b Key Laboratory of Resource Chemistry of Ministry of Education, Shanghai Key Laboratory of Rare Earth Functional Materials, Shanghai Normal University, Shanghai 200234, China;
c State Key Laboratory of Precision Spectroscopy, East China Normal University, Shanghai 200062, China;
d State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, China
Owing to its high sensitivity, fluorescence is widely used in microanalysis, memory media, and bioimaging [1-14]. In the past few decades, a variety of fluorescent compounds based on BODIPY, porphyrin, naphthalimide, and tetraphenylethylene have been developed and exhibited extensive applications in organic light emitting diodes (OLEDs), molecular sensing and bioimaging [15-30]. Recently, the construction of fluorescent compounds showing tunable fluorescence characteristics upon external stimuli has attracted increasing interests due to their applications in sensing, super-resolution fluorescence imaging, and information storage [31-40].
Triphenylamine (TPA) derivative, in which three benzene moieties were connected with a nitrogen atom, has been developed as an emerging fluorescent compound during past few decades [41, 42, 43]. Due to their high quantum yield, easy functionalization as well as aggregation-induced emission (AIE) property, TPA derivative has gained increasing attention and a large number of TPA derivatives have been synthesized with applications in molecular sensing, photocatalysis, supramolecular self-assembly, bioimaging and therapyetc. [44-50]. In general, the TPA core could act as a moderate electron-donating group. When the electron-withdrawing groups such as pyridine, trifluoromethyl and aldehyde were connected with TPA core, a push-pull electron system formed and the resultant molecules could emit bright fluorescence. In addition, the fluorescence characteristics of TPA derivatives can be readily adjusted by simply changing the substituent groups [51]. Furthermore, when external stimuli such as acid, base, and anions were involved with the substituent groups, a dramatic fluorescence change was observed due to the high sensitivity of TPA core. Thus, it was facile to realize tunable fluorescence emission for TPA derivatives through substituent effect and external stimuli [52]. In this work, a series of dipyridyl-containing TPA derivatives with various substituent groups (electron-withdrawing -CF3, -CHO, -CH=C(CN)2 groups and electron-donating -H, -CH3, -OCH3 groups) were designed and prepared (Fig. 1a) and their photophysical properties were comprehensively studied. Moreover, acid-induced fluorescence regulation of the TPA derivatives was also investigated and white light emission was observed, which was helpful for constructing white light emission materials.
|
Download:
|
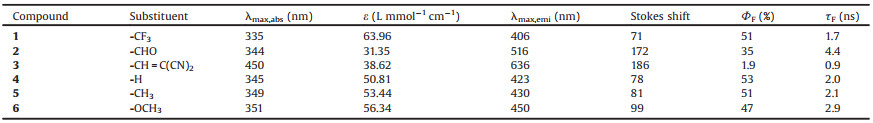
| Fig. 1. (a) Chemical structures of the TPA derivatives 1–6. (b) Crystal structures of compounds 1, 5 and 6. (c) Normalized absorption spectra of 1–6 in CH2Cl2. (d) Normalized emission spectra of 1–6 in CH2Cl2. Photographs of 1–6 (10−5 mol/L in CH2Cl2) under (e) visible light and (f) 365 nm UV light. | |
The TPA derivatives 1, 2, 4–6 were synthesized through Suzuki-Miyaura coupling from the iodine-substituted precursors with 3-pyridinylboronic acid in yields of 46%−74% (Fig. 1). Further treating 2 with malononitrile afforded 3 in a yield of 86% (Detailed preparation information can be seen in Supporting information). By solvent diffusion method, crystal structures of 1, 5 and 6 were obtained and X-ray diffraction analysis indicated the three benzene rings of TPA core adopted a propeller-like conformation and the existence of noncovalent interactions like C-H⋯F hydrogen bond in the solid state (Fig. 1b). In addition, compounds 1–6 were well characterized by 1H and 13C NMR and mass spectrometry (Figs. S1−S13 in Supporting information).
With TPA derivatives 1–6 in hand, their photophysical properties were investigated. As shown in Fig. 1c and Table 1, all compounds exhibited maximum absorption peaks between 335 nm and 351 nm except that 3 showed a maximum absorption peak at 450 nm. Compounds 1, 4, 5 and 6 emitted bright blue fluorescence between 400 nm and 450 nm. For compounds 4, 5, 6, electron-donating groups -H, -CH3, -OCH3 were incorporated into the triphenylamine (TPA) unit, respectively, which made them with weak internal charge transfer (ICT) effect. Thus, the blue emission was observed as shown in Fig. 1f. However, compound 3 showed the maximum absorption peak and emission peak at 450 nm and 636 nm, respectively, which might be due to the effective internal charge transfer (ICT) from the electron push unit to the electron pull unit -CH=C(CN)2. The flexibility of substituent group -CH=C(CN)2 endows the compound 3 with high non-radiative decay rate, which resulted in the low quantum yield of compound 3. However, for compound 2, electron-pull unit -CHO was incorporated into the TPA unit, which endows compound 2 with a moderate internal charge transfer (ICT) effect, thus compound 2 displayed green emission with shorter wavelength than compound 3 and longer wavelength than compounds 4, 5 and 6. In addition, the quantum yields of 1–6 were also determined and the results indicated that most of the compounds exhibited a high quantum yield up to 53% except 3 showed a low quantum yield of 1.9%, which was consistent with the fluorescent photographs (Fig. 1f). Furthermore, the lifetimes of 1–6 were determined to be within 10ns, suggesting all of them displayed fluorescence emission (Fig. S14 in Supporting information andTable 1).
|
|
Table 1 Photophysical properties of compounds 1-6. |
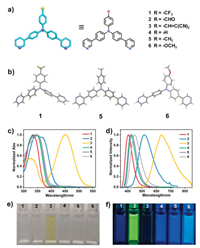
Due to the pyridine moieties were sensitive to acid, the acid-induced fluorescence regulation behavior of compounds 1–6 was further investigated. As shown in Fig. 2, upon adding trifluoroacetic acid (TFA) into 1 in CH2Cl2, a noticeable decrease in the absorption at 335 nm accompanied by an increase in the absorption at 380 nm were observed. The existence of isoabsorptive point at 352 nm also indicated the formation of a new species. In addition, addition of TFA into 1 induced a significant fluorescence quenching at 406 nm, accompanied by the appearance of a new peak at 549 nm (Fig. 2b), corresponding to the protonation form of 1 (1 + H+). The red-shift of absorption and emission wavelengths and decrease of fluorescence intensity might be due to the stronger electron-withdrawing ability of protonated pyridine than that of free pyridine, indicating intramolecular charge transfer (ICT) was mainly involved into the fluorescence mechanism [53]. Interestingly, when 7.0 equiv. TFA was added to 1 in CH2Cl2, the solution showed an obvious white light emission (CIE: 0.31, 0.33) resulting from the complex of blue fluorescence of 1 and yellow fluorescence of 1 + H+ (Figs. 2c and d). It should be noted that the common white light emission materials need the combination of multiple chromophores in one system with accurate ratios [54-56], which was high-cost and unaccessible. Thus, the acid-induced white light emission of 1 afforded a simple way to construct white light emission materials by making use of the single fluorophore. Additionally, 1H NMR spectroscopy was performed to investigate the protonation process and the signals Ha-d corresponding to the protons of pyridine rings displayed an apparent down-field shift upon the addition of TFA, resulting from the decreased electron densities upon protonation (Fig. S15 in Supporting information). Furthermore, upon addition of trimethylamine (TEA) into the solution of 1 + H+, the fluorescence spectrum could be almost completely recovered to the initial state, revealing the protonation process was reversible (Fig. S16 in Supporting information). Compound 4 showed a similar absorption and fluorescence response to TFA with 1 (Fig. S19 in Supporting information). However, the acid-induced absorption and fluorescence change of 3 was different to 1 or 4 (Fig. S18 in Supporting information). For example, the absorption spectrum of 3 was slightly blue-shifted and the fluorescence spectrum showed a 49 nm blue shift accompanied by an increase in fluorescence emission upon addition of TFA, which revealed that the PET process was inhibited upon protonation [51]. Moreover, the absorption spectrum of 2, 5 and 6 was also red-shifted and the fluorescence was quenched without wavelength changes upon acidification due to the enhanced ICT character upon addition of TFA (Figs. S17, S20 and S21 in Supporting information). Therefore, the substituent effect had a great effect on the acid-induced fluorescence regulation of TPA derivatives and tunable multicolor fluorescence emission including white light emission was achieved through simple acidification.
|
Download:
|
| Fig. 2. (a) Absorption spectral changes of compound 1 (10−5 mol/L in CH2Cl2) upon addition of TFA (0–10 equiv.). (b) Emission spectral changes of compound 1 (10−5 mol/L in CH2Cl2, λex=350 nm) upon addition of TFA (0–10 equiv.). The insets show the amplifying emission spectral changes from 520 nm to 690 nm. (c) CIE chromaticity diagram of 1 upon addition of TFA (0–10 equiv.). (d) Photographs of compound 1 (10−5 mol/L in CH2Cl2) upon addition of different equivalents of TFA under UV light. | |
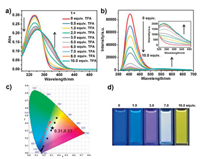
DFT simulations were performed to gain further insight into the mechanism of the acid-induced fluorescence regulation. The results indicated that the electron densities of HOMO of 1 were distributed over the whole molecule and the electron densities of LUMO of 1 were localized on the benzene rings of TPA core and pyridine moieties suggesting –CF3 group almost showed no participation in the fluorescence of 1 (Fig. 3). Thus, the electron-withdrawing pyridine moieties and electron-donating TPA core formed a push-pull electron system and 1 was a typical ICT molecule, which was consistent with the results from above spectroscopic experiments. 1 + H+ showed similar electron densities of HOMO but somewhat different electron densities of LUMO to 1. The electron densities of LUMO of 1 + H+ were mainly localized on the protonated pyridine moieties, probably due to the enhanced electron-withdrawing ability of protonated pyridine. Thus, the enhanced ICT character was observed in 1 + H+, resulting in the fluorescence quenching and red-shift of absorption and emission wavelength. In addition, analysis of hole and electron distribution of 1 indicated holes and electrons were mainly located at the TPA core and the part of pyridine moieties, respectively, suggesting the electron transfer from the TPA core to pyridine moieties at the excited S1 state (Fig. S23 and Table S4 in Supporting information). While for 1 + H+ at the excited S1 state, the hole was similar to 1 and the electron was almost completely located at pyridine moieties, indicating the strong electron-withdrawing ability of protonated pyridines.
|
Download:
|
| Fig. 3. HOMO and LUMO localizations in 1 and 1 + H+. | |
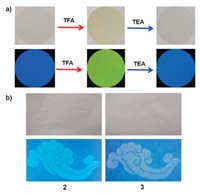
In view of the obvious fluorescence changes of 1–6 upon protonation in solution, the visual experiments in solid state were further performed. As shown in Fig. 4a, 1 was doped into a polyvinylidene fluoride (PVDF) film (3wt%). The PVDF film was initially colorless and exhibited bright blue fluorescence, which was similar to that in the solution state. After slow diffusion of TFA vapor into the PVDF film loaded with 1, the color of the film became light-yellow and the fluorescence gradually changed from blue to yellow-green, indicating that the protonation of compound 1 could also occur in the film. Subsequently, upon diffusion of TEA vapor into the acidified PVDF film, the fluorescence color could be completely recovered, revealing that the protonation and deprotonation was reversible. Moreover, inkjet printing was also conducted to create highly-defined patterns [57]. For instance, the "Xiang-Yun" patterns of 2 and 3 could not be observed under room light, but they could be obviously seen under a UV lamp (Fig. 4b). These results indicated that the TPA derivatives could be applied in anti-counterfeit and information storage.
|
Download:
|
| Fig. 4. (a) Color and fluorescence changes of the PVDF film loaded with 1 after sequential diffusion of TFA and TEA vapors. (b) Fluorescent patterns by inkjet printing from compounds 2 and 3 under room light and 365 nm UV light. | |
In conclusion, we have synthesized a series of dipyridyl-containing TPA derivatives with various substituent groups, which had a significant effect on their photophysical properties. Furthermore, by taking advantage of the protonation of pyridines, acid-induced multicolor fluorescence regulation including white light emission was realized. Theoretical calculations revealed the enhanced ICT was involved into the fluorescence mechanism of protonated TPA derivatives. Moreover, stimuli-responsive fluorescent films and fluorescent inks for inkjet printing were fabricated using these TPA derivatives. These investigations demonstrated TPA was an ideal backbone for constructing multicolor fluorescent materials including white light emission materials. Further preparing new TPA derivatives with strong NIR absorption and emission is under way.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentThis work was supported by the State Key Laboratory of Fine Chemicals (No. KF1801).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.10.012.
| [1] |
E. Betzig, R.J. Chichester, Science 262 (1993) 1422-1425. DOI:10.1126/science.262.5138.1422 |
| [2] |
Z. Xu, X. Chen, H.N. Kim, J. Yoon, Chem. Soc. Rev. 39 (2010) 127-137. DOI:10.1039/B907368J |
| [3] |
X. Sun, B.M. Chapin, P. Metola, et al., Nat. Chem. 11 (2019) 768-778. DOI:10.1038/s41557-019-0314-x |
| [4] |
W. Xu, D. Wang, B.Z. Tang, Angew. Chem. Int. Ed. 60 (2021) 7476-7487. DOI:10.1002/anie.202005899 |
| [5] |
J. Zhu, X. Liu, J. Huang, L. Xu, Chin. Chem. Lett. 30 (2019) 1767-1774. DOI:10.1016/j.cclet.2019.08.027 |
| [6] |
C.B. Huang, L. Xu, J.L. Zhu, et al., J. Am. Chem. Soc. 139 (2017) 9459-9462. DOI:10.1021/jacs.7b04659 |
| [7] |
J. Zhu, P. Jia, N. Li, et al., Chin. Chem. Lett. 29 (2018) 1445-1450. DOI:10.1016/j.cclet.2018.09.002 |
| [8] |
X. Luo, J. Li, J. Zhao, et al., Chin. Chem. Lett. 30 (2019) 831-846. |
| [9] |
Y.X. Hu, X. Hao, L. Xu, et al., J. Am. Chem. Soc. 142 (2020) 6285-6294. DOI:10.1021/jacs.0c00698 |
| [10] |
L. Li, Y. Chen, W. Chen, et al., Chin. Chem. Lett. 30 (2019) 1689-1703. DOI:10.1016/j.cclet.2019.04.017 |
| [11] |
W. Chen, F. Song, Chin. Chem. Lett. 30 (2019) 1717-1730. DOI:10.1016/j.cclet.2019.08.032 |
| [12] |
L.J. Chen, S. Chen, Y. Qin, et al., J. Am. Chem. Soc. 140 (2018) 5049-5052. DOI:10.1021/jacs.8b02386 |
| [13] |
Z. Zhang, F. Wang, X. Chen, Chin. Chem. Lett. 30 (2019) 1745-1757. DOI:10.1016/j.cclet.2019.08.035 |
| [14] |
Y.F. Kang, L.Y. Niu, Q.Z. Yang, Chin. Chem. Lett. 30 (2019) 1791-1798. DOI:10.1016/j.cclet.2019.08.013 |
| [15] |
R. Paolesse, S. Nardis, D. Monti, M. Stefanelli, C.D. Natale, Chem. Rev. 117 (2017) 2517-2583. DOI:10.1021/acs.chemrev.6b00361 |
| [16] |
H. Lu, J. Mack, Y. Yang, Z. Shen, Chem. Soc. Rev. 43 (2014) 4778-4823. DOI:10.1039/C4CS00030G |
| [17] |
J. Mei, N.L.C. Leung, R.T.K. Kwok, J.W.Y. Lam, B.Z. Tang, Chem. Rev. 115 (2015) 11718-11940. DOI:10.1021/acs.chemrev.5b00263 |
| [18] |
M. Liu, S. Ma, M. She, et al., Chin. Chem. Lett. 30 (2019) 1815-1824. DOI:10.1016/j.cclet.2019.08.028 |
| [19] |
X. Sun, T.D. James, E.V. Anslyn, J. Am. Chem. Soc. 140 (2018) 2348-2354. DOI:10.1021/jacs.7b12877 |
| [20] |
T. Wei, S. Lu, J. Sun, et al., J. Am. Chem. Soc. 142 (2020) 3806-3813. DOI:10.1021/jacs.9b11357 |
| [21] |
L. Zhou, L. Xie, C. Liu, Y. Xiao, Chin. Chem. Lett. 30 (2019) 1799-1808. DOI:10.1016/j.cclet.2019.07.051 |
| [22] |
J. Wang, Z. Huang, X. Ma, H. Tian, Angew. Chem. Int. Ed. 59 (2020) 9928-9933. DOI:10.1002/anie.201914513 |
| [23] |
G. Qu, Y. Zhang, X. Ma, Chin. Chem. Lett. 30 (2019) 1809-1814. DOI:10.1016/j.cclet.2019.07.042 |
| [24] |
Q. Sun, D. Sun, L. Song, et al., Anal. Chem. 88 (2016) 3400-3405. DOI:10.1021/acs.analchem.6b00178 |
| [25] |
Z. Xu, X. Huang, M.X. Zhang, et al., Anal. Chem. 91 (2019) 11343-11348. DOI:10.1021/acs.analchem.9b02458 |
| [26] |
Y. Wen, F. Huo, C. Yin, Chin. Chem. Lett. 30 (2019) 1834-1842. DOI:10.1016/j.cclet.2019.07.006 |
| [27] |
M. She, Z. Wang, T. Luo, et al., Chem. Sci. 9 (2018) 8065-8070. DOI:10.1039/c8sc03421d |
| [28] |
J.L. Zhu, Z. Xu, Y. Yang, L. Xu, Chem. Commun. 55 (2019) 6629-6671. DOI:10.1039/c9cc03299a |
| [29] |
G.F. Huo, Q. Tu, X.L. Zhao, X. Shi, H.B. Yang, Chin. Chem. Lett. 31 (2020) 1847-1850. DOI:10.1016/j.cclet.2020.02.010 |
| [30] |
H. Yang, H. Qi, T. Liu, et al., Chin. Chem. Lett. 30 (2019) 977-980. DOI:10.1016/j.cclet.2019.01.023 |
| [31] |
W. Sun, S. Guo, C. Hu, J. Fan, X. Peng, Chem. Rev. 116 (2016) 7768-7817. DOI:10.1021/acs.chemrev.6b00001 |
| [32] |
B. Roubinet, M. Weber, H. Shojaei, et al., J. Am. Chem. Soc. 139 (2017) 6611-6620. DOI:10.1021/jacs.7b00274 |
| [33] |
T. Fleetham, J. Ecton, Z. Wang, N. Bakken, J. Li, Adv. Mater. 25 (2013) 2573-2576. DOI:10.1002/adma.201204602 |
| [34] |
D. Liu, Z. Zhang, H. Zhang, Y. Wang, Chem. Commun. 49 (2013) 10001-10003. DOI:10.1039/c3cc45991h |
| [35] |
K. Yamaguchi, T. Murai, J.D. Guo, T. Sasamori, N. Tokitoh, Chem. Open 5 (2016) 434-438. DOI:10.1002/open.201600059 |
| [36] |
Y. Shiraishi, C. Ichimura, S. Sumiya, T. Hirai, Chem. Eur. J. 17 (2011) 8324-8332. DOI:10.1002/chem.201101048 |
| [37] |
S. Achelle, J. Rodríguez-López, N. Cabon, F. Robin-le Guen, RSC Adv. 5 (2015) 107396-107399. DOI:10.1039/C5RA21514E |
| [38] |
Y.X. Hu, W.J. Li, P.P. Jia, et al., Adv. Optical Mater. 8 (2020) 2000265. DOI:10.1002/adom.202000265 |
| [39] |
Y. Qin, L.J. Chen, Y. Zhang, et al., Chem. Commun. 55 (2019) 11119-11122. DOI:10.1039/c9cc05377h |
| [40] |
Y. Qin, Y. Zhang, G. Yin, et al., Chin. J. Chem. 37 (2019) 323-329. DOI:10.1002/cjoc.201800577 |
| [41] |
F. Ding, Y. Zhan, X. Lu, Y. Sun et al., Chem. Sci. 9 (2018) 4370-4380. DOI:10.1039/c8sc01153b |
| [42] |
M. Klikar, P. Solanke, J. Tydlitát, F. Bureš, Chem. Rec. 16 (2016) 1886-1905. DOI:10.1002/tcr.201600032 |
| [43] |
N. Hammer, T.A. Schaub, U. Meinhardt, M. Kivala, Chem. Rec. 15 (2015) 1119-1131. DOI:10.1002/tcr.201500202 |
| [44] |
Y. Sun, F. Ding, Z. Zhou, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 1968-1973. DOI:10.1073/pnas.1817021116 |
| [45] |
C. Chen, X. Ni, S. Jia, et al., Adv. Mater. 31 (2019) 1904914. DOI:10.1002/adma.201904914 |
| [46] |
W. Xu, M.M.S. Lee, Z. Zhang, et al., Chem. Sci. 10 (2019) 3494-3501. DOI:10.1039/c8sc05805a |
| [47] |
X. Kang, X. Wu, X. Han, et al., Chem. Sci. 11 (2020) 1494-1502. DOI:10.1039/c9sc04882k |
| [48] |
G.F. Huo, X. Shi, Q. Tu, et al., J. Am. Chem. Soc. 141 (2019) 16014-16023. DOI:10.1021/jacs.9b08149 |
| [49] |
L. Zhang, W. Che, Z. Yang, et al., Chem. Sci. 11 (2020) 2369-2374. DOI:10.1039/c9sc06310b |
| [50] |
Y. Qu, B. Jin, Y. Liu, et al., Tetrahedron Lett. 54 (2013) 4942-4944. DOI:10.1016/j.tetlet.2013.07.011 |
| [51] |
J.L. Zhu, L. Xu, Y.Y. Ren, et al., Nat. Commun. 10 (2019) 4285. DOI:10.1038/s41467-019-12204-7 |
| [52] |
J. Tydlitát, S. Achelle, J. Rodríguez-López, et al., Dyes Pigm. 146 (2017) 467-478. DOI:10.1016/j.dyepig.2017.07.043 |
| [53] |
Y. Li, T. Liu, H. Liu, M.Z. Tian, Y. Li, Acc. Chem. Res. 47 (2014) 1186-1198. DOI:10.1021/ar400264e |
| [54] |
X.Y. Lou, N. Song, Y.W. Yang, Chem. Eur. J. 25 (2019) 11975-11982. DOI:10.1002/chem.201902700 |
| [55] |
N.M. Sarih, P. Myers, A. Slater, et al., Sci. Rep. 9 (2019) 11834. DOI:10.1038/s41598-019-47847-5 |
| [56] |
J. Wang, Y. Zhang, Y. Yu, et al., Opt. Mater. 89 (2019) 209-213. DOI:10.2112/si93-029.1 |
| [57] |
K. Yamada, T.G. Henares, K. Suzuki, D. Citterio, Angew. Chem. Int. Ed. 54 (2015) 5294-5310. DOI:10.1002/anie.201411508 |
 2021, Vol. 32
2021, Vol. 32