.201705611 |
| [32] |
Z.B. Sun, H.H. Xie, S.Y. Tang, et al., Angew. Chem. Int. Ed. 54 (2015) 11526-11530. DOI:10.1002/anie.201506154 |
| [33] |
Q.S. Zheng, T.R. Lin, H.Y. Wu, et al., Int. J. Pharm. 463 (2014) 22-26. DOI:10.1016/j.ijpharm.2013.12.045 |
| [34] |
W. Cheng, J.P. Nie, N.S. Gao, et al., Adv. Funct. Mater. 27 (2017) 1704135. DOI:10.1002/adfm.201704135 |
| [35] |
B. Poinard, S. Kamaluddin, A.Q.Q. Tan, et al., ACS Appl. Mater. Inter. 11 (2019) 4777-4789. DOI:10.1021/acsami.8b18107 |
| [36] |
W. Tao, X.B. Zhu, X.H. Yu, et al., Adv. Mater. 29 (2017) 1603276. DOI:10.1002/adma.201603276 |
| [37] |
L. Wang, Y.J. Hu, Y.W. Hao, et al., J. Control. Release 286 (2018) 74-84. DOI:10.1016/j.jconrel.2018.07.028 |
| [38] |
S. Ji, J.Y. Lee, J. Schror, et al., Cancer Lett. 416 (2018) 109-123. DOI:10.1016/j.canlet.2017.12.011 |
| [39] |
S. Park, W. Lim, S. You, G. Song, Toxicol. Lett. 313 (2019) 42-49. DOI:10.1016/j.toxlet.2019.05.021 |
 2021, Vol. 32
2021, Vol. 32 

