b Indian Institute of Technology Guwahati, Guwahati 781039, India
Emerging organic contaminants (EOCs) are a kind of contaminants that are not regularly detected but they have high tendency to spread into the environment, which exhibit great potential to cause adverse effects to the ecology and human health. The EOCs are mainly divided into endocrine disruptors, pharmaceuticals and personal care products, surfactants, and various industrial additives as well as hormones [1-3]. Bisphenol A (BPA), classified as an endocrine disruptor, is a tricky pollutant since it has been widely applied in the production of polycarbonates, epoxy resins, and other plastics over the past decade. More importantly, some traditional treatment technologies including adsorption, membrane filtration, and biological treatment are usually restricted to treat them owing to the ultralow concentration, high chemical stability and low biodegradability of BPA [4, 5]. As an alternative, advanced oxidation processes (AOPs) have attracted tremendous research interest for the decomposition and mineralization of them through some strong oxidative species such as ·OH, ·O2 [6-9].
Among various AOPs, Fenton-like process based on Fe2+ and peroxymonosulfate (PMS) has recently triggered tremendous research interest because of its environmental benignity and cost effectiveness [10-12]. However, the practical application of it is still limited by several drawbacks, such as the narrow operating pH range, hard to recycle the soluble metal ions and necessity for further treatment of sludge [13, 14]. To address these problems, the fabrication of a magnetic catalyst is desirable. Up to now, magnetite nanoparticles (NPs) have been widely investigated as one of the most efficient heterogeneous Fenton catalysts among Fe-based nanomaterials due to their higher content of structural Fe2+ [15-17]. However, compared with the homogenous Fenton reaction, the catalytic performance of Fe3O4 NPs based one is still far from expected. Recently, the hybridization of Fenton reaction with photocatalytic process has shown great potential in this field [18-20]. However, the development of a catalyst that could achieve both high Fenton-like and photocatalytic performance is still challenging.
Herein, a unique yolk-shell structured magnetic mesoporous TiO2 has been synthesized through a facile ultrasound assisted etching method and demonstrated as a highly efficient solar photocatalytic Fenton-like catalyst for degrading BPA. The resultant material exhibits a unique yolk-shell structure with uniform mesopores (~4 nm), a large Brunauer-Emmet-Teller (BET) surface area (~166.7 m2/g), a high pore volume (~0.56 cm3/g) and a strong magnetic susceptibility (~51 emu/g). More importantly, the void space can be facilely tuned from 20 nm to 90 nm by changing the thickness (10−46 nm) of SiO2 middle layer, which can act as a superior micro-reactor for the confined photocatalytic Fenton-like degradation of BPA.
The schematic illustration for the synthesis of yolk-shell structured magnetic mesoporous TiO2 micro-reactor are illustrated in Scheme 1. The uniform magnetite NPs are first fabricated via a facile solvothermal method, which involves the reduction of Fe(Ⅲ) salts with ethylene glycol at a high temperature (200 ℃) in the presence of sodium citrate as a capping agent. Scanning electron microscope (SEM) images clearly show that the Fe3O4 NPs exhibit uniform spherical shapes with an average size of ~130 nm (Figs. S1A and B in Supporting information). Owing to the presence of abundant citrate groups on the surface, the NPs possess outstanding dispersibility in water or ethanol, which is very favorable for the subsequent high-quality coating with SiO2 and TiO2. After the first Stöber coating, the Fe3O4@SiO2 nanospheres with a smooth surface and a particle size of ~200 nm can be obtained (Figs. S1C and D in Supporting information). Transmission electron microscopy (TEM) images reveal that a ~35 nm thick SiO2 layer is evenly wrapped on the Fe3O4 core, forming a well-defined core-shell structure. The further Stöber coating gives rise to a sandwich-like Fe3O4@SiO2@TiO2 nanostructure with a uniform TiO2 layer (~35 nm) coated on the Fe3O4@SiO2 cores (Figs. S1E and F in Supporting information). Following an ultrasound (US) assisted etching process in a weakly basic condition, the SiO2 middle layer in the Fe3O4@SiO2@TiO2 nanospheres can be completely removed, thus forming the void space between Fe3O4 core and TiO2 shell. Note that the US can also cause the further hydrolysis of Ti-OR moiety in the TiO2 shell to become complete hydrolyzed form of Ti-OH, leading to the formation of mesoporous structure. The TEM images clearly show that the well-defined yolk-shell nanostructures with a movable black core is obtained (Figs. 1A and B) The overall spacing is measured to be ~70 nm, that equals the thickness of double silica interlayers, indicating that the silica was completely removed in NaHCO3 solution under US irradiation. No Si peak was detected in the X-ray photoelectron spectroscopy (XPS) analysis, further suggesting the complete removal of SiO2 (Fig. S2 in Supporting information). After calcination at 550 ℃ for 2h, mesoporous TiO2 micro-reactor with uniform yolk-shell structure was obtained with a highly crystalline TiO2 shell. High resolution TEM (HRTEM) image of the Fe3O4@SiO2@c-mTiO2-3 clearly shows that the TiO2 shell is well crystalized with a d-spacing of 0.35 nm, which is a typical reflection of anatase TiO2 (Fig. 1C). Moreover, the size of the void space can be facilely tuned from 20 nm to 90 nm by adjusting the thickness of SiO2 interlayer from 10 nm to 45 nm (Figs. 1D–F). In previous studies [11], the collapse could occur if the etching of SiO2 did prior to the calcination. However, in our study, the collapse was not observed possibly because the post-hydrolysis of TiO2 shell lead to the formation of void space in the network, which could buffer the thermal expansion during calcination, thus increasing the thermal stability of TiO2 shell. The yolk-shell structured sample with different void space size of 20, 40, 70, 90 nm are denoted as Fe3O4@void@c-mTiO2-1, Fe3O4@void@c-mTiO2-2, Fe3O4@void@c-mTiO2-3, Fe3O4@void@c-mTiO2-4, respectively.

|
Download:
|
| Scheme 1. Schematic illustration for the synthesis of magnetic mesoporous TiO2 micro-reactor. | |

|
Download:
|
| Fig. 1. TEM (A and B) HRTEM (C) images of Fe3O4@SiO2@c-mTiO2-3 and TEM images of Fe3O4@SiO2@c-mTiO2-1 (D), Fe3O4@SiO2@c-mTiO2-2 (E) and Fe3O4@SiO2@c-mTiO2-4 (F). | |
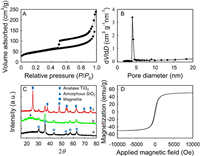
N2 adsorption-desorption isotherms of the yolk-shell Fe3O4@void@c-mTiO2-3 NPs (Fig. 2A) depict an IV curve with a hysteresis loops close to H1-type, indicating that the TiO2 shells contain uniform mesopores. The BET surface area and pore volume of the Fe3O4@void@c-mTiO2-3 sample are measured to be ~166.7m2/g and ~0.56 cm3/g, respectively. Correspondingly, the pore size distribution (Fig. 2B) calculated from the adsorption branch using the Barrett-Joyner-Halenda (BJH) method reveals a uniform pore size centered at ~4.2 nm.
|
Download:
|
| Fig. 2. N2 sorption isothermals (A) and pore size distribution (B) of yolk-shell structured mesoporous TiO2 with a void space of 35 nm. XRD patterns (C) of Fe3O4 (a), Fe3O4@SiO2 (b), and Fe3O4@void@mTiO2-3 (c). The magnetic hysteresis loops at 300K of Fe3O4@void@mTiO2-3 (D). | |
X-ray diffraction (XRD) pattern of the Fe3O4 nanoparticles (Fig. 2C) exhibits several well resolved characteristic diffraction peaks, typical for Fe3O4 crystalline phase. A broad amorphous silica peak can be clearly observed in the XRD pattern of Fe3O4@SiO2 compared with that of pure Fe3O4 NPs, suggesting that the SiO2 was coated. No amorphous SiO2 peak was observed in the sample of Fe3O4@void@mTiO2-3, suggesting that the SiO2 middle layer was well removed. In addition, several sharp peak attributed to anatase TiO2 was detected, proving that the mesoporous TiO2 shell was well crystalized. The magnetic property of the Fe3O4@void@c-mTiO2-3 micro-reactor was evaluated by measuring the magnetization saturation value (Fig. 2D). As a result of the high magnetization (~51 emu/g), the Fe3O4@void@c-mTiO2-3 nanospheres in their homogeneous dispersion can be quickly recycled by applying a hand-held magnetic bar (< 30s, Fig. S3 in Supporting information).
The photocatalytic Fenton-like degradation performance of BPA in the presence of the resultant magnetic mesoporous TiO2 was tested at a neutral pH (Fig. 3A). Before the irradiation of solar light, the system was mechanically stirred in dark for 1h to achieve an adsorption-desorption equilibrium between the catalyst and BPA. As a control, the degradation performances of BPA under PMS, solar/PMS, solar/catalyst, and PMS/catalyst conditions were also examined, showing the removal efficiencies of 17.0%, 44.5%, 76.1%, 87.1%, respectively. The larger degradation efficiency of solar/PMS process than PMS alone was due to the dissociation of PMS into sulfate radicals through light [21]. When Fe3O4@void@c-mTiO2-3 micro-reactor were directly used as a Fenton-like catalyst without solar light, the efficiency was significantly improved resulted from the activation of PMS by the surface Fe2+. Interestingly, when combine solar light, PMS and Fe3O4@void@c-mTiO2 catalyst together, the degradation performance of BPA can be greatly promoted, which can achieve a complete degradation within 40min, demonstrating the priority of this combined process. To better illustrate the priority of the combined process, a synergetic factor ( S) has been introduced, which can be calculated based on Eq. 1 [22-24].
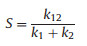
|
(1) |

|
Download:
|
| Fig. 3. Photocatalytic Fenton degradation performance for BPA with different processes (A) and corresponding kinetic constant value (B); in B, PMS (a), Solar+PMS (b), Solar+catalyst (c), PMS+catalyst (d), and Solar+catalyst+PMS (e). Photocatalytic Fenton degradation performance for BPA (C) and corresponding kinetic constant value (D) in the presence of different catalysts; in D, Fe3O4 (a), Hollow c-mTiO2-2 (b), and Fe3O4@void@c-mTiO2-3 (c), Fe3O4@void@c-mTiO2-4 (d) and Fe3O4@SiO2@c-mTiO2 (e). Photocatalytic Fenton degradation performance for BPA (E) and corresponding kinetic constant value (F) in the presence of catalysts with different void space; in F, Fe3O4@void@c-mTiO2-1 (a), Fe3O4@void@c-mTiO2-2 (b), Fe3O4@void@c-mTiO2-3 (c), Fe3O4@void@c-mTiO2-4 (d) and Fe3O4@SiO2@c-mTiO2 (e). | |
Where, k1 and k2 is the kinetic constants of Fenton-like and photocatalytic process, respectively. k12 is the kinetic constant of the combined photocatalytic Fenton-like process. The kinetic constants were calculated by fitting the experimental data with Langmuir-Hinshelwood model. The pseudo first order reaction equation was assumed because the concentration of BPA is very low. The correlation coefficients ( R2) for all the process are above 0.95, suggesting that the assumption is reasonable (Fig. 3B). The S is calculated to be 2.9, much larger than 1, suggesting that the combined process is not a simple sum of photocatalytic and Fenton-like process. The total leached iron ions concentration after the reaction was also measured at the neutral condition (Fig. S4 in Supporting information). We found that when only the catalyst was added to the system, negligible iron ions was detected, suggesting that the catalyst is stable. After the irradiation of solar light, the concentration of leached Fe ions increased, which is possibly due to that the structural Fe3+ ions on the surface of Fe3O4 lose their stability after scavenging the photo-excited electrons and thus get detached from the surface and then leached into the solution (Fig. S4b). The combination of PMS with the catalyst could also lead to the generation of a small amount of leached Fe ions, resulted from the mutual activation of PMS and catalyst surface. When combining these three together, the concentration of more than 20 times larger for leached Fe ions was detected, suggesting a strong synergistic interaction between these elements. This agrees well with the performance results. To demonstrate the superiority of the micro-reactor system, the degradation performances of individual Fe3O4 and hollow structured mesoporous c-mTiO2 (Fe3O4 was etched out by a hot HCl solution) under solar light in the presence of PMS were also examined. It is clearly shown that both the photocatalytic Fenton-like degradation performances of Fe3O4 and hollow structured mesoporous c-mTiO2 are much smaller than that of the Fe3O4@void@c-mTiO2-3 micro-reactor, indicating that necessity to construct such a catalyst (Fig. 3C). In addition, the photocatatyic performance of hollow structured mesoporous c-mTiO2 in the presence of PMS is larger than that without PMS, revealing that PMS also promote the photocaltyic reaction owing to the scavenge of photo-excited electrons (Fig. S5 in Supporting information). According to the calculated kinetic constant (Fig. 3D), the synergistic factor is derived to be 2.5, further revealing the great enhancement after combining Fe3O4 and hollow c-mTiO2 together.
The effect of void size on the degradation performance was also evaluated (Figs. 3E and F). It is clear that the core-shell structured Fe3O4@SiO2@c-mTiO2 without etching out the SiO2 interlayer shows a much smaller photocatalytic Fenton-like degradation performance than that the samples with void space, suggesting that the void space plays an important role in promoting the catalytic performance. This is because the presence of void space can not only increase the number of exposed reactive sites of inner core, thus facilitating the Fenton-like catalytic performance, but also promote the enrichment of the organic pollutants and PMS in the void space, bringing the synergistic effects between the photocatalytic and Fenton-like catalytic process. We also found that the photocatalytic Fenton-like degradation rate of BPA increased with the increase of void space size. This result is reasonable because larger void possess more space for the confined reaction between the photocatalytic and Fenton-like reaction. However, further increasing the void space beyond 70 nm did not lead to a significant increase in the degradation reaction rate, possibly owing to that too large void space will also reduce the synergistic interaction between the two reactions. Therefore, the Fe3O4@void@c-mTiO2-3 was used for the subsequent test.
The pH is an important factor to be considered for the practical application of Fenton based process. Thus, the degradation performance of Fe3O4@void@c-mTiO2-3 was also investigated at different pH (Fig. 4A). We found that in this system, the removal efficiency in both acidic and neutral condition is similar, indicating that this catalyst can work in a neutral pH, which is favorable for the practical application. However, the removal efficiency sharply decreased when the pH was adjusted to 10 possibly owing to the change of reaction mechanism. It is speculated that on the Fe3O4 nanoparticles, the mechanism could be proposed as follows (Eqs.2-4)[25-27]. Where, the symbol indicates the species on the surface of Fe3O4 nanoparticles.
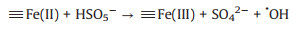
|
(2) |
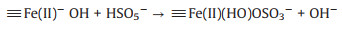
|
(3) |
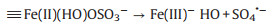
|
(4) |

|
Download:
|
| Fig. 4. Photocatalytic Fenton degradation performance for BPA with at different pH (A) and in the presence of different scavengers (B). Schematically illustrate the mechanism for photocatalytic Fenton degradation of BPA in the presence of yolk-shell structured mesoporous TiO2 as the micro-reactor (C). | |
According to these equations, the reaction mechanism could be different at acidic and basic condition. At an acidic/neutral condition, the ferric ions could directly react with PMS to generate ·OH (Eq. 2). While in a basic condition, the system is abundant with negatively charged OH groups, which could be adsorbed on 
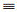
Based on the results above, the mechanism can be explained by Fig. 4C that BPA molecules are mainly degraded by ·OH, which are produced by several pathways. The irradiation of solar light could excite the outer TiO2 shell to generate photo-excited electrons and holes at the conduction band (CB) and valance band (VB), respectively. Then, the holes can react with the water molecules or OH− to generate ·OH. At the same time, the ferric ion can activate the PMS to generate sulfate radicals and ferrous ions. At this moment, the ferrous ions plays an important role in keeping the interaction between the TiO2 shell based photocatalytic and Fe3O4 based Fenton process because on one hand, the Fe3+ can scavenge the photo-excited electrons, thus greatly inhibiting the recombination of electrons and holes. Note that the PMS could also scavenge the photo-excited electrons, but from the experiment result (Fig. S5), the scavenge effect is not significant; on the other hand, the Fe3+ can be recovered quickly to generate Fe2+ to promote the Fenton-like reaction. It is well known that the lifetime of these radicals is very short, which can be easily recombined to form H2O2 or SO42−, exhibiting a much smaller oxidation potential. The presence of void spaces can effectively reduce this effect because they can provide enough space to enrich the BPA modules to get close to the radicals, thus achieving an excellent degradation performance. Finally, the radicals can oxide the BPA molecules to small intermediates and then partially be mineralized into CO2 and H2O, which has been proved by the total organic carbon (TOC) degradation test.
In summary, we have successfully prepared the yolk-shell structured Fe3O4@void@c-mTiO2 micro-reactor through a combined Stöber coating method and an ultrasound assisted etching process. The Fe3O4@void@c-mTiO2 NPs with a high specific surface area and a well-defined yolk-shell structure exhibit a remarkable performance for degrading BPA due to the synergic effect between the TiO2 shells based photocatalytic process and the Fe3O4 cores based Fenton-like reaction. More importantly, the void space can act as a micro-reactor, providing a special place for the catalytic reaction. Furthermore, the catalyst can work efficiently in both acidic and neutral condition. Owing to the high magnetization, the catalyst can be conveniently recovered by a hand-held magnet and be recycled for 5 times without significant loss of activity. This study provides a novel way for the design of unique micro-reactor catalyst for the photocatalytic Fenton-like reaction.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work is supported by the National Natural Science Foundation of China (Nos. 5182220221972163 and 51772050), the Fundamental Research Funds for the Central Universities (No. 2232020D-02), Shanghai Sailing Program (No. 20YF1400500), Shanghai Natural Science Foundation (No. 20ZR1401500), Shanghai Rising-Star Program (No. 18QA1400100), Youth Top-notch Talent Support Program of Shanghai, Science and Technology Commission of Shanghai Municipality (No. 19520713200), Shanghai Scientific and Technological Innovation Project (No. 19JC1410400), Key Basic Research Program of Science and Technology Commission of Shanghai Municipality (No. 20JC1415300), DHU Distinguished Young Professor Program and Fundamental Research Funds for the Central Universities.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.09.061.
| [1] |
A. Pal, K.Y.H. Gin, A.Y.C. Lin, M. Reinhard, Sci. Total Environ. 408 (2010) 6062-6069. DOI:10.1016/j.scitotenv.2010.09.026 |
| [2] |
D.J. Lapworth, N. Baran, M.E. Stuart, R.S. Ward, Environ. Pollut. 163 (2012) 287-303. DOI:10.1016/j.envpol.2011.12.034 |
| [3] |
R. Loos, R. Carvalho, D.C. António, et al., Water Res. 47 (2013) 6475-6487. DOI:10.1016/j.watres.2013.08.024 |
| [4] |
I. Bautista-Toledo, M.A. Ferro-García, J. Rivera-Utrilla, C. Moreno-Castilla, F.J. Vegas Fernández, Environ. Sci. Technol. 39 (2005) 6246-6250. DOI:10.1021/es0481169 |
| [5] |
D.P. Mohapatra, S.K. Brar, R.D. Tyagi, R.Y. Surampalli, Chemosphere 78 (2010) 923-941. DOI:10.1016/j.chemosphere.2009.12.053 |
| [6] |
I. Gültekin, N.H. Ince, J. Environ. Manage. 85 (2007) 816-832. DOI:10.1016/j.jenvman.2007.07.020 |
| [7] |
M. Cheng, G. Zeng, D. Huang, et al., Chem. Eng. J. 284 (2016) 582-598. DOI:10.1016/j.cej.2015.09.001 |
| [8] |
N.N. Mahamuni, Y.G. Adewuyi, Ultrason. Sonochem. 17 (2010) 990-1003. DOI:10.1016/j.ultsonch.2009.09.005 |
| [9] |
Y. Yang, J.J. Pignatello, J. Ma, W.A. Mitch, Environ. Sci. Technol. 48 (2014) 2344-2351. DOI:10.1021/es404118q |
| [10] |
H. Lu, M. Sui, B. Yuan, J. Wang, Y. Lv, Chem. Eng. J. 357 (2019) 140-149. DOI:10.1016/j.cej.2018.09.111 |
| [11] |
C. Zhu, F. Liu, C. Ling, et al., Appl. Catal. B: Environ. 242 (2018) 238-248. |
| [12] |
L. Dong, T. Xu, W. Chen, W. Lu, Chem. Eng. J. 357 (2019) 198-208. DOI:10.1016/j.cej.2018.09.094 |
| [13] |
E. Neyens, J. Baeyens, J. Hazard. Mater. 98 (2003) 33-50. DOI:10.1016/S0304-3894(02)00282-0 |
| [14] |
S. Caudo, G. Centi, C. Genovese, S. Perathoner, Top. Catal. 40 (2006) 207-219. DOI:10.1007/s11244-006-0122-6 |
| [15] |
P. Qiu, K. Kang, K. Kim, et al., RSC Adv. 5 (2015) 96201-96204. DOI:10.1039/C5RA15693A |
| [16] |
S.Q. Liu, L.R. Feng, N. Xu, Z.G. Chen, X.M. Wang, Chem. Eng. J. 203 (2012) 432-439. DOI:10.1016/j.cej.2012.07.071 |
| [17] |
Y. Ling, M. Long, P. Hu, Y. Chen, J. Huang, J. Hazard. Mater. 264 (2014) 195-202. DOI:10.1016/j.jhazmat.2013.11.008 |
| [18] |
H. Yi, M. Jiang, D. Huang, et al., J. Taiwan Inst. Chem. Eng. 93 (2018) 184-192. DOI:10.1016/j.jtice.2018.06.037 |
| [19] |
L. Xu, J. Wang, Environ. Sci. Technol. 46 (2012) 10145-10153. DOI:10.1021/es300303f |
| [20] |
X. Qian, M. Ren, Y. Zhu, et al., Environ. Sci. Technol. 51 (2017) 3993-4000. DOI:10.1021/acs.est.6b06429 |
| [21] |
R.R. Solís, F.J. Rivas, A.M. Chávez, D.D. Dionysiou, J. Hazard. Mater. 400 (2020) 123118. DOI:10.1016/j.jhazmat.2020.123118 |
| [22] |
Y. Xu, J. Ai, H. Zhang, J. Hazard. Mater. 309 (2016) 87-96. DOI:10.1016/j.jhazmat.2016.01.023 |
| [23] |
S. Luo, L. Duan, B. Sun, et al., Appl. Catal. B: Environ. 164 (2015) 92-99. DOI:10.1016/j.apcatb.2014.09.008 |
| [24] |
H. Sun, C. Kwan, A. Suvorova, et al., Appl. Catal. B: Environ. 154- 155 (2014) 134-141. |
| [25] |
Z. Wu, Y. Wang, Z. Xiong, et al., Appl. Catal. B: Environ 277 (2020) 119136. DOI:10.1016/j.apcatb.2020.119136 |
| [26] |
W. Oha, Z. Dong, T. Lima, Appl. Catal. B: Environ. 194 (2016) 169-201p. DOI:10.1016/j.apcatb.2016.04.003 |
| [27] |
C. Tan, N. Gao, Y. Deng, et al., J. Hazard. Mater. 276 (2014) 452-460. DOI:10.1016/j.jhazmat.2014.05.068 |
| [28] |
T. Zeng, X. Zhang, S. Wang, et al., Chem. Eur. J. 20 (2014) 6474-6481. DOI:10.1002/chem.201304221 |
| [29] |
D. Du, W. Shi, L. Wang, J. Zhang, Appl. Catal. B: Environ. 200 (2017) 484-492. DOI:10.1016/j.apcatb.2016.07.043 |
| [30] |
J. Wang, S. Wang, Chem. Eur. J. 334 (2018) 1502-1517. |
 2021, Vol. 32
2021, Vol. 32 

