b School of Environment, Nanjing Normal University, Nanjing 210023, China;
c Jiangsu Key Laboratory for Molecular and Medical Biotechnology, School of Life Sciences, Nanjing Normal University, Nanjing 210023, China
Cellulosic biomass is the largest source of organic matter on earth, offering a renewable feedstock for production of biofuels and chemicals that address the issues of energy sustainability and environmental protection [1, 2]. The main bottleneck in cellulosic industry is the low conversion efficiency of cellulose hydrolysis, leading to high costs for production [3-5]. To better understand the bioconversion mechanism and improve the conversion rate, it is imperative but challenging to develop ultrasensitive method for cellulase activity assay, which is paramount to monitor the saccharification process.
Currently, the filter paper assay (FPA) [6], carboxymethyl cellulase assay for endo- β-1, 4 glucanase [7-9] and fluorescent assay [10] are the most employed methods for cellulase activity tests. In these methods, cellulase activity is either detected based on the initial hydrolysis rate or the end-point accumulation of hydrolytic products [11, 12]. Their performance is hampered by the limited enzyme accessibility to the substrate [13, 14], and the complexity of cellulase enzyme systems (synergy or competition) [11, 15]. As a result, many of these methods are time-consuming, labour-intensive, and costly. Although improvements have been made in newly developed approaches such as miniaturized colorimetric assay, automated FPA, and the combination with quartz crystal microbalance (QCM) technique [10, 16-18], some important issues are not well addressed in these assays. Especially, the sensitivity and reproducibility are inadequate when characterizing newly isolated cellulases [17]. Because most isolated natural cellulase complexes tend to have a shortage of downstream β-glucosidase activity, which may result in product inhibitory effect on the upstream hydrolytic enzymes in the cellulase complex ( e.g., endoglucanases (EGs) and cellobiohydrolases (CBHs)). The activity of EGs and CBHs could not be detected when the cellulose is not degraded into reductive sugars [17, 19].
Unlike the above mentioned methods, electrochemical sensor that fabricated at the solid/liquid interface on an electrode [20-25], has unique merits for the assay of cellulase activity. Firstly, the product inhibition effect can be reduced by removing hydrolytic products via interfacial diffusion process. Additionally, it possesses high sensitivity, fast response, low cost, and designable sensing interface [26-32]. To the best of our knowledge, electrochemical techniques are rarely used for the assay of cellulase activity [33, 34], and some important issues ( e.g., limited enzyme accessibility to cellulosic materials and reproducibility) are not well addressed until now. Considering the above merits of electrochemical sensors and further utilizing the dynamic linkage between boronic acid and sugar [35] that triggered by pH, in this work, we try to create the boronate-modified cellulose nano-network interface and fabricate an ultrasensitive electrochemical sensor with the aim to efficiently detect the cellulase activity.
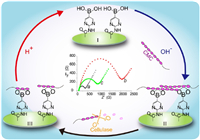
As shown in Scheme 1, boronic acid is first immobilized at the surface of a glassy carbon electrode (GCE). The celluloses are bonded to boronic acid under alkaline condition, forming a substrate nano-network layer through covalent linkage between boronic acids and sugar chains. The carboxymethyl cellulose (CMC), an anionic cellulose polymer, is selected as a general substrate of cellulases. Coupled with a negatively charged electrochemical indicator, ferricyanide ([Fe(CN)6]3-/4-), the electrochemical signals are enhanced on the basis of electrostatic repulsion and steric hindrance effects between [Fe(CN)6]3-/4- and CMC. Hence, impedance effect at the electrode surface will be enhanced and recorded by the electrochemical impedance spectroscopy (EIS) in a label-free manner. The nano-network causes a significant impedance effect at the sensing interface. The subsequent degradation of the nano-network that catalyzed by cellulase may reduce the impedance effect. Thus, a relationship between EIS signal and cellulase activity will be established. To regenerate the sensing nanointerface, acidic treatment of the electrode can break the covalent bonding of cellulose [36-38]. That is to say, the sensor can be easily renewable triggered by the pH value.
|
Download:
|
| Scheme 1. Schematic illustration of the renewable electrochemical sensor for cellulase activity assay. | |
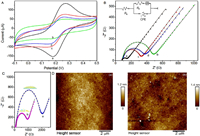
The stepwise modification of the boronate-affinity electrode surface were characterized by cyclic voltammetry (CV) and EIS measurements. As shown in Fig. 1A, the bare GCE exhibits a pair of stable and well-defined redox peaks (curve a). After being electrochemical oxidized, the carboxylated GCE results in a CV with obvious peak current decreasing (curve b), due to the inhibition effect towards electron transfer caused by the resistance of carboxyl groups at the GCE surface. After GCE has been modified with boronic acid pinacol ester, the related CV (curve c) shows the higher peak currents than that of carboxylated GCE. Further treatment of the electrode in H2SO4 will make the peak currents decrease (curve d), indicating the transformation from ester to boronic acid.
|
Download:
|
| Fig. 1. (A) The CV curves of bare GCE (a), carboxylated GCE (b), boronic acid pinacol ester modified GCE (c), and boronic acid modified GCE (d) in 10 mmol/L [Fe(CN)6]3-/4- containing 0.1mol/L KCl, respectively. (B) The corresponding experimental (dot) and fitted (line) EIS spectra for bare GCE (a), carboxylated GCE (b), boronic acid pinacol ester modified GCE (c), and boronic acid modified GCE (d) in the same electrolyte. The inset shows the related equivalent circuit: Rs is the ohmic resistance of the electrolyte; Rp is the polarization resistance, means the electron transfer resistance; CPE is the constant phase element; and W is the Warburg impedance. (C) The experimental (dot) and fitted (line) EIS spectra for the fabricated CMC-modified sensor (a), and the sensor interface after digestion with cellulase solution (1 mU/mL) for 1h at room temperature (b). (D) The AFM images for the mimicked CMC-modified sensor that fabricated on silicon wafer with the sputtering of Au (a), and the mimicked sensor after digestion with cellulase solution (1 mU/mL) for 1h at room temperature (b). | |
To further verify the successful construction of the boronate-affinity electrode surface, the EIS tests are also employed to provide the corroborative evidence for the stepwise changes of the modified electrode surface. As shown in Fig. 1B, the oxidation treatment of the GCE electrode causes the increase of polarization resistance ( Rp), showing an obvious variation of the semicircle diameter ( Rp=76 Ω with curve a, and Rp=376 Ω with curve b in Fig. 1B). This indicates that carboxylic acid (-COOH) groups have been introduced onto the GCE surface, as the negatively charged -COOH groups may cause electrostatic repulsion effect to the redox indicator, [Fe(CN)6]3-/4-. When 2-(aminopyrimidin-5-yl)boronic acid pinacol ester reacts with the -COOH group to form the 2-(aminopyrimidin-5-yl)boronic acid pinacol ester modified GCE, the Rp value (247 Ω) is greatly reduced (curve c in Fig. 1B). After further treatment in H2SO4, 2-aminopyrimidin-5-yl)boronic acid pinacol ester will be transformed into 2-aminopyrimidine-5-boronic acid, which causes the slight increase of Rp value (294 Ω, curve d in Fig. 1B). The amount of boronate on the electrode surface were assessed through utilizing the boronic acid-glucose-ferroceneboronic acid sandwich-type electrochemical test [39]. Assuming the amount of boronate on the electrode surface and the bonded ferroceneboronic acid suits the 1:1 ratio, the approximate amount of boronate on the electrode surface is 1×1011 molecules in GCE surface (d = 3mm).
The modification of CMC on the electrode surface has been confirmed through EIS tests. After the incubation of boronic acid modified GCE with NaCMC, a big increase in the semicircle diameter can be observed ( Rp=2086 Ω with curve a in Fig. 1C), indicating the successful grafting of celluloses via boronate-affinity on the GCE surface. To test the feasibility of the CMC grafted electrochemical impendence sensor towards cellulase activity, the CMC-modified GCE is further incubated with cellulase solution for 1h at room temperature. As shown in curve b in Fig. 1C, the obvious decrease of semicircle diameter can be seen ( Rp=1026 Ω), which results from the digestion of CMC that promotes the interfacial electron transfer. The related atomic force microscopy (AFM) images (Fig. 1D) can further confirm the formation of CMC nano-network and the digestion of CMC nano-network by cellulase treatment. To mimic the electrode surface, the silicon wafer that sprayed with gold is chosen as the substrate, on which the mercaptobenzoic acid is casted for providing the boronate-affinity sites. The subsequent NaCMC modification and cellulase digestion are the same as that of the actual electrode fabrication process. The imaging results demonstrate that a nano-network is formed on the substrate (Fig. 1D, image a) and the cellulase digestion leads to the reduction of surface height (Fig. 1D, image b), thus changing the surface geography. That is to say, both the EIS tests and AFM characterization verify the feasibility on fabrication of electrochemical impendence sensor towards cellulase activity assay.
After optimizing the digestion temperature and time (Fig. S1 in Supporting information, ), the CMC nano-network modified sensor has been employed to quantitatively examine the cellulase activity. As shown in Fig. 2A, the EIS response of the CMC-modified sensor varies with different cellulase activities. The corresponding relationship between Rp value and cellulase activity is illustrated in Fig. 2B. As can be seen, the Rp value decreases with the increase of cellulase activity from 5×10-8 U/mL to 0.1 U/mL. The Rp value is linearly related to the logarithm of cellulase activity with the detection range from 10-7 to 0.05 U/mL. And the related equation is Rp (Ω)=−99.32–247.1lg[cellulase activity (U/mL)]. These results reveal that our proposed CMC nano-network modified electrochemical impedance sensor can efficiently examine the cellulase activity with a wide detection range.

|
Download:
|
| Fig. 2. (A) The EIS response of the CMC-modified sensor towards different cellulase activities in 10 mmol/L [Fe(CN)6]3-/4- containing 0.1mol/L KCl. (a-k: 5×10-8, 1×10-7, 1×10-6, 1×10-5, 5×10-5, 1×10-4, 5×10-4, 1×10-3, 0.005, 0.05, 0.1 U/mL). (B) The calibration curve between the Rp value and cellulase activity. Each experiment was repeated three times to obtain the average data value. | |
In order to verify the accuracy of our proposed method, samples with different cellulase activities are measured by our electrochemical impedance sensor, and compared to the FPA method. As shown in Figs. S2 and S3 (Supporting information), the detection results obtained by our proposed sensor are almost in consistent with those measured by FPA method, showing a good accuracy. The slightly higher cellulase activity obtained by our proposed sensor may due to the better enzyme accessibility to the cellulosic nanointerface.
Five samples (spiked purified cellulase from Trichoderma sp.) were measured via our proposed sensor to compare the results of cellulase activity, and Sample No. 5 was also tested by FPA method. The results showed a good correlation (Fig. 3A), which further confirmed the promising practical usability of our electrochemical sensor.

|
Download:
|
| Fig. 3. (A) Comparison of cellulase activity of five spiked purified cellulase samples measured by our proposed sensor and FPA method. (B) The variation of electrochemical impedance after reversibly forming and breaking the cellulose nano-network at the electrode surface under the alkaline condition (pH 9.2) and acidic condition (pH 4.0), respectively. | |
On the other hand, the renewability is desirable in sensor construction, and is a key point to estimate the cost of sensor in the saccharification process. The renewability of our proposed electrochemical impedance sensor is further examined. Under the alkaline condition (pH 9.2), the CMC is grafted to the electrode surface through boronate-affinity interaction, showing a large interfacial electron transfer resistance. However, after acidic treatment (pH 4.0), the decrease of interfacial electron transfer resistance is observed. After washing several times and incubating with NaCMC under pH 9.2, the large interfacial electron transfer resistance will re-occur, implying the reforming of the cellulose nano-network at the electrode surface. As shown in Fig. 3B, the impedance can be reversibly switched at least 12 times, revealing that our electrochemical impedance sensor manifests the good renewability. The recycled electrodes exhibited satisfied sensing performance (Fig. S4 in Supporting information), and intra-assay coefficient variation (3.26% for 2×10-4 U/mL cellulase) also confirmed the electrode-to-electrode reproducibility.
In summary, a renewable electrochemical sensor for cellulase activity determination has been successfully fabricated by anchoring boronate-affinity sites at the electrode surface for bonding celluloses to generate nano-network-like sensing interface. Such sensor possesses the following advantages: (1) The boronate-affinity sites facilitate to capture the celluloses and generate nano-network-like motifs, which can increase the enzyme accessibility to the substrate; (2) the product inhibition effect is greatly reduced by removing hydrolytic products via interfacial diffusion process; (3) the dynamic linkage between boronic acids and celluloses modulated by pH makes the sensor renewable for reliable detection and cost savings with the market demand (one modified electrode can be renewably used at least 6 times, the preparation process is rather facile and does not need expensive reagents, and the low detection limit suits very well for the actual cellulases screen with low concentration). The fabrication process is facile and repeatable, and the obtained electrochemical sensor can be employed for ultrasensitive label-free detection of cellulase activity with a wide detection range, showing great potential for activity screen of new enzymes in saccharification conversion.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21625502, 21705079, 21671105 and 21974070) and the Natural Science Foundation of Jiangsu Province (Nos. BK20192008 and BK20171033). We appreciate the financial support from the PAPD.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.10.003.
| [1] |
B.E. Dale, J.E. Anderson, R.C. Brown, et al., Environ. Sci. Technol. 48 (2014) 7200-7203. DOI:10.1021/es5025433 |
| [2] |
A. Demirbas, Appl. Energy 86 (2009) S108-S117. DOI:10.1016/j.apenergy.2009.04.036 |
| [3] |
M.E. Himmel, S.Y. Ding, D.K. Johnson, et al., Science 315 (2007) 804-807. DOI:10.1126/science.1137016 |
| [4] |
L.R. Lynd, P.J. Weimer, W.H. van Zyl, I.S. Pretorius, Microbiol. Mol. Biol. Rev. 66 (2002) 506-577. |
| [5] |
D.S. Xue, J.B. Wang, S.J. Yao, Chin. Chem. Lett. 26 (2015) 1011-1015. DOI:10.1016/j.cclet.2015.05.019 |
| [6] |
H.L. Griffin, Anal. Biochem. 56 (1973) 621-625. DOI:10.1016/0003-2697(73)90234-0 |
| [7] |
E. Ximenes, Y. Kim, N. Mosier, B. Dien, M. Ladisch, Enzyme Microb. Technol. 48 (2011) 54-60. DOI:10.1016/j.enzmictec.2010.09.006 |
| [8] |
D.E. Eveleigh, M. Mandels, R. Andreotti, C. Roche, Biotechnol. Biofuels 2 (2009) 1-8. DOI:10.1186/1754-6834-2-1 |
| [9] |
W.J. Liu, J. Hong, D.R. Bevan, Y.H.P. Zhang, Biotechnol. Bioeng. 103 (2009) 1087-1094. DOI:10.1002/bit.22340 |
| [10] |
W. Helbert, H. Chanzy, T.L. Husum, M. Schulein, S. Ernst, Biomacromolecules 4 (2003) 481-487. DOI:10.1021/bm020076i |
| [11] |
Y.H.P. Zhang, M.E. Himmel, J.R. Mielenz, Biotechnol. Adv. 24 (2006) 452-481. DOI:10.1016/j.biotechadv.2006.03.003 |
| [12] |
B. Wu, Y. Zhao, P.J. Gao, BioResources 1 (2006) 189-200. DOI:10.15376/biores.1.2.189-200 |
| [13] |
M. Dashtban, M. Maki, K.T. Leung, C.Q. Mao, W.S. Qin, Crit. Rev. Biotechnol. 30 (2010) 302-309. DOI:10.3109/07388551.2010.490938 |
| [14] |
R.H. Atalla, D.L. Vanderhart, Science 223 (1984) 283-285. DOI:10.1126/science.223.4633.283 |
| [15] |
M. Dashtban, H. Schraft, W.S. Qin, Int. J. Biol. Sci. 5 (2009) 578-595. |
| [16] |
Z.Z. Xiao, R. Storms, A. Tsang, Biotechnol. Bioeng. 88 (2004) 832-837. DOI:10.1002/bit.20286 |
| [17] |
G. Coward-Kelly, C. Aiello-Mazzari, S. Kim, C. Granda, M. Holtzapple, Biotechnol. Bioeng. 82 (2003) 745-749. DOI:10.1002/bit.10620 |
| [18] |
G. Hu, J.A. Heitmann, O.J. Rojas, Anal. Chem. 81 (2009) 1872-1880. DOI:10.1021/ac802318t |
| [19] |
M. Maki, K.T. Leung, W.S. Qin, Int. J. Biol. Sci. 5 (2009) 500-516. |
| [20] |
C.Z. Zhu, G.H. Yang, H. Li, D. Du, Y.H. Lin, Anal. Chem. 87 (2015) 230-249. DOI:10.1021/ac5039863 |
| [21] |
J. Zhu, H.Y. Gan, J. Wu, H.X. Ju, Anal. Chem. 90 (2018) 5503-5508. DOI:10.1021/acs.analchem.8b01217 |
| [22] |
M. Li, M. Lv, L.H. Wang, C.H. Fan, X.L. Zuo, Curr. Opin. Electrochem. 14 (2019) 71-80. DOI:10.1016/j.coelec.2019.01.001 |
| [23] |
W. Zhang, R.G. Wang, F. Luo, P.L. Wang, Z.Y. Lin, Chin. Chem. Lett. 31 (2020) 589-600. DOI:10.1016/j.cclet.2019.09.022 |
| [24] |
Q. Zhou, G.H. Li, Y.J. Zhang, et al., Anal. Chem. 88 (2016) 9830-9836. DOI:10.1021/acs.analchem.6b02945 |
| [25] |
Q. Wang, Z. Nie, Y.F. Hu, S.Z. Yao, Acta Chim. Sinica 75 (2017) 1109-1114. DOI:10.6023/A17070321 |
| [26] |
Z.Q. Xu, P. Zhang, Y.Q. Chai, H.J. Wang, R. Yuan, Chem. Commun. 54 (2018) 8741-8744. DOI:10.1039/C8CC04953J |
| [27] |
T. Gao, F.Z. Liu, D.W. Yang, et al., Anal. Chem. 87 (2015) 5683-5689. DOI:10.1021/acs.analchem.5b00816 |
| [28] |
T.T. Yan, L.Y. Zhu, H.X. Ju, J.P. Lei, Anal. Chem. 90 (2018) 14493-14499. DOI:10.1021/acs.analchem.8b04338 |
| [29] |
S. Manavalan, J. Ganesamurthi, S.M. Chen, P. Veerakumar, K. Murugana, Nanoscale 12 (2020) 5961-5972. DOI:10.1039/C9NR09148C |
| [30] |
Y.Y. Zhang, Y.X. Song, Y.F. Shen, et al., CCS Chem. (2020). DOI:10.31635/ccschem.020.202000361 |
| [31] |
Y.Q. Lv, S.Y. Chen, Y.F. Shen, et al., J. Am. Chem. Soc. 140 (2018) 2801-2804. DOI:10.1021/jacs.8b00515 |
| [32] |
Y.L. Ying, J.J. Wang, A.R. Leach, et al., Sci. China Chem. 63 (2020) 589-618. DOI:10.1007/s11426-020-9716-2 |
| [33] |
L. Hilden, L. Eng, G. Johansson, S.E. Lindqvist, G. Pettersson, Anal. Biochem. 290 (2001) 245-250. DOI:10.1006/abio.2000.4959 |
| [34] |
D. Fapyane, E.E. Ferapontova, Anal. Chem. 89 (2017) 3959-3965. DOI:10.1021/acs.analchem.6b04391 |
| [35] |
Z. Liu, H. He, Acc. Chem. Res. 50 (2017) 2185-2193. DOI:10.1021/acs.accounts.7b00179 |
| [36] |
G. Springsteen, B.H. Wang, Tetrahedron 58 (2002) 5291-5300. DOI:10.1016/S0040-4020(02)00489-1 |
| [37] |
M. Li, W.H. Zhu, F. Marken, T.D. James, Chem. Commun. 51 (2015) 14562-14573. DOI:10.1039/C5CC04976H |
| [38] |
C. Battilocchio, F. Feist, A. Hafner, et al., Nat. Chem. 8 (2016) 360-367. DOI:10.1038/nchem.2439 |
| [39] |
J. Li, Z.M. Bai, Y.J. Mao, et al., Electroanalysis 29 (2017) 2307-2315. |
 2021, Vol. 32
2021, Vol. 32 

