Temperature is the most frequently measured fundamental parameter and plays critical roles in many biological and biotechnological processes [1, 2]. Generally, cellular activities and events including cell division, gene expression and enzymatic reactions can be affected by the temperature of living cells. Medical studies have also suggested some pathological cells show higher temperatures than normal cells because of their faster metabolic rates [3, 4]. For a cell or tissue, temperature variations of even a few degrees may mean the difference between life and death. Therefore, the precise and real-time sensing of the temperature within the physiological range would facilitate the biomedical diagnostic and treatment processes [5-7]. The development of fluorescent thermometry is a popular approach to measure intracellular temperature in biomedicine due to their unique features of noninvasive, high sensitivity, fast response [8]. Although several luminescent probes based on fluorescent dyes, quantum dots and metal complexes or clusters have been developed for this purpose [9-12], they suffer from one or more limitations, including cytotoxicity, photobleaching and UV-excited intracellular auto-fluorescence disturbance. In addition, such temperature sensors usually operate by exploiting temperature-dependent luminescence intensity mechanism [13]. However, the measurement of luminescence intensity is also heavily affected by any changes in probe concentration, excitation or detection efficiency, thus reducing the sensing accuracy.
In order to overcome this limitation, some ratiometric thermometers based on the luminescence intensity ratio of two emission centers were developed. In previous study, we have demonstrated the luminescent ratiometric thermometers by rationally assembling different lanthanide ions (e.g., Tb3+ and Eu3+ or Yb3+ and Nd3+) with organic bridging linkers into the isostructural metal-organic frameworks (MOFs) [14-22] or encapsulating the organic dyes within the pores of lanthanide MOF [23, 24]. Because these MOF thermometers are excited in UV–vis range, the strong auto-fluorescence from biological tissues and low penetration depth hinder their application in biomedical field. Two-photon excited fluorescence is a third-order nonlinear opticical (NLO) behavior, which has strong ability to penetrate the tissues with weak light absorption and scattering effects compared with one-photon fluorescence [25-27]. In addition, two-photon fluorescence can reduce the light injury, light toxicity and prevent photo bleaching because it is pumped by near-infrared light. Such attractive features make two-photon excited fluorescent materials promising as biosensors and in vivo imaging. However, to date, ratiometric thermometers based two-photon excited fluorescence has not yet been developed. Considering the second harmonic generation (SHG) response, which is also a type of nonlinear optical behaviour, remains stable at most conditions unless crystal structure changes [28], it can be used as a reference signal to realized ratiometric thermometry. Thus, in this paper, we demonstrate a novel ratiometric thermometer by encapsulating a two-photon fluorescence dye 4-(4-(dimethylamino)styryl)-1-ethylpyridinium (DMASE) into a porous MOF, Zn-TCOMA, to form the MOF⊃dye composite, and utilizing the intensity ratio between the two-photon excited fluorescence from the dye DMASE and SHG signal of the MOF Zn-TCOMA to achieve temperature reading. As expected, the Zn-TCOMA⊃DMASE combine the advantages of two-photon excited fluorescence without auto-fluorescence and SHG with stable signal, and display high water stability, good biocompatibility and strong temperature dependence in intensity ratio over the physiological range (20~60 ℃) when excited at 1064 nm. These properties make the Zn-TCOMA⊃DMASE superior ratiometric thermometer for sensing of physiological temperatures.
Reaction of the ligand tetrakis[4-(carboxyphenyl)oxamethyl]methane acid (H4TCOMA) with Zn(NO3)3 via a solvothermal method affords the colorless crystals of Zn2TCOMA(H2O)·(H2O)(DMF)0.5 (Zn-TCOMA). As shown in Fig. 1, Zn-TCOMA crystallizes Fdd2 space group, which is a point group with no symmetry. Zn2+ has two modes of coordination, one is each Zn2+ connected to four O atoms originated from three different ligands and a H2O molecule. The other is connected to five O atoms originated from four different ligands and a H2O molecule (Scheme S1b in Supporting information). There is a one-dimensional penetrating channel with pore size of 0.39 nm and the interpenetration structure is composed of two sets of frameworks with the same architecture but different directions (Schemes S1c and d in Supporting information).
|
Download:
|
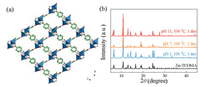
| Fig. 1. (a) Three-dimensional structure composed of two independent framework of Zn-TCOMA. (b) PXRD patterns of Zn-TCOMA and Zn-TCOMA after being treated with different pH aqueous solutions at 100 ℃ for one day. | |
Crystal powder X-ray diffraction (PXRD) test was conducted on the synthesized Zn-TCOMA (Fig. S1 in Supporting information). PXRD patterns of synthesized Zn-TCOMA are consistent with one simulated from single crystal, indicating that Zn-TCOMA was synthesized successfully. The stability of Zn-TCOMA was characterized and it was shown that Zn-TCOMA was intact in H2O environment whether with strong acid or strong alkali or even at boiling environment, which expanding its scope of application in some extent and it is rare in category of MOFs with Zn as metal atom (Fig. 1 and Fig. S2 in Supporting information) [29]. Through the analysis of its structure, we conclude that its excellent chemical stability is attributed to the following factors: (1) the framework forms a double interpenetrating structure through the flexible ligand, which makes the structure stable, and part of the hydrophilic surface in one network can be covered by the hydrophobic phenyl ligand in the other network, which increases the hydrophobicity of the framework. (2) There are few hydrogen bond donor sites on the pore surface, so it can be considered as hydrophobic MOFs. (3) The oxygen atom on the ether bond in the ligand can be regarded as an effective hydrogen bond receptor, making a great contribution to the high acid-base stability of Zn-TCOMA. The oxygen on the side chain prevents the attack of H+/OH- from the external environment, further limiting the dissociation of the coordination bond around the connection site.
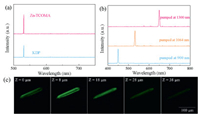
The second-order nonlinear optical properties of Zn-TCOMA crystals are evaluated by compared with the commonly used nonlinear optical crystal potassium dihydrogen phosphate (KDP). Fig. 2 is nonlinear optics property of Zn-TCOMA and KDP with the same size of 170–240 meshes. The SHG intensity of Zn-TCOMA is 1.3 times of KDP, and this is pretty strong in MOFs reported because of its particular ligand [30]. As we can see from Scheme S1a (Supporting information), the ligand L has four symmetrical flexible branched chain and alkoxy group provides effective electron transport as electron donor cooperated with carboxyl as strong electron withdrawal group. Moreover, the diamonded topology provides more effective polarization and electron transport than 1-D chains or 2-D frameworks, and effectively enhances the SHG effect [31]. Besides, the interpenetration of two identical frameworks increases the SHG performance of the material to a certain extent as well [32]. The microscope picture of Zn-TCOMA was photographed at 1064 nm laser pumped. Green spot can be seen by naked eye in one columnar crystal, representing an emission light of 532 nm wavelength (Scheme S6c in Supporting information). Moreover, 450 nm and 650 nm signal can be collected in Zn-TCOMA at 900 nm and 1300 nm pumped, respectively (Fig. 2), and the corresponding picture were obtained as blue and red light by naked eye (Schemes S6a and e in Supporting information). In addition, from the confocal laser scanning microscope picture, the SHG signal in Zn-TCOMA is uniform in all scanning depth (Fig. 2c). Similarly, Zn-TCOMA⊃DMASE composite was obtained using the same method as synthesis of Zn-TCOMA but addition of different amount of the two-photon fluorescence dye DMASE. The dye DMASE has a size of 0.31×0.46×1.64 nm3 and possesses high conjugation degree and efficient electron transport pathways, resulting big two-photon absorption (TPA) cross section and two-photon fluorescence (TPF) intensity [33, 34]. The XRD patterns of Zn-TCOMA and Zn-TCOMA⊃DMASE are in good agreement, which indicates that encapsulation of dye does not influence crystallization structure (Fig. S3 in Supporting information). The thermal stability of dye is improved almost 140 ℃ after loading according to the thermo-gravimetric analysis and the compound can be stabilized up to 400 ℃, expanding its scope of application and it is feasible for the sensing of physiological temperature (Fig. S5 in Supporting information). Additionally, pH stability of Zn-TCOMA⊃DMASE composite was experimented as well and the result revealed that the composite is as stable as MOFs (Fig. S4 in Supporting information). Besides, it can also be stabilized in biological analogue solution like PBS for one day and might be able to be applied to the biological environment in the future.
|
Download:
|
| Fig. 2. (a) Nonlinear spectroscopy of Zn-TCOMA and KDP, pumped at 1064 nm laser. (b) Nonlinear spectroscopy of Zn-TCOMA, pumped at 900 nm, 1064 nm and 1300 nm. (c) The confocal laser scanning microscope picture of Zn-TCOMA in a depth scanning mode, pumped at 1000 nm laser. | |
The one-photon fluorescence and the quantum efficiency of Zn-TCOMA, DMASE and Zn-TCOMA⊃DMASE at room temperature were studied (Fig. S6 and Table S2 in Supporting information). The quantum efficiency of dye is obviously improved after loading, as a result of reduced fluorescence quenching caused by the interaction between dye molecules, therefore improving the quantum efficiency of the dye. However, when the dye content continues to increase, the aggregation caused quenching (ACQ) effect will play a role, resulting in quantum efficiency decreasing. The fluorescent photograph of Zn-TCOMA⊃DMASE was collected at 436 nm excitation by fluorescence microscope and an orange crystal can be observed which is exactly accorded with the emission wavelength of Zn-TCOMA⊃DMASE (Scheme S3 in Supporting information). What is more, the steadiness of dye loaded in framework of Zn-TCOMA was characterized by testing the fluorescence spectra of the supernatant after soaking for a week (Scheme S4 in Supporting information). After immersing in DMF for a week, the supernatant shows the same fluorescence spectra as pure DMF and is colourless by naked eye, proving that dye has suitable size which can be encapsulated in framework and the composite is relatively stable to be utilized in practical application.
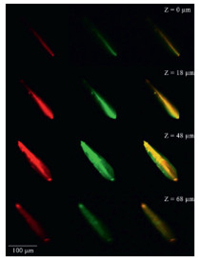
The nonlinear optical property of Zn-TCOMA⊃DMASE was measured in the same method taking KDP as the reference. It can be observed that Zn-TCOMA⊃DMASE samples simitanniously exhibits two-photon fluorescence emission at 599 nm and SHG signal originated from MOFs which is not affected by the encapsulation of dye (Fig. 3a). When pump wavelength increases, the multi-photon emission appeared and the corresponding SHG signal were not affected compared to Zn-TCOMA (Fig. 3b). The blue and orange lights were obvious in Zn-TCOMA⊃DMASE when pumped at 900 nm, while the red lights were also clear when pumped at 1300 nm (Schemes S6b and f in Supporting information). The uniformity of the dye distribution can be confirmed as shown in Fig. 4, the two-photon fluorescence of dye (red) and SHG signal of Zn-TCOMA (green) are uniform and basically overlapped in all scanning depth. Moreover, the nonlinear optical performance of the pure dye was tested and no two-photon fluorescence can be obtained in DMF solution, which further explaining the necessity of loading by MOFs (Fig. S7 in Supporting information) [24]. Considering that the two-photon fluorescence of Zn-TCOMA⊃DMASE is sensitive to temperature, a self-calibrated nonlinear optical thermometer can be designed.

|
Download:
|
| Fig. 3. (a) Nonlinear spectroscopy of Zn-TCOMA⊃DMASE and KDP, pumped at 1064 nm. (b) Nonlinear optics of Zn-TCOMA⊃DMASE, pumped at 900 nm, 1064 nm and 1300 nm. | |
|
Download:
|
| Fig. 4. The confocal laser scanning microscope picture of Zn-TCOMA⊃DMASE in a depth scanning mode, pumped at 1000 nm laser, The first column (red): signal of two-photon fluorescence of DMASE, the second column (green): signal of SHG of Zn-TCOMA, the third column: merge of red and green. | |
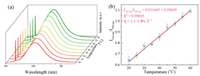
Ratiometric temperature sensing property of Zn-TCOMA⊃DMASE samples at physiological temperature region were investigated (Fig. 5). Pumping Zn-TCOMA⊃DMASE by 1064 nm pulse laser, the samples glow in orange light with two-photon emission peak near 600 nm. Due to the different nonlinear optical mechanism, the intensity of the SHG signal and the two-photon emission are not consistent with increasing of temperature. With the increasing of temperature, the SHG emission originated from MOFs crystal is basically stable because there is not much change in crystal structure of Zn-TCOMA in physiological temperature range. However, the intensity of the two-photon emission peak near 600 nm obviously decrease, attributed to the increasing of the stretching vibration and rotation of the dye molecules, which further results in fluorescence quenching. Taking Zn-TCOMA⊃DMASE (DMASE: 8.45 wt%) for example, there is a good linear relationship between the temperature and the ratio defined as SHG intensity divided by two-photon emission intensity pumped at 1064 nm in temperature range of 20~60 ℃ (Fig. 5b), expressed as follows:

|
(1) |
|
Download:
|
| Fig. 5. (a) Temperature-dependent nonlinear optics of Zn-TCOMA⊃DMASE (DMASE: 8.45wt%) in a range of 20~60 ℃, pumped at 1064 nm laser. (b) Relationship between ratio of SHG intensity divided by two-photon emission intensity and temperature. | |
Δ = I532 nm/I599 nm, I532 nm is the SHG emission intensity and I599 nm is the two-photon emission peak at 1064 nm pumped. T is Celsius temperature. Besides, we calculated the relative sensitivity of the material and its value is 1.1%~1.8% ℃-1. In addition, the fibre spectrometer is able to measure the changes of fluorescence intensity of 0.02%. According to this calculation, the resolution of Zn-TCOMA⊃DMASE (DMASE: 8.45 wt%) can achieve at 0.018 ℃ and can be applied to the measurement of the temperature in biology.
In order to verify the precision of nonlinear optical temperature sensing properties in physiological temperature range, we studied the ratio of emission intensity at 532 nm and 599 nm repeated and reversible changing with temperature. As shown in Fig. S8 (Supporting information), in five cycles from 20 ℃ to 60 ℃, SHG and two-photon emission peak intensity ratio of Zn-TCOMA⊃DMASE with the change of temperature is reversible, indicating that the material can be reused. Temperature dependent emission spectra of Zn-TCOMA⊃DMASE (DMASE: 7.54 wt%) and Zn-TCOMA⊃DMASE (DMASE: 20.22 wt%) pumped at 1064 nm were also noted down for proving that this material could be commonly utilized with different loaded concentrations of DMASE (Figs. S9 and S10 in Supporting information). They all have good linearity of the fitting line and relatively high relative sensitivity, showing that this system is universal for temperature sensing. Cytotoxicity (Fig. S11 in Supporting information) were tested for studying the potential application of Zn-TCOMA⊃DMASE in biology. Taking adrenal nerve tumour cells (PC12 cells) of mouse as research object, with different content of DMASE or Zn-TCOMA⊃DMASE (5~100 μg/mL), the cell survival rate of samples treated with Zn-TCOMA⊃DMASE are above 80% and are higher than that of samples treated with DMASE, indicating biocompatibility of the material is very good and the cell toxicity of the DMASE can be reduced by loading. In order to observe the cell survival state and the optical signal of Zn-TCOMA⊃DMASE in the cell more intuitively, the confocal laser scanning microscope picture of Zn-TCOMA⊃DMASE co-cultured with PC12 cell for 4 h were obtained, displaying that the cells grow well and Zn-TCOMA⊃DMASE can generate SHG and two-photon fluorescence signals simultaneously in the cell environment. The excellent biocompatibility of Zn-TCOMA⊃DMASE makes it possible for application in vivo sensing/imaging.
In summary, a MOF Zn-TCOMA with good SHG response was synthesized and exhibits excellent thermal and pH stability which is rare in Zn-based MOFs. When encapsulated by a two-photon fluorescent dye DMASE into the pores, the composite Zn-TCOMA⊃DMASE was obtained. Utilizing the intensity ratio between two-photon fluorescence of DMASE and SHG signal of Zn-TCOMA, Zn-TCOMA⊃DMASE exhibits ratiometric temperature sensing property at physiological temperature region of 20~60 ℃ with high sensitivity. This MOF thermometer also shows excellent repeatability, good biocompatibility, and high temperature resolution of 0.018 ℃, opening a new avenue to develop diverse optical thermometric or thermographic applications in biotechnology or other areas.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 51632008, 51772268, U1609219 and 61721005) and Zhejiang Provincial Natural Science Foundation of China (No. LD18E020001).
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.10.015.
| [1] |
X.D. Wang, O.S. Wolfbeis, R.J. Meier, Chem. Soc. Rev. 42 (2013) 7834-7869. DOI:10.1039/c3cs60102a |
| [2] |
C.D.S. Brites, P.P. Lima, N.J.O. Silva, et al., Adv. Mater. 22 (2010) 4499-4504. DOI:10.1002/adma.201001780 |
| [3] |
J. Qiao, Y.H. Hwang, D.P. Kim, L. Qi, Anal. Chem. 92 (2020) 8579-8583. DOI:10.1021/acs.analchem.0c01534 |
| [4] |
G. Ke, C. Wang, Y. Ge, et al., J. Am. Chem. Soc. 134 (2012) 18908-18911. DOI:10.1021/ja3082439 |
| [5] |
E.N. Ceron, D.H. Ortgies, B. Del Rosal, et al., Adv. Mater. 27 (2015) 4781-4787. DOI:10.1002/adma.201501014 |
| [6] |
A.M. Kaczmarek, M.K. Kaczmarek, R.V. Deun, Nanoscale 11 (2019) 833-837. DOI:10.1039/C8NR08348G |
| [7] |
R.N. Bastos, C.D.S. Brites, P.A. Rojas-Gutierrez, et al., Adv. Funct. Mater. 29 (2019) 1905474. DOI:10.1002/adfm.201905474 |
| [8] |
H.S. Wang, Coord. Chem. Rev. 349 (2017) 139-155. DOI:10.1016/j.ccr.2017.08.015 |
| [9] |
A.E. Albers, E.M. Chan, P.M. McBride, et al., J. Am. Chem. Soc. 134 (2012) 9565-9568. DOI:10.1021/ja302290e |
| [10] |
A. Cadiau, C.D. Brites, P.M. Costa, et al., ACS Nano 7 (2013) 7213-7218. DOI:10.1021/nn402608w |
| [11] |
E.J. McLaurin, V.A. Vlaskin, D.R. Gamelin, J. Am. Chem. Soc. 133 (2011) 14978-14980. DOI:10.1021/ja206956t |
| [12] |
D. Ananias, F.A. Paz, D.S. Yufit, L.D. Carlos, J. Rocha, J. Am. Chem. Soc. 137 (2015) 3051-3058. DOI:10.1021/ja512745y |
| [13] |
D. Jaque, F. Vetrone, Nanoscale 4 (2012) 4301-4326. DOI:10.1039/c2nr30764b |
| [14] |
Y. Zhang, H. Dong, Y. Liu, et al., Chem. Commun. 55 (2019) 3445-3448. DOI:10.1039/C8CC10232E |
| [15] |
Y. Yao, Z. Gao, Y. Lv, et al., Angew. Chem. Int. Ed. 58 (2019) 13803-13807. DOI:10.1002/anie.201907433 |
| [16] |
Y. Zhang, S. Yuan, G. Day, et al., Coord. Chem. Rev. 354 (2018) 28-45. DOI:10.1016/j.ccr.2017.06.007 |
| [17] |
Y. Cui, J. Zhang, H. He, G. Qian, Chem. Soc. Rev. 47 (2018) 5740-5785. DOI:10.1039/C7CS00879A |
| [18] |
J. Hao, X. Xu, H. Fei, L. Li, B. Yan, Adv. Mater. 30 (2018) 1705634. DOI:10.1002/adma.201705634 |
| [19] |
H. Liu, C. Xu, D. Li, H.L. Jiang, Angew. Chem. Int. Ed. 57 (2018) 5379-5383. |
| [20] |
X. Fang, Q. Shang, Y. Wang, et al., Adv. Mater. 30 (2018) 1705112. DOI:10.1002/adma.201705112 |
| [21] |
W.P. Lustig, S. Mukherjee, N.D. Rudd, et al., Chem. Soc. Rev. 46 (2017) 3242-3285. DOI:10.1039/C6CS00930A |
| [22] |
Y. Cheng, Y. Ying, S. Japip, et al., Adv. Mater. 30 (2018) 1802401. DOI:10.1002/adma.201802401 |
| [23] |
Y. Cui, H. Xu, Y. Yue, et al., J. Am. Chem. Soc. 134 (2012) 3979-3982. |
| [24] |
Y. Cui, R. Song, J. Yu, et al., Adv. Mater. 27 (2015) 1420-1425. DOI:10.1002/adma.201404700 |
| [25] |
B. Situ, M. Gao, X. He, et al., Mater. Horiz. 6 (2019) 546-553. DOI:10.1039/C8MH01293H |
| [26] |
Y. Wan, T. Xia, Y. Cui, Y. Yang, G. Qian, ChemPlusChem 82 (2017) 1320-1325. DOI:10.1002/cplu.201700438 |
| [27] |
J. Su, J. Zhang, X. Tian, et al., J. Mater. Chem. B 5 (2017) 5458-5463. DOI:10.1039/C6TB03321K |
| [28] |
R.L. Tang, C.L. Hu, F.F. Mao, J.H. Feng, J.G. Mao, Chem. Sci. 10 (2019) 837-842. DOI:10.1039/C8SC04342F |
| [29] |
S.S. Kaye, A. Dailly, O.M. Yaghi, J.R. Long, J. Am. Chem. Soc. 129 (2007) 14176-14177. DOI:10.1021/ja076877g |
| [30] |
C. Wang, T. Zhang, W. Lin, Chem. Rev. 112 (2012) 1084-1104. DOI:10.1021/cr200252n |
| [31] |
D.W. Fu, W. Zhang, R.G. Xiong, Dalton Trans. 30 (2008) 3946-3948. |
| [32] |
L.R. Mingabudinova, V.V. Vinogradov, V.A. Milichko, E. Hey-Hawkins, A.V. Vinogradov, Chem. Soc. Rev. 45 (2016) 5408-5431. DOI:10.1039/C6CS00395H |
| [33] |
H. Dong, C. Zhang, J. Yao, Y.S. Zhao, Chem. Asian J. 11 (2016) 2656-2661. DOI:10.1002/asia.201600387 |
| [34] |
H.S. Kim, K.W. Sohn, Y. Jeon, et al., Adv. Mater. 19 (2007) 260-263. DOI:10.1002/adma.200602101 |
 2021, Vol. 32
2021, Vol. 32 

