b Key Laboratory of Drug Targeting and Drug Delivery System of the Education Ministry, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, West China School of Pharmacy Sichuan University, Chengdu 610041, China
Tumor is still one of the most serious threats of human being. Although various new chemical drugs, antibodies and even gene drugs are developed for different targets of tumor, the overall survival of tumor bearing patients is far from satisfactory. Among various reasons, the poor drug delivery is considered a big serious challenge for tumor treatment. The drugs suffer from short blood circulation time, high distribution volume, low tumor accumulation and poor stability in circulation, etc. The development of nanotechnology provides powerful tools to carry the drugs to tumor with many benefits, including protecting drugs from degradation, improving blood circulation time, elevating tumor targeting capacity, responsive release of drugs to target sites, and co-delivery different drugs to same target for synergistic therapy [1, 2]. Therefore, the nanoparticles-based drug delivery systems, also known as nanomedicines, have been attracted increasing attention in the past two decades.
Nowadays, several nanomedicines have approved for clinical application, such as doxorubicin loaded liposomes, albumin-bound paclitaxel nanoparticles, and paclitaxel micelles [1, 3]. What is more, there are many nanomedicines in the pipeline that are under clinical evaluation [4]. However, clinical data show that the main advantage of nanomedicines is low side effect, for example, liposomal doxorubicin has lower cardiotoxicity, albumin-bound paclitaxel nanoparticles have higher tolerance dose. At same dose, these nanomedicines show comparable antitumor effect of the free drug solution [5]. Therefore, it is important to analysis the tumor targeting drug delivery procedure, and find ways to improve the drug delivery.
The tumor targeting drug delivery procedure could be divided into blood circulation, tumor accumulation, tumor penetration, cell internalization and drug release [6]. The final drug delivery efficiency depends on the efficiency of each part of the procedure. Among the five parts, tumor penetration is considered an important shortage of the nanoparticles-based drug delivery. Generally, properties of both tumor and nanoparticles greatly influence tumor penetration. Compared with normal tissues, tumor contains distinguished characters, termed tumor microenvironment (TME). Typical TME includes leaky and torturous tumor vasculature, dense intratumor extracellular matrix (ECM), high interstitial fluid pressure (IFP), altered enzyme level, acidic microenvironment, and hypoxia, etc. [7, 8]. The TME provides strict barriers for drug delivery, but the distinguished characters also provide stimuli for responsive drug delivery [9-11].
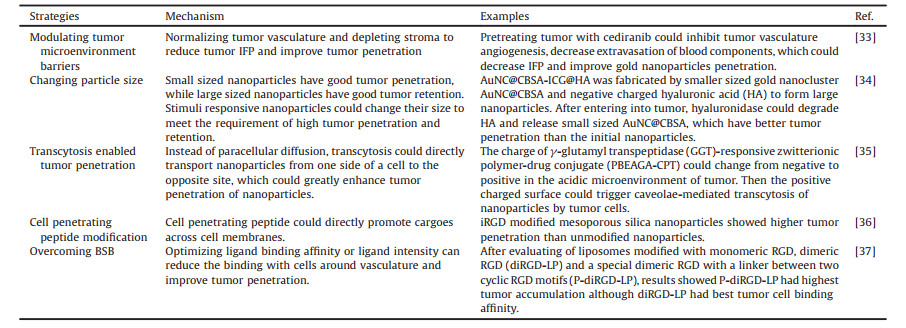
In the review, we first summarized the barriers that restrict the tumor penetration, and then reviewed the strategies to improve tumor penetration of nanoparticles, both through modulating TME and changing properties of nanoparticles (Fig. 1). Lastly, we further discussed the shortages of the studies and provided a perspective of the area.
|
Download:
|
| Fig. 1. Illustration of barriers that restrict tumor penetration, and the corresponding strategies to improve tumor penetration. | |
In various TME characters, IFP and tumor stroma are main components of TME barriers for nanoparticles penetration. Normally, a compound or a particle could be transported to a distant area by diffusion or convection, which tend to transport from area with higher concentration and pressure to lower area. The leaky vasculature and hampered lymph system result in enhanced permeability and retention (EPR) effect, which is the basic rationale for passive tumor targeting of nanoparticles. However, the accumulated extravasation of blood components in the tumor interstitium leads to evaluated IFP, which can reach over 100 mmHg in some cases while the number in normal tissue is only 0-3 mmHg [8, 12]. What is worse, the aberrant tumor vasculature decreases blood flow and pressure. As a result, pressure gradient from blood to tumor is near zero even negative in some high tumor, which considerably hinders the convection of interstitium and penetration of nanoparticles. Therefore, restoring the pressure gradient from blood to tumor is a promising strategy to improve tumor penetration, which could be achieved by both normalizing tumor vasculature and lymph system.
Tumor stroma refers to all the non-cell components in tumor, which is mainly composed of various collagen, glycosaminoglycan and growth factor molecules [13]. The tumor stroma could support the growth, resistance and metastasis of tumor cells. Among the components, collagen is the main barrier that restricts tumor penetration. A study showed the diffusion coefficient and hydraulic conductivity of immunoglobulin in different tumors were positively related to the collagen levels rather than glycosaminoglycan levels, demonstrating the dominant role of collagen [13, 14]. Therefore, reducing the level of collagen in tumor will improve tumor penetration.
2.2. Properties of nanoparticlesThe properties of nanoparticles, such as size, potential, shape and surface moieties, all influence their in vivo behaviors. For tumor neovasculature, the cutoff size of the pores is about 300-500 nm, which means particles larger than the size could not extravasate from blood, while smaller particles could extravasate into tumor more easily. After extravasated to tumor vasculature periphery, further penetration is still greatly influenced by the particle size. The interstitial gap is greatly reduced due to the dense tumor ECM and quick proliferation of tumor cells. Therefore, nanoparticles with smaller size have better tumor penetration capacity. Dancy et al. compared the penetration of polystyrene particles with 20 nm, 40 nm and 100 nm in mimic tumor extracellular matrix [15]. Compared with 100 nm particles, 20 nm and 40 nm particles showed more diffusive trajectories. Similarly, in breast tumor slices, 20 nm and 40 nm particles showed rapid diffusion, while 100 nm particles were immobilized within the tumor ECM. Although small sized nanoparticles have good tumor penetration capacity, the retention is poor. For example, 300 nm liposomes showed higher tumor accumulation than 100 nm liposomes [16]. There is a size dilemma that both small and large sized nanoparticles could not achieve high tumor penetration and retention simultaneously, which greatly hinders the tumor targeting drug delivery and treatment.
Shape is another important factor that influences tumor penetration. Lee et al. found that 300×18 nm-sized tobacco mosaic virus with a tubular structure had better penetration capacity than 30 nm-sized spherical cowpea mosaic virus [17]. However, it is not true that particles with higher aspect ratio have better penetration property. Agarwal et al. found that low aspect ratio (H/D=0.3) cylindrical nanoparticles showed higher intratissue delivery and more uniform distribution than other cylindrical (H/D=0.45), rod-shaped nanoparticles (H/D=4, 8) and sphere counterpart [18]. The nanoparticles density also influences permeability through endothelial cells. Tay et al. prepared a series of consistently sized silica nanoparticles (SiNPs) with density from 1.45 g/cm3 to 2.13 g/cm3. Results showed that a cumulative gravitational-mediated force of around 1.8 nN/μm along the endothelial cells, while the particle density was between 1.57 g/cm3 to 1.72 g/cm3, was a critical threshold force for nanoparticles to perturb endothelial cell junction, resulting in effective penetration through endothelial cell layers [19]. Other properties, such as rigidity of nanoparticles could also influence penetration in biological hydrogels [20, 21]. Therefore, shape-changeable nanoparticles have superior tumor targeting delivery capacity than particles with a fixed shape [22, 23].
Charge also influences tumor penetration. Normally, negative charged nanoparticles have longer blood circulation time than positive charged nanoparticles due to the adsorption of protein corona [24, 25], but positive charged nanoparticles have better affinity with tumor cells due to the negative charged cell membrane. What is more, positive charged nanoparticles could be internalized by caveolae-mediated pathway and then be transcytosis to another side of tumor cells, which greatly improve tumor penetration of nanoparticles [26]. Additionally, surface PEGylation is widely used to improve blood circulation time, but it could inhibit the interaction between nanoparticles and tumor cells. Therefore, charge reversal and PEG detachment strategies are developed to improve tumor penetration [27, 28].
2.3. Interaction barrier between tumor and nanoparticlesAs discussed above, smaller sized nanoparticles have better tumor penetration, and then it can deduce that free drugs, with molecular weight of several hundreds to thousands, should have very good tumor penetration because their size is normally smaller than 1 nm. However, most drugs tend to accumulate near blood vasculature. The reason is that the drugs are easy to bind with and be taken up by cells around vasculature, which hinders their further penetration [29]. Similarly, nanoparticles may also bind with vasculature peripheral cells, trapping them in the vasculature periphery, which called binding site barrier (BSB). Tsumura et al. evaluated the influence of dissociation rate constant (Kd) of antibody-drug conjugates (ADC) on tumor penetration [30]. Results showed the ADC with higher Kd had better tumor penetration and higher tumor concentration, leading to better antitumor effect. The BSB may also contribute by unintended binding with stroma cells. Tumor associated fibroblast cells (TAF) are major component in desmoplastic tumor stroma and preferentially localize near blood vessels. Miao et al. demonstrated the uptake of anisamide modified nanoparticles by TAF was 6-fold higher than other cells [31]. Therefore, decreasing the binding affinity of nanoparticles with cells could improve tumor penetration.
The binding of nanoparticles with ECM also hinders tumor penetration. Dancy et al. compared polystyrene particles with and without PEG modification. Both in Matrigel and breast cancer slices, PEGylated particles showed better diffusion capacity, while uncoated particles were immobilized in the ECM due to their high binding affinity [15]. Authors further evaluated the particles with different density of PEGs. High (with Γ/Γ* of 3.6, Γ is the PEG surface coverage and Γ* is the total surface area) and medium (with Γ/Γ* of 1.4) PEG density showed lower binding affinity with Matrigel than that of low (with Γ/Γ* of 0.8) PEG density. But in vivo, the medium PEG density showed highest penetration in breast tumor xenografts. Similarly, Nance et al. found that the PEGylated nanoparticles with 70 nm diffused 100-fold faster in brain than the nanoparticles without PEG [32].
3. Strategies to improve tumor penetrationAccording to the barriers discussed above, many strategies are developed to improve tumor penetration (Table 1) [33-37].
|
|
Table 1 Strategies to improve tumor penetration. |
The aberrant tumor vasculature contributes a lot to tumor high IFP, therefore, vasculature normalization is proposed to reduce tumor IFP and improve tumor penetration [38]. Normally, vasculature normalization could be achieved by anti-angiogenesis treatment. For example, Wang et al. used thalidomide to normalize the vasculature and improve the tumor penetration of RGD modified liposomes [39]. However, the anti-angiogenesis treatment should be carefully optimized because the decreased tumor vasculature intensity could reduce IFP as well as tumor blood perfusion, while the latter could hinder drug delivery. Our group used cediranib for tumor vasculature normalization. Results showed oral administration of cediranib of 0.375, 1.5 and 6 mg/kg/d could effectively improve tumor penetration, but 24 mg/kg/d of cediranib would decrease the nanoparticles accumulation in tumor [33]. Meanwhile, two days treatment improved drug delivery but three days treatment decreased. Therefore, the normalization strategy should be carefully optimized before combination with nanoparticles. Additionally, directly disruption tumor vasculature could reduce blood and oxygen supply, which is useful for antitumor therapy [10, 38]. However, the reduced blood supply would inhibit nanoparticles distribution from blood to tumor.
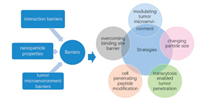
Depleting stroma could improve tumor penetration. Metformin (MET) is a widely used antidiabetic drug, which could suppress tumor progression by activating adenosine monophosphate-activated kinase (AMPK) pathway and p53 pathway, inhibiting adenosine triphosphate (ATP) synthesis and suppressing ERK/P70S6K signaling [40, 41], while AMPK pathway plays an important role in inhibiting desmoplastic reaction [42]. Han et al. demonstrated 24 h incubation of Pancreatic ductal adenocarcinoma (PDAC) cells with 5 mmol/L MET led to 2.3-fold increase in p-AMPK level and 47.6% decrease of the expression of TGF-β [43]. After co-incubation with pancreatic stellate cells (PSCs) for 24 h, the secreted level of α-smooth muscle actin (α-SMA) and collagen I decreased by 58.1% and 60.4% respectively. In vivo, MET treatment decreased the collagen level in PADC tumor by 40%, suggesting the MET could effectively deplete ECM of PDAC. Consequently, pretreatment tumor with MET significantly improved the penetration of gemcitabine loaded Fe3O4 nanoparticles (Fig. 2). Similarly, hydralazine [44], losartan [45] and cyclopamine [46] all could reduce ECM content of tumor, thus improving penetration of nanoparticles and the corresponding antitumor effect.
|
Download:
|
| Fig. 2. Illustration of MET-induced stromal depletion for enhancing the penetration and cathepsin B-triggered release of gemcitabine carried by Fe3O4 nanoparticles in the lysosome of PADC cells. Reproduced with permission [43]. Copyright © 2020, American Chemical Society. | |
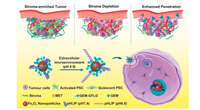
Particle size greatly influences tumor penetration of nanoparticles. Normally, nanoparticles with smaller size have better penetration. However, the application of small sized nanoparticles is hindered by the quickly renal filtration and easy distribution to tumor vasculature due to high interstitial fluid pressure of tumor. Therefore, size changeable strategies are proposed to improve both tumor penetration and retention, which has been summarized in previous reviews (Fig. 3) [47, 48]. The nanoparticles could shrink or aggregate under various stimuli, such as pH [49], light [50], enzyme [51] and redox condition.
|
Download:
|
| Fig. 3. A brief illustration of stimuli-induced size-changeable strategies with their potential applications. Reproduced with permission [48]. Copyright © 2020, American Chemical Society. | |
Enzyme is a widely used stimuli for responsive drug delivery. In tumor, there are several enzymes overexpressed, such as matrix metalloproteinases (MMPs), hyaluronidase, legumain, caspase-3 and furin. For example, hyaluronic acid (HA) could be degraded by hyaluronidase [52], which could be used to construct size-changeable nanoparticles. Our group used small sized cationic albumin-protected gold nanocluster (AuNC@CBSA) and HA to fabricate a large size nanoparticle (AuNC@CBSA-ICG@HA), while indocyanine green (ICG) was loaded as photothermal agent and imaging probe [34]. The AuNC@CBSA-ICG@HA could shrink from about 200 nm to 50 nm in the presence of hyaluronidase, realizing good penetration in tumor spheroids and subcutaneous breast cancer. To further improve the targeting capacity and antitumor effect, our group coated the particles with red blood cell membrane and loaded with photosensitizer pheophorbide A (PheoA), paclitaxel and a PD-L1 peptide antagonist (dPPA) [53]. The particles, pPP-mCAuNCs@HA, showed a reduced uptake by macrophages, long blood circulation time (about 38 h) and good tumor penetration compared with controls. In combination of photodynamic therapy, chemotherapy and immunotherapy, both the primary 4T1 breast cancer and lung metastasis were significantly inhibited, with elevated CD3+CD8+ T cell percentage and tumor cell apoptosis. Furthermore, coating the size-changeable nanoparticles with macrophage membrane could further expand the tumor targeting drug delivery because of the tumor homing capacity of macrophage [54]. Similarly, Zhou's group developed a kind of near-infrared (NIR) light triggered size-reducible liposomes [55]. Upon laser irradiation, the small sized PAMAM was released, and the size changed from 162 nm to 8.6 nm, which greatly enhanced tumor penetration.
The application of large sized nanoparticles is restricted by their poor tumor penetration. Although the shrinkage of the large sized nanoparticles could improve tumor penetration, shrinking procedure is time-consuming, which could not improve tumor penetration as soon as the particles extravasated from tumor microvessels. To overcome the problem, aggregation strategy is proposed. According to the strategy, small sized nanoparticles are used to deliver drugs to tumor with high tumor penetration and homogenous intratumor distribution. Then under the triggering of various stimuli, the particles aggregate to much larger size, which have better retention capacity both in tumor and cells. For example, our group conjugated Ala-Ala-Asn-Cys-Lys (AK) peptide and 2-cyano-6-aminobenzothiazole (CABT) onto 20 nm gold nanoparticles (AuNPs-A & C) [56]. After incubation with legumain, the Ala-Ala-Asn could be cleaved by legumain and then click cycloaddition occurred between 1, 2-thiolamino groups and the contiguous cyano group, which could form 300 nm aggregates at 12 h. The nanoparticles showed good penetration into tumor spheroids and the area far from tumor microvessels, and also the accumulation in tumor was improved. The percentage injected dose per gram glioma (%ID) of AuNPs-A & C was 7.52% at 24 h after intravenous injection, which was much higher than PEGylated gold nanoparticles (3.22%). After treatment with doxorubicin (DOX)-loaded AuNPs-A & C, the median survival time of glioma bearing mice increased from 17 days to 66 days. The tumor targeting and penetration could be further improved by surface modification with RRGD peptide and tumor stroma depletion [57], as discussed in previous sections.
Although the size-changeable nanoparticles have achieved improved tumor penetration, the effect can be further improved by combination with other strategies [58]. Tumor microenvironment modulation could improve the penetration of particles, which is also applicable for size-changeable nanoparticles. For example, losartan pretreatment could decrease the level of collagen in tumor, so combining losartan pretreatment with shrinkable nanoparticles could greatly improve the penetration of large sized nanoparticles initially [45]. After size reduction triggered by enzyme or other stimuli, the tumor penetration further improved, resulting in good drug delivery efficiency and antitumor effect. Similarly, tumor vasculature normalization by cediranib could effectively reduce IFP of 4T1 tumor, which can increase tumor penetration of AuNPs-A & C [33].
3.3. Transcytosis enabled tumor penetrationDue to high IFP and dense ECM in tumor, the diffusion and convection of nanoparticles are greatly hindered, which attenuates the tumor penetration of nanoparticles. Promisingly, transcytosis, a process that are directly actively transports ligands and receptors from one side of a cell to the opposite site, is considered a useful pathway to improve tumor penetration of nanoparticles [26]. Cationization of nanoparticles could trigger adsorptive-mediated transcytosis, but cationic nanoparticles often have short blood circulation time due the quickly clearance by MPS. To overcome the problem, Zhou et al. developed a γ-glutamyl transpeptidase (GGT)-responsive zwitterionic polymer-drug conjugate (PBEAGA-CPT), which has a long blood circulation time [35]. The GGT on tumor cells or tumor neovascular endothelial cells could cationize the potential of PBEAGA-CPT from -10 mV to 5 mV. The cationized PBEAGA-CPT could be taken up by tumor cells through caveolae-mediated pathway, which is considered as a transcytosis pathway. Consequently, the penetration through tumor spheroids was greatly elevated, which could be significantly inhibited by caveolae-endocytosis inhibitor genistein and the exocytosis inhibitor Exo1, suggesting the transcytosis enabled penetration relied on the caveolae-mediated endocytosis and exocytosis. Due to the good tumor penetration capacity, the tumor drug concentration of PBEAGA-CPT was 1.8-fold higher than the un-responsive PEAGA-CPT. What is more, the Cy5 labeled PBEAGA-CPT could diffuse away from the vessels, while PEAGA-CPT was mostly retained in the periphery of the vessels. As a result, the PBEAGA-CPT completely kill the subcutaneous and orthotopic pancreatic tumor, indicating good tumor penetration was important for drug delivery.
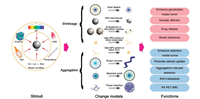
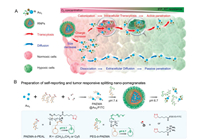
The transcytosis could be combined with size changeable strategy to further improve tumor penetration. Wang et al. developed a nanopomegranate (RNP) that were engineered through the programmed self-assembly of a tumor environment-targeting polymeric matrix and modular building blocks of ultrasmall gold nanoparticles (Au5) (Fig. 4) [59]. The RNP was about 110 nm with neutral surface charge, making it has long blood circulation time. After extravasated from vessels to tumor matrix, the acidic microenvironment quickly protonated the pH sensitive materials of RNP, leading to hydrophobic to hydrophilic change and the disassociation of the RNP. Therefore, the small sized Au5 was released with better tumor penetration. Furthermore, the Au5 with protonated polymers had positive charge, making them actively transcellular transport via caveolae-mediated transcytosis, which further improved tumor penetration. Overall, the strategy could deliver the Au5 to tumor with high and homogenous distribution, which could enrich local irradiation dosage and enhance radiotherapy outcome.
|
Download:
|
| Fig. 4. (A) The programmed RNPs maintain a larger size and negative surface charge for prolonging blood circulation, and are disassociated into Au5 in response to mild tumor acidity. The small size and cationized surface of Au5 enable passive and active tumor penetration into distal hypoxic area via extracellular diffusion and intracellular transcytosis, respectively. (B) Schematic showing the preparation and structural change of RNPs at tumor pH. Reproduced with permission [59]. Copyright 2020, American Chemical Society. | |
Cell penetrating peptide is a kind of peptides that can promote cargoes across cell membranes. Due to the lack of cell type selectivity, many strategies were developed to improve tumor targeting of CPP-modified nanoparticles, such as shielding CPPs in blood circulation and exposure them in tumor [60]. Recently, CPPs with cell selectivity were developed to expand their application. Ruoslahti's group developed a group of peptides that have a typical sequence motif R/KXXR/K, which could bind with neuropilin-1 and improve penetration of both drugs and nanoparticles [61].
iRGD, with the sequence of CRGDKGPD, is a typic and widely used tumor penetrating peptide. The iRGD could bind to αvβ3 and αvβ5 for tumor targeting, and then the peptide is proteolytically to convert the internal R/KXXR/K motif to C-terminal, which could bind with neuropilin-1 for tumor penetration [62]. As a useful ligand, iRGD has been widely used to improve penetration of nanoparticles through both covalent conjugation and physical co-administration [63-66]. Our group developed a hyaluronidase responsive size-reducible and laser-triggered nitric oxide (NO) release nanoparticles (IDDHN) with a HA shell and dendritic-graft-l-lysine (DGL) core, which have good tumor penetration efficiency due to the size reducible capacity and NO enhanced penetration [67]. To further improve the tumor penetration, we coadministered IDDHN with iRGD [68]. Compared with IDDHN, the tumor cell uptake of iRGD+IDDHN significantly improved. In vivo, the tumor distribution of iRGD+IDDHN at 36 h post injection was 2.1-fold higher than that of IDDHN. As a result, the tumor was completely ablated after four times treatment in two weeks, while IDDHN treatment could only decrease the tumor growth. Except coadministration, iRGD could also be conjugated onto nanoparticles to improve tumor penetration. For example, Wang et al. modified the small interfering RNA (siPlk1) and microRNA (miR-200c) coloaded mesoporous silica nanoparticles with iRGD to improve the tumor targeting and penetration, which showed enhanced penetration in tumor spheroids and elevated antitumor effect [36].
3.5. Overcoming BSBThe high binding affinity of nanoparticles with cells around vasculature would trap the nanoparticles in peripheral area, decreasing their further penetration in to deep tumor. Therefore, optimizing ligand binding affinity or ligand intensity would be useful for overcoming BSB. For example, Guo et al. developed stealth liposomes modified with the monomeric RGD, dimeric RGD (diRGD-LP) and a special dimeric RGD with a linker between two cyclic RGD motifs (P-diRGD-LP) [37]. Results showed the diRGD-LP had strongest interaction with B16 cells and highest cellular uptake. However, P-diRGD-LP showed highest tumor accumulation capacity, indicating the highest binding affinity of diRGD-LP with targeted cells may hinder their tumor penetration, while optimizing the binding with cells could improve tumor targeting drug delivery.
Ligand detachment is a useful strategy to avoid trapping in peripheral area of tumor vasculature. After the ligand decorated nanoparticles bound with receptors and triggered endocytosis, detaching ligand from the nanoparticles enabled the nanoparticles escape from ligand-receptor complex and be excytosed [69, 70]. However, the strategy could only actively penetrate one cell layer. To further improve the penetration, Jin et al. developed an MMP-9 cleavable biotin-labeled liposome to carry small sized micelles [71]. After intravenous injection, the biotin could quickly bind with tumor cells around tumor vasculature and be trapped there due to the BSB. Then, the overexpressed MMP-9 would cleave the biotin-labeled polymer and destroy the liposomes, which could release the small sized micelles for further penetration. The tumor distribution of the liposomes was significantly higher than biotin-labeled small sized micelles and un-responsive liposomes.
4. Conclusion and perspectiveThe nanoparticles enabled tumor targeting drug delivery is a complex procedure that is influenced by not only the properties of nanoparticles but also the physical characters and the interaction between nanoparticles and biological environment. In recent years, tumor penetration is gained a lot of attention, and various strategies are developed to improve tumor penetration of nanoparticles. Although a great progress has been made as reviewed above, there are several aspects should be taken into consideration in the future study.
Firstly, tumor is a complex tissue that the characters communicate with and influence each other. For example, in tumor vasculature normalization strategy, several studies showed the vasculature normalization decreased IFP as well as increased MMP expression [72], while the higher MMP level contributes to tumor proliferation and metastasis [73]. Therefore, comprehensive evaluation of various aspects should be performed in modulating TME. Secondly, combination different strategies is a good way to improve overall outcome. Nowadays, more and more studies have designed nanoparticles that can modulate several aspects of TME and have responsive properties simultaneously. For example, combination TME modulation with size changeable nanoparticles significantly improved tumor penetration and antitumor effect. Finally, the tumors are heterogenous, and nanoparticles have different properties and composition. So, the conclusion of one research may be not applicable to other studies, case by case optimization and evaluation are important to get promising drug delivery efficiency.
In conclusion, tumor penetration is important for tumor targeting drug delivery. There are several barriers influence tumor penetration, including TME barriers, nanoparticle properties, and interaction barriers between tumor and nanoparticles. To overcome the barrier, several strategies are developed, which could effectively improve tumor penetration, and finally enhance tumor treatment outcome.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentThe work was supported by 111 Project (No. B18035).
| [1] |
C. Li, J. Wang, Y. Wang, et al., Acta Pharm. Sin. B 9 (2019) 1145-1162. DOI:10.1016/j.apsb.2019.08.003 |
| [2] |
J. Cao, D. Huang, N.A. Peppas, Adv. Drug Deliv. Rev. 167 (2020) 170-188. DOI:10.1016/j.addr.2020.06.030 |
| [3] |
D. Bobo, K.J. Robinson, J. Islam, et al., Pharm. Res. 33 (2016) 2373-2387. DOI:10.1007/s11095-016-1958-5 |
| [4] |
M. Merino, S. Zalba, M.J. Garrido, J. Control. Release 275 (2018) 162-176. DOI:10.1016/j.jconrel.2018.02.015 |
| [5] |
U. Bulbake, S. Doppalapudi, N. Kommineni, et al., Pharmaceutics 9 (2017) 12. DOI:10.3390/pharmaceutics9020012 |
| [6] |
Q. Sun, Z. Zhou, N. Qiu, et al., Adv. Mater. 29 (2017) 1606628. DOI:10.1002/adma.201606628 |
| [7] |
M. De Palma, D. Biziato, T.V. Petrova, Nat. Rev. Cancer 17 (2017) 457-474. DOI:10.1038/nrc.2017.51 |
| [8] |
H. Gao, Curr. Drug Metab. 17 (2016) 731-736. DOI:10.2174/1389200217666160630203600 |
| [9] |
Y. Zhou, X. Chen, J. Cao, et al., J. Mater. Chem. B 8 (2020) 6765-6781. DOI:10.1039/D0TB00649A |
| [10] |
S. Yang, H. Gao, Pharmacol. Res. 126 (2017) 97-108. DOI:10.1016/j.phrs.2017.05.004 |
| [11] |
P. Zheng, Y. Liu, J. Chen, et al., Chin. Chem. Lett. 31 (2020) 1178-1182. DOI:10.1016/j.cclet.2019.12.001 |
| [12] |
C.H. Heldin, K. Rubin, K. Pietras, et al., Nat. Rev. Cancer 4 (2004) 806-813. DOI:10.1038/nrc1456 |
| [13] |
H. Yang, Z. Tong, S. Sun, et al., J. Control. Release 328 (2020) 28-44. DOI:10.1016/j.jconrel.2020.08.024 |
| [14] |
H. Cabral, Y. Matsumoto, K. Mizuno, et al., Nat. Nanotechnol. 6 (2011) 815-823. DOI:10.1038/nnano.2011.166 |
| [15] |
J.G. Dancy, A.S. Wadajkar, C.S. Schneider, et al., J. Control. Release 238 (2016) 139-148. DOI:10.1016/j.jconrel.2016.07.034 |
| [16] |
G. Kibria, H. Hatakeyama, N. Ohga, et al., Biomaterials 34 (2013) 5617-5627. DOI:10.1016/j.biomaterials.2013.03.094 |
| [17] |
K.L. Lee, L.C. Hubbard, S. Hern, et al., Biomater. Sci. 1 (2013) 581-588. DOI:10.1039/c3bm00191a |
| [18] |
R. Agarwal, P. Jurney, M. Raythatha, et al., Adv. Healthc. Mater. 4 (2015) 2269-2280. DOI:10.1002/adhm.201500441 |
| [19] |
C.Y. Tay, M.I. Setyawati, D.T. Leong, ACS Nano 11 (2017) 2764-2772. DOI:10.1021/acsnano.6b07806 |
| [20] |
M. Yu, L. Xu, F. Tian, et al., Nat. Commun. 9 (2018) 2607. DOI:10.1038/s41467-018-05061-3 |
| [21] |
H. Wu, M. Yu, Y. Miao, et al., Acta Pharm. Sin. B 9 (2019) 858-870. DOI:10.1016/j.apsb.2019.02.010 |
| [22] |
R. Liu, Y. An, W. Jia, et al., J. Control. Release 321 (2020) 589-601. DOI:10.1016/j.jconrel.2020.02.043 |
| [23] |
C. Lin, F. Tong, R. Liu, et al., Acta Pharm. Sin. B 10 (2020) 2348-2361. DOI:10.1016/j.apsb.2020.10.009 |
| [24] |
W. Xiao, H. Gao, Int. J. Pharm. 552 (2018) 328-339. DOI:10.1016/j.ijpharm.2018.10.011 |
| [25] |
H. Gao, Q. He, Opin Expert, Drug Deliv. 11 (2014) 409-420. |
| [26] |
Y. Liu, Y. Huo, L. Yao, et al., Nano Lett. 19 (2019) 8010-8020. DOI:10.1021/acs.nanolett.9b03211 |
| [27] |
M. Zhang, X. Chen, C. Li, et al., J. Control. Release 319 (2020) 46-62. DOI:10.1016/j.jconrel.2019.12.024 |
| [28] |
M. Jin, G. Jin, L. Kang, et al., Int. J. Nanomedicine 13 (2018) 2405-2426. DOI:10.2147/IJN.S161426 |
| [29] |
A.J. Primeau, A. Rendon, D. Hedley, et al., Clin. Cancer Res. 11 (2005) 8782-8788. DOI:10.1158/1078-0432.CCR-05-1664 |
| [30] |
R. Tsumura, S. Manabe, H. Takashima, et al., J. Control. Release 284 (2018) 49-56. DOI:10.1016/j.jconrel.2018.06.016 |
| [31] |
L. Miao, J.M. Newby, C.M. Lin, et al., ACS Nano 10 (2016) 9243-9258. DOI:10.1021/acsnano.6b02776 |
| [32] |
E. Nance, C. Zhang, T.Y. Shih, et al., ACS Nano 8 (2014) 10655-10664. DOI:10.1021/nn504210g |
| [33] |
W. Xiao, S. Ruan, W. Yu, et al., Mol. Pharm. 14 (2017) 3489-3498. DOI:10.1021/acs.molpharmaceut.7b00475 |
| [34] |
R. Liu, C. Hu, Y. Yang, et al., Acta Pharm. Sin. B 9 (2019) 410-420. DOI:10.1016/j.apsb.2018.09.001 |
| [35] |
Q. Zhou, S. Shao, J. Wang, et al., Nat. Nanotechnol. 14 (2019) 799-809. DOI:10.1038/s41565-019-0485-z |
| [36] |
Y. Wang, Y. Xie, K.V. Kilchrist, et al., ACS Appl. Mater. Interfaces 12 (2020) 4308-4322. DOI:10.1021/acsami.9b21214 |
| [37] |
Z. Guo, B. He, H. Jin, et al., Biomaterials 35 (2014) 6106-6117. DOI:10.1016/j.biomaterials.2014.04.031 |
| [38] |
J.D. Martin, G. Seano, R.K. Jain, Annu. Rev. Physiol. 81 (2019) 505-534. DOI:10.1146/annurev-physiol-020518-114700 |
| [39] |
D. Wang, J. Fu, Y. Shi, et al., J. Control. Release 238 (2016) 186-196. DOI:10.1016/j.jconrel.2016.07.014 |
| [40] |
X. Sui, Y. Xu, X. Wang, et al., Mol. Pharm. 12 (2015) 3783-3791. DOI:10.1021/acs.molpharmaceut.5b00577 |
| [41] |
F.A. Duca, C.D. Cote, B.A. Rasmussen, et al., Nat. Med. 21 (2015) 506-511. DOI:10.1038/nm.3787 |
| [42] |
S.M.A. Da, J. Abarca-Quinones, B. Guigas, et al., Clin. Sci. 118 (2009) 411-420. |
| [43] |
H. Han, Y. Hou, X. Chen, et al., J. Am. Chem. Soc. 142 (2020) 4944-4954. DOI:10.1021/jacs.0c00650 |
| [44] |
Y. Chen, W. Song, L. Shen, et al., ACS Nano 13 (2019) 1751-1763. |
| [45] |
X. Cun, S. Ruan, J. Chen, et al., Acta Biomater. 31 (2016) 186-196. DOI:10.1016/j.actbio.2015.12.002 |
| [46] |
T. Jiang, B. Zhang, S. Shen, et al., ACS Appl. Mater. Interfaces 9 (2017) 31497-31508. DOI:10.1021/acsami.7b09458 |
| [47] |
W. Yu, M. Shevtsov, X. Chen, et al., Chin. Chem. Lett. 31 (2020) 1366-1374. DOI:10.1016/j.cclet.2020.02.036 |
| [48] |
W. Yu, R. Liu, Y. Zhou, et al., ACS Cent. Sci. 6 (2020) 100-116. DOI:10.1021/acscentsci.9b01139 |
| [49] |
Z. Shi, Q. Li, L. Mei, Chin. Chem. Lett. 31 (2020) 1345-1356. DOI:10.1016/j.cclet.2020.03.001 |
| [50] |
R. Liu, M. Yu, X. Yang, et al., Adv. Funct. Mater. 29 (2019) 1808462. DOI:10.1002/adfm.201808462 |
| [51] |
S. Ruan, X. Cao, X. Cun, et al., Biomaterials 60 (2015) 100-110. DOI:10.1016/j.biomaterials.2015.05.006 |
| [52] |
Z. Luo, Y. Dai, H. Gao, Acta Pharm. Sin. B 9 (2019) 1099-1112. DOI:10.1016/j.apsb.2019.06.004 |
| [53] |
W. Yu, X. He, Z. Yang, et al., Biomaterials 217 (2019) 119309. DOI:10.1016/j.biomaterials.2019.119309 |
| [54] |
C. Hu, T. Lei, Y. Wang, et al., Biomaterials 255 (2020) 120159. DOI:10.1016/j.biomaterials.2020.120159 |
| [55] |
X. Xiong, Z. Xu, H. Huang, et al., Biomaterials 245 (2020) 119840. DOI:10.1016/j.biomaterials.2020.119840 |
| [56] |
S. Ruan, C. Hu, X. Tang, et al., ACS Nano 10 (2016) 10086-10098. DOI:10.1021/acsnano.6b05070 |
| [57] |
S. Ruan, W. Xiao, C. Hu, et al., ACS Appl. Mater. Interfaces 9 (2017) 20348-20360. DOI:10.1021/acsami.7b02303 |
| [58] |
W. Yu, C. Hu, H. Gao, ACS Appl Bio Mater 3 (2020) 5455-5462. DOI:10.1021/acsabm.0c00917 |
| [59] |
L. Wang, W. Jiang, L. Xiao, et al., ACS Nano 14 (2020) 8459-8472. DOI:10.1021/acsnano.0c02674 |
| [60] |
Z. Cong, L. Zhang, S.Q. Ma, et al., ACS Nano 14 (2020) 1958-1970. DOI:10.1021/acsnano.9b08434 |
| [61] |
E. Ruoslahti, Adv. Mater. 24 (2012) 3747-3756. DOI:10.1002/adma.201200454 |
| [62] |
K.N. Sugahara, T. Teesalu, P.P. Karmali, et al., Science 328 (2010) 1031-1035. DOI:10.1126/science.1183057 |
| [63] |
X. Cun, J. Chen, S. Ruan, et al., ACS Appl. Mater. Interfaces 7 (2015) 27458-27466. DOI:10.1021/acsami.5b09391 |
| [64] |
X. Yang, X. Chen, T. Lei, et al., Acta Pharm. Sin. B 10 (2020) 1094-1105. DOI:10.1016/j.apsb.2020.02.011 |
| [65] |
J. Zhao, B. Wen, Z. Tan, et al., J. Drug Target. 28 (2020) 1063-1070. DOI:10.1080/1061186X.2020.1775839 |
| [66] |
S. Zhang, X. Pei, H. Gao, et al., Chin. Chem. Lett. 31 (2020) 1060-1070. DOI:10.1016/j.cclet.2019.11.036 |
| [67] |
C. Hu, X. Cun, S. Ruan, et al., Biomaterials 168 (2018) 64-75. DOI:10.1016/j.biomaterials.2018.03.046 |
| [68] |
C. Hu, X. Yang, R. Liu, et al., ACS Appl. Mater. Interfaces 10 (2018) 22571-22579. DOI:10.1021/acsami.8b04847 |
| [69] |
L. Cai, C. Yang, W. Jia, et al., Adv. Funct. Mater. 30 (2020) 1909999. DOI:10.1002/adfm.201909999 |
| [70] |
S. Ruan, L. Qin, W. Xiao, et al., Adv. Funct. Mater. 28 (2018) 1802227. DOI:10.1002/adfm.201802227 |
| [71] |
Y. Jin, Z. Wu, C. Wu, et al., J. Control. Release 320 (2020) 142-158. DOI:10.1016/j.jconrel.2020.01.040 |
| [72] |
Y. Sakurai, T. Hada, S. Yamamoto, et al., Mol. Ther. 24 (2016) 2090-2099. DOI:10.1038/mt.2016.178 |
| [73] |
M.L. Tang, X.J. Bai, Y. Li, et al., Curr. Med. Sci. 38 (2018) 809-817. DOI:10.1007/s11596-018-1947-5 |
 2021, Vol. 32
2021, Vol. 32