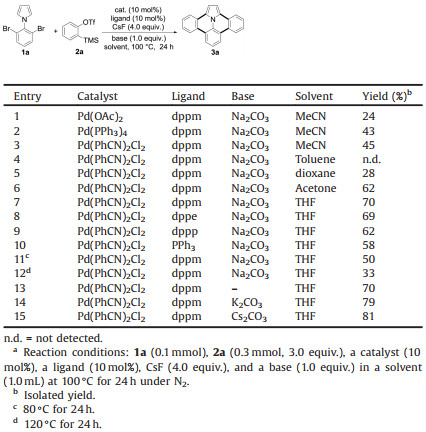
Ullazine core is a small N-doped polycyclic aromatic hydrocarbon (PAH) molecule with 16 π-electrons, which is an isoelectronic structure of pyrene (Figs. 1a and b). Its unique planar and push-pull structure with strong intramolecular charge transfer (ICT) character makes it an attractive building block in organic functional materials, such as dye-sensitized solar cells (DSSCs) [1], organic field-effect transistors (OFETs) [2], and polycyclic heteroaromatic arenes (PHAs) [3]. Therefore, the synthesis of ullazine derivatives have attracted considerable attention.
|
Download:
|
| Fig. 1. Structures of (a) pyrene, (b) ulllazine and (c) dibenzo[d, k]ullazine. | |
Since the first synthesis of ullazines by professors Balli and Zeller in 1983 [4], no improvement has been made for over twenty years until 2005. 3, 4, 8, 9-Tertrasubstituted ullazines were obtained by transition-metal-catalyzed annulation reactions using internal alkynes [5], while 3, 9-disubstituted ullazines were synthesized by Lewis acid mediated intramolecular cyclization reactions [1a] and a photocatalytic annulation reaction utilizing terminal alkynes [6].
Dibenzo[d, k]ullazine is a π-extended ullzaine skeleton fused with two benzene rings at the 3, 4-and 8, 9-positions (Fig. 1c). Its synthetic method is rare. In 2012, Ren and co-workers developed an intramolecular Friedel-Crafts-type arylation reaction of aryl triazenes, by which one 5, 8, 11-trisubstituted dibenzo[d, k]ullazine was synthesized (Scheme 1a) [7]. Later, Ito [8] and Müllen [9] independently reported a 1, 3-dipolar cycloaddition reaction of 8 H-isoquinolino[4, 3, 2-de]phenanthridin-9-ium salts with various dipolarophiles to construct 1, 2-disubstituted dibenzo[d, k]ullazines (Scheme 1b). To the best of knowledge, the parent dibenzo[d, k]ullazine core has not been synthesized yet. A more general and concise synthesis of dibenzo[d, k]ullazines from simple starting materials is still in demand.

|
Download:
|
| Scheme 1. Synthetic strategies towards dibenzo[d, k]ullazines. | |
Arynes are highly active intermediates [10], which are usually generated in situ from 2-(trimethylsilyl)aryl triflates using fluorine anion. They in recently years serve as effective and attractive synthons to construct polycyclic molecules [11]. Inspired by these work and due to our continuous interest in N-doped PAHs [12], we herein report an efficient protocol to prepare dibenzo[d, k]ullazines by a Pd-catalyzed double annulation of 1-(2, 6-dibromophenyl)-1 H-pyrroles with arynes (Scheme 1c). The photophysical properties of these molecules were also studied. It is noted that the parent dibenzo[d, k]ullazine core was obtained for the first time.
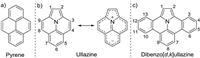
Our study commenced with the reaction between 1-(2, 6-dibromophenyl)-1 H-pyrrole (1a) and 2-(trimethylsilyl)phenyl trifluoromethanesulfonate (2a) as the benzyne precursor in the prescence of Pd(OAc)2 (10mol%), dppm (10mol%), CsF (4.0 equiv.) and Na2CO3 (1.0 equiv.) in CH3CN at 100 ℃ for 24h in a nitrogen atmosphere. The targeted double annulated product 3a was obtained in a low yield of 24% (Table 1, entry 1), and no singly annulated byproduct was detected. The structure of 3a was identified by 1H, 13C NMR and HRMS analyses. Catalyst screening showed that only palladium catalysts worked. Both Pd(PPh3)4 and Pd(PhCN)2Cl2 improved the yield to around 45% (Table 1, entries 2 and 3). For easy handling, Pd(PhCN)2Cl2 was chosen as the catalyst. The reaction did not occur in toluene, but performed much better in THF, giving 3a in 70% yield (Table 1, entries 4–7). Bidentrate phosphine ligands, bis(diphenylphosphino)methane (dppm) and 1, 2-bis(diphenylphosphino)ethane (dppe) gave similar yields, while 1, 3-bis(diphenylphosphino)propane (dppp) was less effective. Monophosphine ligand, triphenylphosphine (PPh3) was also inferior (Table 1, entries 7–10). Lowering and elevating the reaction temperature both significantly decreased the yield of 3a (Table 1, entries 11 and 12). Although addition of extra base sodium carbonate showed no influence, further increase of the basicity could promote the reaction, and the best yield of 81% was achieved when Cs2CO3 was employed as the base (Table 1, entries 13–15). It needs to point out that triphenylene was observed as a byproduct in this reaction.
|
|
Table 1 Optimization of the reaction conditions.a |
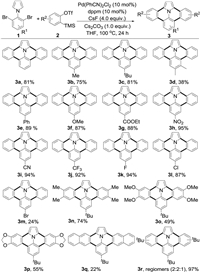
With the optimal conditions in hand, the scope of 2, 6-dibromophenylpyrroles (1) was firstly investigated (Scheme 2). Alkyl and phenyl substituted 1a-e were doubly annulated to afford the desired dibenzo[d, k]ullazines 3a-e in good yields. A methyl group at the 3-position of 1d led to a significant diminished yield of 3d due to the steric hindrance. Electron-donating and electron-withdrawing functional groups such as alkoxyl, ester, nitro, cyano, and trifluoromethyl groups were all tolerated and give the corresponding functionalized dibenzo[d, k]ullazines 3f-j in excellent yields. Halogenated substrates with fluoro-, chloro-and bromo-substituents were compatible, providing opportunity for further functionalization of dibenzo[d, k]ullazine, either as a core or a functionality. However, because of the comparable reactivity at the 2-, 4-and 6-positions of 1-(2, 4, 6-tribromophenyl)-1 H-pyrrole, the reaction went complicatedly and the bromo-substituted product 3m was only isolated in a low yield of 24%.
|
Download:
|
| Scheme 2. Substrate scopes of 2, 6-dibromophenylpyrroles and arynes. Reaction conditions: 1 (0.1 mmol), 2 (0.3 mmol, 3.0 equiv.), Pd(PhCN)2Cl2 (10mol%), dppm (10mol%), CsF (4.0 equiv.), and Cs2CO3 (1.0 equiv.) in THF (1.0 mL) at 100 ℃ for 24 h under N2. Isolated yield. | |
The scope of arynes (2) was subsequently studied in use of 1c as the substrate. When symmetrical 4, 5-disubstituted aryne precusors were employed, the corresponding 4, 5, 11, 12-tetrasubstituted dibenzo[d, k]ullazines could be obtained. The moderate yields for 3o and 3p is probably due to slower generation of arynes from alkoxyl substituted precursors [13]. To prepare a larger π-extended dibenzo[d, k]ullazine derivative, the 2, 3-naphthalyne precursor was synthesized and subjected to the double annulation reaction, which delivered the desired dinaphtho[d, k]ullazine 3q in 22% yield. The regioselectivity was examined by utilizing an unsymmetrical aryne precursor, 4-(tert-butyl)-2-(trimethylsilyl)phenyl trifluoromethanesulfonate. The reaction ran smoothly to provide 3r in an excellent yield as a mixture of three regiomers, which were successfully isolated in a ratio of 2:2:1 (Supporting information).
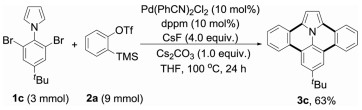
To show the practicability of this methodology, a gram-scale reaction of 1c and 2a was conducted. The targeted product 3c was finally isolated in 63% yield after purification by column chromatography on silica gel (Eq. 1).
|
(1) |
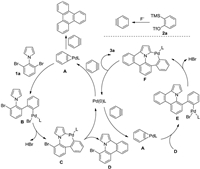
A tentative mechanism was proposed in Scheme 3 based on our preliminary results and previous studies [11]. The reaction initializes with an oxidative addition of the Pd(0) species with the aryne to generate palladacycle A. A reacts with 1a to form intermediate B, which undergoes an intramolecular C–H activation to afford palladacycle C. Reductive elimination then occurs to give the singly annulated intermediate D. Subsequently, a similar catalytic cycle between A and D provides the desired double annulated product 3a. The generation of triphenylene as a byproduct supported this mechanism. However, a pathway starting with an oxidative addition of the Pd(0) species with 1a could not be ruled out at current stage.
|
Download:
|
| Scheme 3. Proposed mechanism. | |
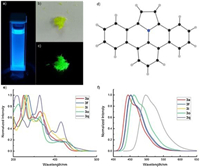
The parent dibenzo[d, k]ullazine core 3a was recrystallized from dichloromethane (DCM) as yellow-colored needle crystals, and its almost planar structure was unambiguously confirmed by the X-ray crystallographic analysis (Figs. 2b and d). The supplementary crystallographic data of 3a (CCDC: 2019567) can be obtained free of charge from The Cambridge Crystallographic Data Centre. 3a in DCM emits blue fluorescence under UV irradiation (Fig. 2a). The fluorescence intensity enhanced along with the increase of concentration from 10− 6 mol/L to 10−4 mol/L, while the shape and wavelength of the emission band kept unchanged. This phenomenon is attributed to the concentration effect of a single-molecule emissive behavior. When the concentration reached 10−3 mol/L, the fluorescence intensity drastically quenched, suggesting a typical aggregation caused quenching (ACQ) (Fig. S2 in Supporting information). In the solid state, 3a exhibits bathochromically-shifted green fluorescence (Fig. 2c), which is in accordance with its π-π stacking interactions as observed in the single crystal structure. Due to the large planar skeleton, dibenzo[d, k]ullazines are barely soluble in common organic solvents. Their photophysical properties were studied and the representative data are displayed inFigs. 2e and f, and Table 2, For all data, see Supporting information.
|
Download:
|
| Fig. 2. (a) Photograph of the DCM solution of 3a under UV irradiation; Photographs of the pristine crystals of 3a (b) under ambient light, and (c) under UV irradiation; (d) X-ray crystal structure of 3a; Normalized (e) UV–vis absorption and (f) fluorescence spectra of 3a, 3f, 3i, 3o and 3q, excited at 365, 360, 365, 370 and 414 nm, respectively. | |
|
|
Table 2 Photophysical data of selected dibenzo[d, k]ullazines. |
All dibenzo[d, k]ullazines showed similar absorption from 310 nm to 370 nm, except that a significant red shift and a much higher molar extinction coefficient of 50256L mol−1cm−1 were found for 3q. Moreover, the emission of 3q shifted bathochromically 53 nm from that of 3a while only small fluorescence variations were observed for others. These results suggested that extension of π-conjugation is a more efficient means to tune the photophysical property of the dibenzo[d, k]ullazine core than substitution on the phenyl rings. In addition, due to the rigid and π-extended core, most of dibenzo[d, k]ullazines have good fluorescence quantum yields in a range from 22% to 37% (Supporting information)
In summary, we have developed an efficient Pd-catalyzed double annulation reaction of 1-(2, 6-dibromophenyl)-1 H-pyrroles with arynes to provide a series of π-extended dibenzo[d, k]ullazines in good to excellent yields. These compounds with good photophysical properties can be used as building blocks to construct novel organic functional materials.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsWe thank the financial support from the National Natural Science Foundation of China (No. 21772134) and the Fundamental Research Funds for the Central Universities (No. 20826041D4117).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.09.057.
| [1] |
(a) J.H. Delcamp, A. Yella, T.W. Holcombe, M.K. Nazeeruddin, M. Grätzel, Angew. Chem. Int. Ed. 52 (2013) 376-380; (b) A. Dualeh, R. Humphry-Baker, J.H. Delcamp, M.K. Nazeeruddin, M. Grätzel, Adv. Energy Mater. 3 (2013) 496-504; (c) L.N. Yang, S.C. Li, Z.S. Li, Q.S. Li, RSC Adv. 5 (2015) 25079-25088; (d) H. Qiao, Y. Deng, R. Peng, et al., RSC Adv. 6 (2016) 70046-70055; (e) S. Mathew, N.A. Astani, B.F.E. Curchod, et al., J. Mater. Chem. A 4 (2016) 2332-2339; (f) C. Cebrián, J. Mater. Chem. C 6 (2018) 11943-11950; (g) D. Miao, C. Aumaitre, J.F. Morin, J. Mater. Chem. C 7 (2019) 3015-3024. |
| [2] |
A. Skabeev, U. Zschieschang, Y. Zagranyarski, et al., Org. Lett. 20 (2018) 1409-1412. DOI:10.1021/acs.orglett.8b00183 |
| [3] |
(a) H. Yokoi, Y. Hiraoka, S. Hiroto, et al., Nat. Commun. 6 (2015) 8215-8224; (b) Y. Tokimaru, S. Ito, K. Nozaki, Angew. Chem. Int. Ed. 56 (2017) 15560-15564; (c) Z. Zhou, Z. Wei, Y. Tokimaru, et al., Angew. Chem. Int. Ed. 58 (2019) 12107-12115; (d) M. Takase, T. Narita, W. Fujita, et al., J. Am. Chem. Soc. 135 (2013) 8031-8040; (e) M. Richter, S. Hahn, E. Dmitrieva, et al., Chem. Eur. J. 25 (2019) 1345-1352; (f) X. Guo, Z. Yuan, Y. Zhu, et al., Angew. Chem. Int. Ed. 58 (2019) 16966-16973; (g) K. Nakamura, Q.Q. Li, O. Krejcí, et al., J. Am. Chem. Soc. 142 (2020) 113639-11369; (h) S. Ito, Y. Tokimaru, K. Nozaki, Angew. Chem. Int. Ed. 54 (2015) 7256-7260; (i) Y. Tokimaru, S. Ito, K. Nozaki, Angew. Chem. Int. Ed. 57 (2018) 9819-9824; (j) H. Yokoi, S. Hiroto, H. Shinokubo, J. Am. Chem. Soc. 140 (2018) 4649-4655. |
| [4] |
H. Balli, M. Zeller, Helv. Chim. Acta 66 (1983) 2135-2139. DOI:10.1002/hlca.19830660724 |
| [5] |
K.I. Kanno, Y. Liu, A. Iesato, K. Nakajima, T. Takahashi, Org. Lett. 7 (2005) 5453-5456. DOI:10.1021/ol052214x |
| [6] |
A. Das, I. Ghosh, B. König, Chem. Commun. 52 (2016) 8695-8698. DOI:10.1039/C6CC04366F |
| [7] |
J. Zhou, W. Yang, B. Wang, H. Ren, Angew. Chem. Int. Ed. 51 (2012) 12293-12297. DOI:10.1002/anie.201206578 |
| [8] |
S. Ito, Y. Tokimaru, K. Nozaki, Chem. Commun. 51 (2015) 221-224. DOI:10.1039/C4CC06643J |
| [9] |
R. Berger, M. Wagner, X. Feng, K. Müllen, Chem. Sci. 6 (2015) 436-441. DOI:10.1039/C4SC02793K |
| [10] |
(a) H.H. Wenk, M. Winkler, W. Sander, Angew. Chem. Int. Ed. 42 (2003) 502-528; (b) S.A. Worlikar, R.C. Larock, Curr. Org. Chem. 15 (2011) 3214-3232; (c) D. Pérez, D. Peña, E. Guitián, Eur. J. Org. Chem. (2013) 5981-6013; (d) J.A. García-López, M.F. Greaney, Chem. Soc. Rev. 45 (2016) 6766-6798; (e) M. Feng, X. Jiang, Synthesis 49 (2017) 4414-4433; (f) I. Pozo, E. Guitián, D. Pérez, D. Peña, Acc. Chem. Res. 52 (2019) 2472-2481. |
| [11] |
(a) Z. Liu, X. Zhang, R.C. Larock, J. Am. Chem. Soc. 127 (2005) 15716-15717; (b) C. Xie, Y. Zhang, Z. Huang, P. Xu, J. Org. Chem. 72 (2007) 5431-5434; (c) Z. Liu, R.C. Larock, J. Org. Chem. 72 (2007) 223-232; (d) M. Feng, B. Tang, N. Wang, H.X. Xu, X. Jiang, Angew. Chem. Int. Ed. 54 (2015) 14960-14964; (e) Z. Chen, X. Han, J.H. Liang, et al., Chin. Chem. Lett. 25 (2014) 1535-1539; (f) Z. Li, W. Wang, H. Jian, et al., Chin. Chem. Lett. 30 (2019) 386-388. |
| [12] |
(a) B. Feng, D. Wan, L. Yan, et al., RSC Adv. 6 (2016) 66407-66411; (b) J. Tang, S. Li, Z. Liu, et al., Org. Lett. 19 (2017) 604-607; (c) V.D. Kadam, B. Feng, X. Chen, et al., Org. Lett. 20 (2018) 7071-7075; (d) Z. She, Y. Wang, D. Wang, et al., J. Am. Chem. Soc. 140 (2018) 12566-12573; (e) X. Zheng, R. Su, Z. Wang, et al., Org. Lett. 21 (2019) 797-801. |
| [13] |
C. Lu, A.V. Dubrovskiy, R.C. Larock, J. Org. Chem. 77 (2012) 8648-8656. DOI:10.1021/jo3016192 |
 2021, Vol. 32
2021, Vol. 32