b School of Materials Science and Engineering, Shandong University of Science and Technology, Qingdao 266590, China;
c School of Materials Science and Engineering, Zhengzhou University, Zhengzhou 450001, China;
d Shenzhen HUASUAN Technology Co., Ltd., Shenzhen 518055, China
The archives are generally kept as important data of each department, which are mostly paper files and are often directly put into a file cabinet for storage. The archives are all covered with dust and bacteria after long-term storage. If we do not remove dust and bacteria in time, the archives' lives will be reduced, even cause healthy danger to the archives' conservator. The current file storage cabinet only realizes the single placement function, which cannot effectively protect the archives. Thus, it is urgently needed to solve the archive's dust removal and sterilization in storage.
Ultraviolet (UV) disinfection, chlorination and ozonation have been used to sterilize [1]. However, this application was limited by the energy-cost, the operationally intensive and the potential formation of the hazardous disinfection byproduct [2]. In comparison, photocatalysts, utilizing solar light and forming less or even no disinfection byproducts, are considered as a promising approach for the oxidative disinfection to various bacteria [3]. Among the various photocatalysts, TiO2 possesses efficient sterilization functions under UV irradiation, meanwhile, TiO2 has the advantages of being non-toxic, chemically stable, non-irritating, inert and inexpensive [4]. During UV irradiation, highly reactive oxygen species (ROS) such as superoxide radical anions (·O2–) and hydroxyl radicals (·OH), are generated; these species could lead to oxidative damage of bacterial cell components, such as protein, nucleic acid and lipid [5]. However, UV light accounts only ~4% of total solar radiation incident on the earth's surface; TiO2 is inactive in the visible and near-infrared (NIR) light region [6]. Therefore, it is necessary to improve its photoabsorption range and increase the antibacterial rate.
Very recently, plasmonic noble metal nanoparticles or nanorods (NRs) have offered a new chance to overcome the low photocatalytic antibacterial efficiency through the surface plasmon resonance (SPR) effect [7]. The combination of plasmonic noble metal with semiconductor photocatalysts can improve the photocatalytic antibacterial activity. SPR can effectively improve the photocatalytic property of semiconductor by (1) enhancing light absorption to visible and NIR region [8] and (2) preventing electron-hole recombination through hot-electron separation [9]. Au NRs can absorb visible and NIR light owing to their transversal (TSPR) and longitudinal (LSPR) surface plasmon resonance peak positions (500–600 and 700–900 nm) [10]. We believe that Au NR is an excellent candidate to improve the photocatalytic antibacterial activities of TiO2 photocatalysts.
In this work, we demonstrate a rational design of a composite that integrates Au nanorods (NRs) with TiO2 nanobelts (NBs) prepared by a hydrothermal method, followed by a photoreduction approach, which can be more effective to harvest the full solar spectrum (UV, visible and NIR) light and present effective photocatalytic oxidation of Escherichia coli (E. coli) under UV, visible and NIR excitation. The excellent antibacterial performance can be ascribed to large special surface areas and the SPR effect of Au NRs, which has a wide range of visible and NIR light harvesting. Inspired this, we design a sterilization file cabinet with Au NR/TiO2 NB heterostructures as the photocatalytic coating plates. This Au NR/TiO2 NB heterostructures coating can be prepared easily at low cost, and it can kill bacteria for rapid disinfection. This work provides a potential candidate for sterilization of bacteria and archives protection.
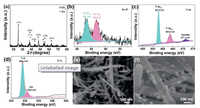
To obtain Au NR/TiO2 NB heterostructures, TiO2 NBs were first synthesized by a hydrothermal method [11], and then were immersed in HAuCl4/NaOH aqueous solution by a photoreduction method. Finally, the Au NR/TiO2 NB heterostructures were obtained. Fig. 1a and Fig. S1 (Supporting information) display the XRD patterns of TiO2 NBs and Au NR/TiO2 NB heterostructures. Pure TiO2 NBs (Fig. S1) show the diffraction peaks of anatase TiO2 (JCPDS No. 21-1272) [12]. For Au NR/TiO2 NB heterostructures (Fig. 1a), the characteristic peaks of anatase TiO2 still exist without obvious peak shift, in addition, there are two peaks at 38.0° and 44.8°, corresponding to the (111) and (200) peaks of Au NRs (JCPDS No.04−0784) [13]. Figs. 1b–d and Fig. S2 (Supporting information) show the X-ray photoelectron spectra (XPS) of Au NR/TiO2 NB heterostructures, which is performed to examine the surface elements and their valence states. XPS survey scan (Fig. S2) clearly shows the Au signal detected from Au NR/TiO2 NB heterostructures, indicating that Au NRs are successfully loaded onto the surface of TiO2 NBs. The XPS spectrum of Au 4f (Fig. 1b) has been identified with doublets at 83.3 eV (4f7/2) and 86.8 eV (4f5/2) characteristic of metallic Au° [14]. As can be seen in Fig. 1c, the main Ti 2p peak is deconvolved into three peaks centered at 457.9 (Ti 2p3/2), 463.6 (Ti 2p1/2) and 471.4 (satellite) eV, respectively, indicating the Ti4+ state of titanium in TiO2 nanobelts [15]. For O 1s XPS spectrum (Fig. 1d), the primary peaks located at 529.2 and 530.8 eV are indexed to the Ti−O bonds and Ti−OH groups in the sample [16]. These results further suggest the existence of TiO2 and Au in the heterostructures.
|
Download:
|
| Fig. 1. (a) X-ray diffraction (XRD) pattern (b) Au 4f, (c) Ti 2p and (d) O 1s X-ray photoelectron spectroscopy (XPS) spectra of Au NR/TiO2 NB heterostructures. Scanning electron microscope (SEM) images of (e) TiO2 NBs and (f) Au NR/TiO2 NB heterostructures. | |
The structures of TiO2 NBs and Au NR/TiO2 NB heterostructures can be characterized by scanning electron microscope (SEM) analysis. Fig. 1e indicates that the as-prepared TiO2 NBs present good belt structures. It is observed from Fig. 1f that the Au NRs are randomly distributed on the surface of TiO2 NBs, most of them are monodisperse but a few are aggregated.
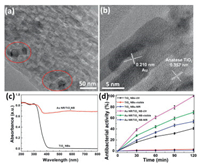
To investigate the structure of TiO2 NBs, Au NRs and Au NR/TiO2 NB heterostructures in detail, the transmission electron microscope (TEM) images were recorded, as shown in Figs. 2a and b, and Figs. S3 and S4 (Supporting information). TEM (Fig. S3a) images show that most of TiO2 NBs are belt-shaped. High resolution transmission electron microscope (HRTEM) image (Fig. S3b) confirms the anatase crystal structure of TiO2 NB [17]. TEM image (Fig. S4a) indicates Au NRs have good rod structure with an aspect ratio of 3 (long axis of 70 nm and short axis of 23 nm). The HRTEM (Fig. S4b) image revealed that a lattice spacing of 0.210 nm could be attributed to the (111) plane of Au nanorods [18]. After assembling Au NRs on the surface of TiO2 NBs, the Au NR/TiO2 NB heterostructures retain a 1D belt-like structure and some Au NRs are dispersed over the TiO2 NBs (Fig. 2a). Fig. 2b shows the HRTEM image of Au NR/TiO2 NB heterostructures. The interplanar space of 0.357 nm and 0.210 nm correspond to the (101) plane of anatase TiO2 and (111) plane of Au, respectively. These results revealed the compact contact interface between Au NRs and TiO2 NBs.
|
Download:
|
| Fig. 2. (a, b) Transmission electron microscope (TEM) images of Au NR/TiO2 NB heterostructures; (c) UV–vis absorption spectra of TiO2 NBs and Au NR/TiO2 NB heterostructures; (d) Photocatalytic antibacterial efficiency against E. coli for TiO2 NBs and Au NR/TiO2 NB heterostructures under UV, visible, and NIR light irradiation. | |
Fig. 2c and Fig. S5 (Supporting information) display the UV–vis DRS spectra of Au NRs, TiO2 NBs and Au NR/TiO2 NB heterostructures. As shown in Fig. S5, Au NRs display a typical TSPR and LSPR absorption peaks at 520 nm and 910 nm, respectively. The measured spectrum of Au NR indicates that it has good visible and NIR light absorption. Pristine TiO2 NBs (black curve in Fig. 2c) only show absorption of UV light associated with wide band gap and no visible and NIR light absorption. After the Au NRs are assembled onto the surface of the TiO2 NBs (red curve in Fig. 2c), Au NR/TiO2 NB heterostructures show a full solar spectrum absorption in the UV, visible and NIR region. The SPR effects of Au NRs play a major role in the improvement of visible and NIR light absorption of Au NR/TiO2 NB heterostructures.
The parameters that govern the efficiency of a photocatalyst include its surface area, which is determined using Brunauer-Emmett-Teller (BET) analysis and the nitrogen adsorption-desorption isotherms (Fig. S6 in Supporting information). TiO2 NBs possess a BET surface area of 22.645m2/g. After being coupled with Au NRs, the Au NR/TiO2 NB heterostructures possess a higher specific surface area of 27.044m2/g. The larger surface area of the Au NR/TiO2 NB heterostructures implies that it can provide more activity sites and possess better photocatalytic antibacterial activity.
To prove the photocatalytic antibacterial activity of TiO2 NBs and Au NR/TiO2 NB heterostructures, the sterilization of E. coli was measured under UV, visible and NIR light irradiation (Fig. 2d). In the light control experiment (Fig. S7 in Supporting information), the cell density of E. coli presents no obvious decrease without photocatalysts except UV light, indicating the negligible effect of the visible and NIR light on the bacterial cells. As shown in Fig. 2d, TiO2 NB is a good UV photocatalyst. The sterilization rate of E. coli for TiO2 NBs under UV light irradiation for 120min is 39%. However, TiO2 NBs present no visible and NIR photocatalytic antibacterial activity. After assembling Au NRs onto the surface of TiO2 NBs, the UV photocatalytic property of the Au NR/TiO2 NB heterostructures is greatly improved. This is ascribed to the carrier transfer from the semiconductor to metal. In addition to the improved specific surface area beneficial to the contact between the catalyst and bacteria. The corresponding sterilization degree of Au NR/TiO2 NB heterostructures increases to 100% after 120min under UV light irradiation. Similar to the previous result [19], TiO2 NBs have no visible and NIR photocatalytic antibacterial activities. Unexpectedly, the visible and NIR antibacterial activities of the Au NR/TiO2 NB heterostructures are enhanced, and the sterilization rate increases to 65% and 49% after 120min under visible and NIR light irradiation. This improvement of visible and NIR photocatalytic antibacterial performance is attributed to the transverse and longitudinal SPR effects of Au NRs (Fig. S5), which induces the enhanced visible and NIR light absorption. We also checked the photocatalytic antibacterial property of the catalysts under simulated solar light irradiation (Fig. S8 in Supporting information). After irradiation of simulated solar light for 120min, theE. coli sterilization rate of TiO2 NBs and Au NR/TiO2 NB heterostructures is 59% and 100%, respectively.
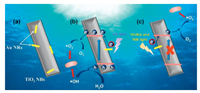
On the basis of the above discussion, a possible photocatalytic antibacterial mechanism over Au NR/TiO2 NB heterostructures is proposed in Scheme 1. Under UV light illumination (Scheme 1b), both TiO2 NBs and Au NRs are excited. The hot electrons of Au NRs leap over the Schottky barrier to TiO2 NBs and then captured by dissolved oxygen in aqueous solution to produce ·O2–. The photoexcited holes in the VB of TiO2 NBs move to the surface and react with H2O to produce ·OH, finally participate in the inactivation of E. coli cells. Under visible and NIR light illumination (Scheme 1c), TiO2 NBs can only work as a semiconductor upon excitation with UV light and is therefore inactive when excited with visible and NIR light. Only Au NRs get the energy to generate hot electrons. Therefore, when TiO2 NBs and plasmonic Au NRs are in contact, the hot electrons from Au NRs can be injected into the CB of TiO2 NBs to produce ·O2–, promoting the charge transfer process. The strong oxidizing reactive oxygen species (ROS, e.g. ·O2– and ·OH) are generated in the photocatalytic reaction of Au NR/TiO2 NB heterostructures, confirmed by the reactive species trapping experiments (Fig. S9 in Supporting information), which triggers oxidative stress and cell damage in bacteria [20, 21].
|
Download:
|
| Scheme 1. The possible mechanism for enhancing photocatalytic antibacterial activity of Au NR/TiO2 NB heterostructures. | |
Besides, because the TSPR and LSPR wavelength of Au NRs is located in the visible and NIR region, the TiO2 NB will display significant absorption in this region (Fig. 2c) in the photocatalytic process. So it significantly improves the full solar light photocatalytic antibacterial activity of the heterostructure. Therefore, the formed Au NR/TiO2 NB heterostructures (Scheme 1a) as well as TSPR and LSPR effects of Au NRs play important roles to improve the photocatalytic antibacterial performance by promoting the separation of carriers and improving visible and NIR light absorption.
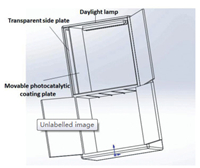
Inspired the excellent photocatalytic antibacterial activity of Au NR/TiO2 NB heterostructures, we design a sterilization file cabinet with Au NR/TiO2 NB heterostructures as the photocatalytic coating plates (Scheme 2). As shown in Scheme 2, the sterilization file cabinet includes the file cabinet body, the movable photocatalytic coating plates and the transparent side plates. The movable photocatalytic coating plates are parallel to the side plates. The movable photocatalytic coating plates are detachably mounted in the file cabinet body, in which the Au NR/TiO2 NB heterostructures are coated on the plates and can be used to absorb dust and sterilize bacteria under the light illumination. The side plates are set to be transparent so that the light can penetrate through and irradiate on the movable photocatalytic coating plates in the daytime. The daylight lamps are further arranged in the file cabinet body and can provide light to irradiate the movable photocatalytic coatings in the evenings.
|
Download:
|
| Scheme 2. Schematic illustration of sterilization file cabinet with Au NR/TiO2 NB heterostructures as the photocatalytic coating panels. | |
In summary, we have synthesized a novel Au nanorod/TiO2 nanobelt (Au NR/TiO2 NB) heterostructure via a hydrothermal method, followed by a photoreduction approach for preferentially loaded Au NRs. The results show that when Au NRs are combined with TiO2 NBs, the visible and NIR absorption of TiO2 NBs is significantly improved. Under light illumination, the visible and NIR light is utilized by the Au NRs via the TSPR and LSPR effect to generate and inject hot electrons into the conduction band of TiO2. These hot electrons, together with the photo-excited electrons and holes in the TiO2 NBs irradiated by the UV light, would react with O2 and H2O to generate produce a large amount of ROS, which can bring serious damage to bacterial membranes, resulting in bacterial death. Due to this reason, the Au NR/TiO 2 NB heterostructures present enhanced full solar spectrum (UV, visible and NIR) photocatalytic antibacterial properties. Inspired this, we also design a sterilization file cabinet with Au NR/TiO2 NB heterostructures as the photocatalytic coating plates. This Au NR/TiO2 NB heterostructures can be prepared easily, and it can kill bacteria efficiently. Therefore, it is a potential candidate for sterilization of bacteria and archives protection.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThe authors are thankful for fundings from the National Natural Science Foundation of China (Nos. 51872173, 51772176), Taishan Scholars Program of Shandong Province (Nos. tsqn201812068, tspd20161006), Higher School Youth Innovation Team of Shandong Province (No. 2019KJA013), Key Research and Development Program of Shandong Province (No. 2018GGX102028), Science and Technology Special Project of Qingdao City (No. 20-3-4-3-nsh), the Opening Fund of State Key Laboratory of Heavy Oil Processing (No. SKLOP202002006), Cooperative Education Project of the Ministry of Education (No. 201902195026), Humanities and Social Sciences Program (GoMoruo Studies) of the Education Department of Sichuan Province (No. GY2020C01), and Shandong Archives Science and Technology Project (No. 2020-33).
Appendix A. Supplementary dataSupplementarymaterial related to this article can befound, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.10.020.
| [1] |
H. Li, J. Zhong, H. Zhu, et al., ACS Appl. Bio Mater. 2 (2019) 4892-4903. DOI:10.1021/acsabm.9b00644 |
| [2] |
B.E. Nagay, C. Dini, J.M. Cordeiro, et al., ACS Appl. Mater. Interfaces 11 (2019) 18186-18202. DOI:10.1021/acsami.9b03311 |
| [3] |
J. Xu, N. Liu, D. Wu, et al., ACS Nano 14 (2020) 337-346. DOI:10.1021/acsnano.9b05386 |
| [4] |
J. Xu, X. Zhou, Z. Gao, Y.Y. Song, P. Schmuki, Angew. Chem. Int. Ed. 128 (2016) 603-607. DOI:10.1002/ange.201508710 |
| [5] |
J. Zhao, J. Xu, X. Jian, et al., ACS Appl. Mater. Interfaces 12 (2020) 23606-23616. DOI:10.1021/acsami.0c04260 |
| [6] |
Z. Liang, X. Bai, P. Hao, et al., Appl. Catal. B-Environ. 243 (2019) 711-720. DOI:10.1016/j.apcatb.2018.11.017 |
| [7] |
J. Xu, Y. Zhang, T.T. Zhai, et al., ACS Nano 12 (2018) 6895-6903. DOI:10.1021/acsnano.8b02292 |
| [8] |
H. Tian, X. Liu, Z. Liang, et al., J. Colloid Interf. Sci. 557 (2019) 700-708. DOI:10.1016/j.jcis.2019.09.075 |
| [9] |
J. Pan, L. Zhang, S. Zhang, et al., ACS Appl. Nano Mater. 2 (2019) 1516-1524. DOI:10.1021/acsanm.9b00002 |
| [10] |
X. Hu, J. Tian, Y. Xue, Y. Li, H. Cui, ChemCatChem 9 (2017) 1511-1516. DOI:10.1002/cctc.201601719 |
| [11] |
J. Tian, X. Hu, N. Wei, et al., Sol. Energy Mater. Sol. Cells 151 (2016) 7-13. DOI:10.1016/j.solmat.2016.02.017 |
| [12] |
L. Ding, S. Yang, Z. Liang, et al., J. Colloid Interf. Sci. 567 (2020) 181-189. DOI:10.1016/j.jcis.2020.02.014 |
| [13] |
Y. Liu, F. Chen, Q. Wang, et al., Nanoscale 11 (2019) 8812-8824. DOI:10.1039/c9nr00361d |
| [14] |
Z. Li, L. Gao, W. Ma, Q. Zhong, Appl. Surf. Sci. 497 (2019) 143749. DOI:10.1016/j.apsusc.2019.143749 |
| [15] |
Y. Guo, X. Kong, Z. Liang, et al., J. Colloid Interf. Sci. 571 (2020) 412-418. DOI:10.1016/j.jcis.2020.03.066 |
| [16] |
Y. Li, L. Ding, Z. Liang, Y. Xue, H. Cui, J. Tian, Chem. Eng. J. 383 (2020) 123178. DOI:10.1016/j.cej.2019.123178 |
| [17] |
X. Xu, Y. Guo, Z. Liang, H. Cui, J. Tian, Int. J. Hydrogen Energ. 44 (2019) 27311-27318. DOI:10.1016/j.ijhydene.2019.08.174 |
| [18] |
D. Li, J. Lao, C. Jiang, et al., Int. J. Hydrogen Energ. 44 (2019) 30876-30884. DOI:10.1016/j.ijhydene.2019.10.041 |
| [19] |
Y. Cai, M. Strømme, K. Welch, PLoS One 8 (2013) e75929. DOI:10.1371/journal.pone.0075929 |
| [20] |
B. Moongraksathum, J.Y. Shang, Y.W. Chen, Catalysts 8 (2018) 352. DOI:10.3390/catal8090352 |
| [21] |
P. Li, M. Guo, Q. Wang, et al., Appl. Catal. B -Environ. 259 (2019) 118107. DOI:10.1016/j.apcatb.2019.118107 |
 2021, Vol. 32
2021, Vol. 32 

