b Chengdu Kanghua Biological Products Co., Ltd., Chengdu 610100, China;
c College of Biochemical Engineering, Beijing Union University, Beijing 100023, China;
d Galactophore Department, Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu 610072, China;
e Clinical Laboratory, Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu 610072, China
Although reducing the resultant disease burden, the Haemophilus influenzae type b (Hib), Streptococcus pneumoniae and Neisseria meningitidis are still the main causes of invasive diseases [1]. Hib is a human bacterial pathogen, which can cause many serious diseases, including epiglottitis, pneumonia, sepsis, and meningitis. Hib is the most common cause of other invasive bacterial diseases of bacterial meningitis. In developed countries, the annual incidence of bacterial meningitis is about 5–10 cases per 100, 000 population [2]. In the United States, this organism is the main cause of bacterial meningitis. Among children, more than 95% of all diseases related to invasive diseases of Haemophilus species are caused by type b influenza H [3]. In low-income countries, infectious diseases still account for a large proportion of deaths, and vaccination can greatly reduce the burden of infectious diseases [4]. The development of vaccines is a sign of success fight against multiple infectious diseases. Vaccination of pathogen-derived antigens can provide protection against infectious diseases by inducing antigen-specific primary immune responses. Once the same pathogen is encountered again, the immune system will be activated quickly. The discovery of the vaccine began in 1796 that Edward Jenner protected people against smallpox by injecting similar but less toxic vaccinia virus [5]. Currently, vaccines have been widely applicated to prevent and treat diseases [6], clinically approved vaccines are made from attenuated or inactivated pathogens, toxoids, protein or polysaccharide antigens which are isolated from pathogens and etc. [7]. Capsular polysaccharide (CPS) is a component related to immune response on bacterial cells [8], capsular polysaccharides are composed of monosaccharides bonded together by glycosidic bonds, they have excellent biocompatibility and unique biological activity, and have been proven to be the excellent targets for bacterial vaccine development [9, 10], bacteticidal and/or opsonic antibodies directed against capsular polysaccharide (PS) glycotopes can prevent invasive diseases caused by capsular bacteria [11]. In 1977, the United States approved the 14-valent capsular polysaccharides conjugated vaccine against S. pneumoniae developed by Merck Sharp & Dohme, which greatly promoted the development of polysaccharide vaccines [12]. Unfortunately, with the deepening of research, it was observed that polysaccharide vaccine only induces B cell response, even if antibodies are produced, there is no long-term memory, even if repeated vaccination, the immune effect cannot be enhanced, and the antibody concentration may lower than the initial immunization, so-called "hypo-responsiveness" [13]. Studies have also found that polysaccharide vaccines cannot work on children under 2 years old, which may due to the immature B cell function in children under 2 years of age. Therefore, polysaccharide vaccines as T-cell independent antigens cannot protect children under 2 years of age [1]. But coupling bacterial polysaccharides with carrier proteins can produce T cell-dependent antigens [14], can induce protective immunity in children under 2 years of age, and thus polysaccharide conjugate vaccines have been developed.
Before the advent of the Hib conjugate vaccine in 1988, approximately 1 in 200 children under the age of 5 suffered from Hib disease [15, 16]. After the emergence of Hib conjugate vaccines, many developed and developing countries began to introduce Hib conjugate vaccines, and the incidence of Hib disease has dropped sharply. In many countries where all infants are routinely vaccinated against Hib, invasive Hib disease has almost disappeared [17]. Streptococcus pneumoniae is the main pathogen causing pneumonia, otitis media, meningitis, bacteremia, and septicemia, is a major global health problem with the characteristics of high morbidity, mortality, and is associated with global economic burden [18, 19]. According to a survey conducted by the World Health Organization (WHO), Streptococcus pneumoniae (also known as pneumococcus) is the fourth largest microorganism that causes fatal infections. Young children, the elderly, and immunocompromised patients are particularly prone to pneumococcal infections. It is estimated that 14.5 million children are infected with pneumonia every year. In 2013, nearly one million children under the age of 5 died of pneumonia [20]. Neisseria meningitidis (meningococcus) is a gram-negative diplococcus which can cause septicemia and meningitis in susceptible people. Neisseria meningitidis are obligate human pathogens. Obtained through aerosols and oral or nasal secretions during contact, it is a member of the normal nasopharyngeal microbiome of healthy individuals. Once obtained, meningococcal bacteria can be carried briefly or even up to several months [21]. Meningococcal infection is a widespread but not uniform problem. It is a sporadic and epidemic disease. It is estimated that there are about 1.2 million cases of meningococcal infections worldwide each year, and about 135, 000 deaths. The disease patterns vary greatly over time, geographical regions, age groups, and bacterial serogroups [22].
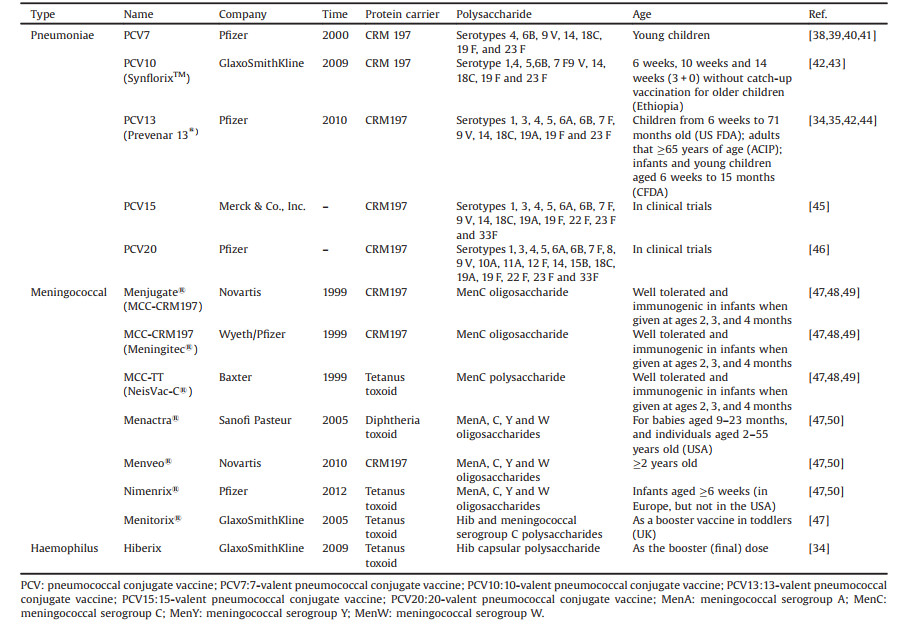
Chemical conjugation with carrier protein can effectively enhance the immunogenicity of poorly immunogenic antigens. This method has been successfully used to produce polysaccharide conjugate vaccines against infectious diseases [23]. The polysaccharide conjugate vaccine covalently couples the protein and polysaccharide together, adds the positive characteristics of the protein antigen to the polysaccharide antigen, improves the immunogenicity of the polysaccharide antigen, and provides an epitope for CD4+T cells, which promotes the memory response to polysaccharides. The polysaccharide conjugate vaccine covalently couple protein and polysaccharide together, which increases the positive characteristics of protein antigen for polysaccharide antigen, improves the immunogenicity of polysaccharide antigen, and provides antigenic determinants for CD4+T cells, thereby promoting the memory of polysaccharides, specifically, the polysaccharide part of the vaccine binds to the B cell receptor (BCR) and is endocytosed into B cells. The carrier protein in the polysaccharide protein conjugate is degraded into polypeptides. The peptide and the major histocompatibility complex combine to form a peptide-MHC II complex. The complex is presented to the surface of B cells and interacts with CD4+T cell receptor (TCR), and further activates T cells through the interaction of T cell-B cell surface molecules. Activated T cells secrete lL-2, lL-4 and other cytokines to stimulate B cell differentiation and maturation, can induce PS-specific IgM-to-IgG switching, memory B-cell development, and long-lived T-cell memory [24-28]. The immune mechanism of polysaccharide conjugate vaccine and the difference between polysaccharide vaccine and polysaccharide conjugate vaccine can be seen in Fig. 1.

|
Download:
|
| Fig. 1. The immune mechanism of polysaccharide conjugate vaccine and the difference from polysaccharide vaccine. (a) Immune mechanism of polysaccharide conjugate vaccine. The polysaccharide conjugate vaccine is taken up by the antigen-presenting cells, and the conjugate molecules are digested to obtain glycan-peptides. Glycan-peptides bind to MHC II and are presented to cells for recognition, thereby stimulating the production of cytokines IL-4 and IL-2, promoting homologous B cells to mature into memory B cells and produce IgG antibodies. (b) The difference between polysaccharide vaccine and polysaccharide conjugate vaccine. Polysaccharides are T cell independent antigens. By combining with appropriate proteins, T cell-dependent antigens can be produced, which can produce immune memory. The applicable age range is wider and the effect is better. BCR: B cell receptor; TCR: T cell receptor; MHC II: major histocompatibility complex class II. | |
This review introduces the development of polysaccharide conjugate vaccines. At first, we introduce the development status of polysaccharide conjugate vaccines and some typical polysaccharide conjugate vaccines that have been on the market. Secondly, we elucidate the protein carriers commonly used in vaccines, and then a brief introduction to the coupling chemistry for preparing polysaccharide conjugate vaccines is given. Finally, the commonly used instruments for detecting polysaccharide conjugate vaccines are introduced. It is hoped that this review can provide ideas for researchers who studying polysaccharide conjugate vaccines and promote the development of polysaccharide conjugate vaccines.
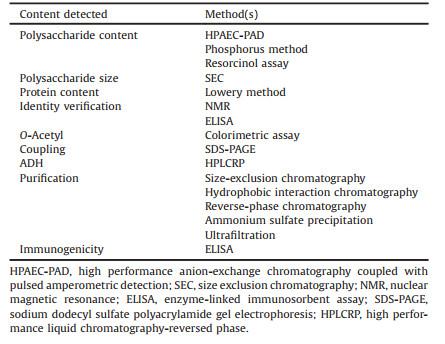
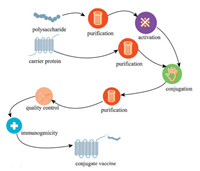
2. Development of polysaccharide conjugate vaccineThere has been a century of research history of polysaccharide vaccine and decades of polysaccharide conjugate vaccine for the treatment of Hib, Streptococcus pneumoniae and Neisseria meningitidis [29], and the treatment effect is satisfactory. The conjugate vaccine consists of carrier protein and bacterial PS [30]. The first conjugate vaccine is the Hib conjugated vaccine, which was licensed for clinical application in 1987 [30], subsequently, research on polysaccharide conjugate vaccines has developed rapidly. In recent years, many polysaccharide conjugate vaccines have been approved for the prevention of Streptococcus pneumoniae, Neisseria meningitidis and Hib, such as Hiberix (Glaxi SmithKline, Rixensart, Belgium), Prevenar 13 (Wyeth Pharmaceuticals Inc., Pearl River, NY, USA), Menjugate (Chiron, Emeryville, California, USA), Pedvax HIB (Merck, Kenilworth, NJ, USA), Menveo® (Novartis). On August 19, 2009, the US Food and Drug Administration (FDA) approved Hiberix (GlaxoSmithKline Biologics, Belgium Rixensart), it can be used as a booster dose in children from 15 months to 4 years old (before 5th birthday) according to the Accelerated Approved Regulation to cope with the continuing shortage of Hib vaccine from December 2007 to July 2009. On January 14, 2016, the Food and Drug Administration (FDA) approved the expansion of Hiberix's indications, which can be used for immunization of infants and children aged 6 weeks to 4 years. Hiberix is a Hib conjugate vaccine composed of Hib capsular polysaccharide and inactivated tetanus toxoid. It contains 10 μg of purified capsular polyribose ribitol phosphate (PRP) and 25 μg of tetanus toxoid (PRP-T), which is available as a single-dose vial of lyophilized vaccine [31, 32]. The Pneumococcal polysaccharide (PnPS) conjugate vaccine Prevenar13® (PCV13) contains 13 serotypes of S. pneumoniae polysaccharide, that are individually conjugated to nontoxic diphtheria protein (cross-reactive material [CRM(197)]) [33]. In February 2010, the US Food and Drug Administration approved PCV13 for use in children from 6 weeks to 71 months old to prevent invasive pneumococcal disease and otitis media [34]. On August 13, 2014, the Advisory Committee on Immunization Practices (ACIP) recommended the use of a 13-valent pneumococcal conjugate vaccine for adults that ≥65 years of age [35]. China Food and Drug Administration (CFDA) approved the application of Prevenar 13 for immunization of infants and young children aged 6 weeks to 15 months in 2016. Menjugate is developed by Chiron and applicated to the treatment of meningococcal infections caused by the pathogen Neisseria meningitidis. It employs CRM coupling technology and uses diphtheria toxoid as the carrier protein for specific antigens of type C meningitis. The vaccine is under development because of its potential to prevent meningitis in adults and infants [36]. MenACWY-CRM197 (Menveo®, Novartis vaccines) is a tetravalent meningococcal conjugate vaccine that can prevent invasive meningococcal disease caused by Neisseria meningitidis antigen A, C, W135 and Y. Menveo® is composed of A, C, W135, Y serogroups and the carrier protein (non-toxic diphtheria toxin CRM197). MenACWY-CRM197 has been licensed for active immunization in the European Union, Canada and Australia and can prevent ≥2 aged people develop invasive meningococcal disease and are authorized in the United States for use between the ages of 2 months and 55 years old [37]. Table 1 [34, 35, 38-50] summarizes the relevant information of Hib, meningococcal and pneumonia polysaccharide conjugate vaccine and shown the preparation process of the polysaccharide conjugate vaccine in Fig. 2.
|
|
Table 1 The vaccines. |
|
Download:
|
| Fig. 2. The preparation steps of polysaccharide conjugate vaccine. First, the polysaccharide is purified and then activated. At the same time, the required carrier protein is purified. The polysaccharide and protein are coupled by coupling chemistry. The conjugate is then purified, quality controlled, and tested for immunogenicity, and finally the conjugate vaccine is obtained. | |
In principle, protein that carries a human T cell epitope and can transform a T cell-independent polysaccharide-specific immune response into a T helper cell response can be used as a carrier for polysaccharide conjugate vaccine [51]. However, there are only a few carrier proteins can be applicated in polysaccharide conjugate vaccines. Diphtheria toxoid (DT), tetanus toxoid (TT), CRM197, Haemophilus protein D (PD) and outer membrane protein complex (OMPC) are currently the most studied protein carriers. OMPC has been used in Hib conjugate vaccine and the first generation of pneumococcal conjugate vaccine. PD is a 40 kDa cell surface protein derived from non-typeable H. influenzae (NTH), which is produced by a recombinant strain of Escherichia coli and has been introduced into a multivalent pneumococcal conjugate vaccine as a carrier for most serotypes [52].
3.1. Diphtheria toxinDiphtheria toxin is a 62 kDa single polypeptide chain, containing two disulfide bonds, synthesized by the Corylzebacterium diphfheriae and released outside the cell. The complete diphtheria toxin molecule is non-enzymatically active. After mild treatment with trypsin or other proteases in the presence of certain thiols, the molecule will be broken down into a 24 kDa fragment A and a 38 kDa fragment B, and the molecule is activated [53]. Fragment A containing the catalytic C domain and fragment B consisting of transmembrane and receptor binding domains [54]. Studies have shown that the role of fragment A and fragment B in immunology is quite different, and they will not cross-react in immunodiffusion against antitoxins. Antifragment A antibody can inhibit the enzymatic activity of the isolated fragment A, but it will not neutralize the toxin in the body. Antifragment B antibody neutralizes the toxin's in vivo toxicity by preventing the toxin from attaching to the surface of sensitive cells [55]. Diphtheria toxin has been widely used as a protein carrier in polysaccharide conjugate vaccines. Faezeh Najafzadeh et al. coupled the detoxified lipopolysaccharide (D-LPS) of Pseudomonas aeruginosa PAO-1 with DT (as a carrier protein) to obtain a conjugate vaccine (D-LPS-DT), and evaluated the immunity in a mouse model. The team covalently coupled D-LPS and DT via amidation, and the molar ratio of LPS to DT in the prepared conjugate was 3:1. The results showed that mice immunized with D-LPS-DT conjugate vaccine produced four times higher IgG antibodies than mice immunized with D-LPS, indicating that the vaccine coupled with DT of Pseudomonas aeruginosa PAO-1 can increase anti-LPS antibodies and have the ability to prevent Pseudomonas, and has a huge potential to prevent infection [56]. A phase 1 clinical study to verify the safety and immunogenicity of Vi-DT typhoid conjugate vaccine was conducted in Filipino healthy adults and children. The vaccine tested is diphtheria toxoid conjugated Vi-polysaccharide vaccine (Vi: Salmonella typhi capsular polysaccharide, Vi-DT), which was obtained by covalent coupling of Vi polysaccharide and DT. The vaccine contains two active ingredients, 25 μg purified Vi polysaccharide and DT formulated with stabilizers. The results showed that Vi-DT has good safety, good tolerance and immunogenicity in all age groups from 2 to 45 years old [57].
3.2. Tetanus toxinTetanus toxin (TT) is a potent neurotoxin produced by Clostridium tetani bacteria, is composed of a light chain of 52.4 kDa responsible for cleaving synaptophysin and a heavy chain of 98.3 kDa. The heavy chain can be further divided N-terminal domain (46.7 kDa) responsible for cell penetration and C-terminal domain (51.6 kDa) controlling TT neuronal specific binding (also known as tetanus toxin fragment C (TTFC)) [58]. TT has been widely used in the research field of polysaccharide conjugate vaccines. A phase II clinical trial evaluated the immunogenicity and safety of Sanofi Pasteur's investigational quadrivalent (serogroups A, C, Y, and W) meningococcal tetanus-toxoid conjugate vaccine: MenACYW-TT. Conducted in healthy infants who have been meningococcal, the licensed conjugate vaccine MCV4-TT (NCT03205358) was used as a control. In this phase II study conducted in Finland, 188 toddlers aged 12–24 months were randomly assigned to MenACYW-TT or MCV4-TT at a 1:1 ratio. In this exploratory study, the MenACYW-TT vaccine is well tolerated and immunogenic. If the results of this exploratory study are confirmed in the Phase III, for toddlers who receive meningococcal vaccination for the first time, a single dose of MenACYW-TT may be considered as an alternative vaccination program [59]. In a phase III trial (NCT02752906), the tetravalent meningococcal tetanus toxoid conjugate vaccine (MenACYW-TT) was evaluated as a booster vaccine. The results of the study show that MenACYW-TT is as safe as licensed vaccine--MCV4 (Menactra®; MCV4-DT). The research data also shows that the booster dose of MenACYW-TT conjugate vaccine can cause a rapid and strong immune response in adolescents and adults who within 4–10 years after the initial dose of MCV4 vaccine [60]. However, TT and DT need to be detoxified with formaldehyde before conjugation with polysaccharides, which will cause more lysine groups in the carrier protein to become potential polysaccharide conjugation sites [61].
3.3. CRM197CRM197 is a non-toxic variant, usually obtained by fermentation of C. diphtheriae C7 (β197) culture and isolation [62, 63]. A single G-A transition occurred in CRM197, resulting in its glycine-52 being replaced by glutamic acid. Although the enzymatic activity is lost, the binding activity is retained, compared with diphtheria toxin, CRM197 has higher purity, uniform structure, and lysine residues are available and non-toxic, so there is no need to detoxify by formaldehyde or glutaraldehyde. The detoxification process of formaldehyde involves intramolecular and intermolecular cross-linking, making the product very heterogeneous and difficult to purify, and this process also can cause significant epitope modification, so CRM197 can avoid the problem of handling a large amount of toxic supernatant, these advantages make CRM197 have better carrier effect and can become an ideal protein carrier of polysaccharide conjugate vaccine [64-67]. CRM197 contains two domains joined together by disulfide bonds, segment A (catalytic domain) and segment B (transmembrane domain). The B domain contains a subdomain that binds to the EGF receptor heparin-binding epidermal growth factor-like growth factor (HB-EGF) and a subdomain that translocation into cells [68]. The role of segment A is still under study. Kaihang Wang et al., proposed that fragment A of CRM197 as an intramolecular adjuvant carrier protein can be fused with non-granular antigens in conventional genetic manipulations, thereby greatly enhancing the immunogenicity of E2 protein. Their findings may provide an example for the design of non-particulate subunit vaccines [54]. Studies have shown that the diphtheria toxin mutant CRM197 may increase the production of Th1 and Th2 secreting T cells during the immune response, and induce the differentiation and maturation of B cells through heterogeneous cytokines. Thus, when the CRM197 is coupled to the capsular polysaccharide, it may cause stronger antibody response and immune memory [54, 69]. Many CRM197-based polysaccharide conjugate vaccines have been developed to protect important bacterial pathogens, such as Streptococcus pneumoniae, Hib, and Neisseria meningitidis serum A, C, Y, W135. Currently, millions of people around the world have received these vaccines. There are also vaccines against Staphylococcus aureus, Streptococcus agalactiae (Streptococcus type B), Salmonella typhi, Salmonella paratyphi, Mycobacterium tuberculosis, and some pneumococcal conjugate vaccines are in preclinical or clinical evaluation [62]. Yunsong Chang et al. covalently combined Arabinomannan (AM) with CRM197 to obtain a conjugate and investigated its immune activity. Preliminary immunization studies have shown that the AM-CRM197 immunogen can induce strong specific antibodies after systemic administration, indicating that AM-CRM197 has the potential to be a good candidate for a new carbohydrate-based vaccine and can trigger tuberculosis protective immune response [70]. However, the method of producing CRM197 can be through Pfenex technology, that is, to express CRM197 in Pseudomonas fluorescens, or by fermentation of C7 diphtheriae C7 (β197) strain containing CRM197 gene, the low yield and management limitations make it unable to meet the needs of large-scale production of vaccines. Because of this, we should focus on increasing their production or looking for better protein carriers to improve the development of polysaccharide conjugate vaccine [71].
3.4. Carrier-induced epitopic suppressionAlthough polysaccharide conjugate vaccines have many advantages, vaccines of this type may have carrier-induced epitopic suppression (CIES) effect. Research reports indicate that pre-exposure to the carrier may affect the effectiveness of subsequent inoculation of the polysaccharide conjugate vaccine containing the same or antigen-related carrier, enhancing or inhibiting the anti-CPS immune response. The CIES might be caused by anti-carrier antibodies facilitating conjugate capture by antigen presenting cells (APCs) and expansion of carrier-specific B cells [72]. The mechanism of CIES can be caused by one mechanism or multiple mechanisms. Pre-existing antibodies against carriers may hinder and/or promote the absorption of antigen-antibody complexes by antigen-presenting cells, thereby preventing hapten-specific B cells from approaching their epitopes, which is beneficial to anti-carrier B cell reactions but not conducive to anti-hapten B cell response [69]. Dominant carrier-specific memory B cells may deprive the necessary resources of hapten-specific B cells, such as T cells, through a competitive mechanism, and the regulatory T cells produced by the carrier may interfere with the anti-hapten response [69]. This phenomenon should attract the attention of researchers, and should take into account the possible influencing factors for the development of such vaccines.
4. Coupling chemistryBioconjugates play a vital role in life sciences and drug development, because it provides the ability to combine biomolecules with drugs, polymers, nanoparticles, and other compounds with different compositions and functions. As a result, they have become a major component of many biochemical and pharmaceutical applications, including protein stabilization, drug delivery, and gene therapy [73]. The methods used to prepare the conjugated vaccine mainly include reductive amination, amidation and etherification reactions, and the conjugated vaccine obtained by the method combination shows a high degree of stability [74].
Reductive amination is an indispensable method for analyzing glycomic, which can greatly promotes glycan characterization and quantification by coupling functional tags at reducing ends of glycans, and sodium cyanoborohydride (NaBH3CN) and sodium triacetoxyborohydride (NaBH(OAc)3) are two important reagents to achieve this reaction [75, 76]. However, compared with the cyanidation chemical method using 1-cyano-4-dimethylaminopyridinium tetraflouroborate (CDAP), the yield of the reductive amination chemical method is not satisfactory, the coupling by reductive amination usually has lower PS-protein conjugate yields, and an excessive amount of sodium cyanoborohydride (which must be removed later) needs to be added, which increased the cost of vaccines [77].
Cyanylation chemistry was originally created using CNBr to create reactive cyanoester groups on PS. It was used in the first conjugate reported by Dr. John Robbins in 1980, and refined by Merieux using the 1983 version of Robbins [30]. Cyanylation chemistry usually achieved by activating polysaccharides with reagents such as CNBr, introducing spacers, activating spacer's end groups, and coupling activated polysaccharide-spacer complexes to natural or activated proteins. The resulting conjugates are cross-linked and have a very high molecular weight [78]. Before coupling the PS to the protein, it must be activated by chemical modification. The methods currently used to activate the PS are mainly periodate oxidation and cyanation. However, the periodate oxidation method may affect the ring structures of PS, change its physical structure and lose important epitopes, which is not the case when using the cyanide method [30]. However, the efficiency of cyanide chemistry method using CNBr is very low, and the direct coupling between carbohydrate and protein, peptide or lipid usually has the characteristics of low yield and complex mixture, especially when the protein and glycans participating in the reaction have multiple reaction sites or steric hindrance.
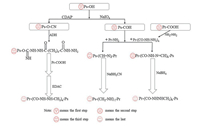
Therefore, the basic method of preparing homogeneous glycoconjugates is to installate an appropriate linker between protein and polysaccharid, reduce steric hindrance and control conjugation through highly regio- and stereos elective manner [79], thus requiring reactive linker. Adipic acid dihydrazide (ADH) is a commonly used linker, which reacts specifically with the reducing end of carbohydrates through reductive amination [79]. Meanwhile, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDAC) is requred in the coupling process as a catalyst. Subsequently, Douglas EShafer et al. found that the organic cyanation reagent 1-cyano-4-dimethylaminopyridinium tetraflouroborate (CDAP) can be used to activate soluble polysaccharides. CDAP is a water-soluble cyanation reagent with higher electrophilicity than CNBr, thus allows the cyanation reaction to be carried out under relatively mild conditions [80]. Compared with CNBr, CDAP has the characteristics of less toxicity, more friendly to environment and personnel, simple to operate, can be used at a lower pH, and has fewer side reactions. The activated polysaccharide is functionalized by exanediamine or adipic dihydrazide, which can be directly coupled to proteins. The conjugated vaccine prepared by this method has shown a better immune response, and most researchers prefer to use CDAP to activate polysaccharides [81, 82]. Dain Kim et al. reduced the size of PnPS through microfluidization, and applicated ADH as a linker, EDAC as a catalyst to couple polysaccharides with CRM 197, they successfully obtained polysaccharide conjugates, and conducted further research on it. The results were satisfactory [83]. The technical route for preparing polysaccharide protein conjugate vaccine can be seen in Fig. 3 [84, 85]. Khadijeh Ahmadi et al., prepared immunoconjugates by adding ADH (as an efficient linker) and EDAC (as a carboxyl activating agent) to the purified polysaccharide in turn, and then subjected to a series of treatments. The conventional polysaccharide/protein content determination was performed, and the immunoconjugates were characterized by reversed-phase chromatography and FTIR spectroscopy. Finally, the immunogenicity of the conjugated molecules was tested in a mouse model. The results showed that specific antibodies (total IgG) titers against conjugate vaccine were higher than nonconjugated capsular polysaccharides. The conditioning activity of conjugate vaccine antiserum is significantly higher than polysaccharides alone. In addition, the bacterial load of the conjugate vaccine group challenged with S. aureus COL strain cells was significantly reduced compared to the PBS and nonconjugated control groups. In conclusion, their results indicate that the immunoconjugates could be developed as a potential vaccine candidate against S. aureus [86]. Tiansen Li et al., obtained applicated immunogenic conjugate antigens by using ADH as a bridge, EDAC as a coupling agent to couple polysaccharides and proteins. The conjugate was immunized in mice by and determinate the antibody titer by indirect enzyme-linked immunosorbent assay (ELISA), the results showed that antibodies appeared on the 14th day after immunization and persisted [87]. Although ADH has been widely used as an effective linker, as a six-carbon spacer arm, ADH cannot effectively reduce the steric shielding between PS and protein, moreover, the spacer arm may produce a new antigen structure, which may be harmless or toxic, and may lead to the production of unnecessary non-protective antibodies. Therefore, the non-toxic and non-immunogenic long spacer arm is an ideal candidate for the development of conjugate vaccines, and studies have shown that polyethylene glycol (PEG) may be a good choice [88]. PEG hydrogels are easy to modify to allow degradation and inclusion of biomolecules and other cell adhesion ligands to promote specific cell functions, its biological purification effect is widely used for surface modification of artificial medical materials. Additionally, it can also inhibit protein adhesion [89, 90].
|
Download:
|
| Fig. 3. Common technical route for preparing polysaccharide conjugate vaccine. i) Activate the polysaccharide by CADP, add the linker ADH, then add EDAC and protein to couple the polysaccharide to the protein to obtain a polysaccharide conjugate vaccine, ii) or use NaIO4 to oxidize the polysaccharide to obtain an aldehyde group, and then react with the protein to obtain a carbon-nitrogen double bond, the polysaccharide protein conjugated vaccine is obtained under the action of the reducing agent NaBH3CN, iii) or the protein is activated at the same time as the polysaccharide is activated, the carboxyl group of the protein reacts with hydrazine, and then reacts with the activated polysaccharide, and finally the polysaccharide conjugate vaccine is obtained under the action of NaBH4. Ps, polysaccharide; Pr, protein. | |
In future research, we should look for better linkers, and improve the synthesis method at the same time, so as to obtain polysaccharide conjugate vaccine with better immune activity.
5. Quality controlGlycoconjugates involve several important aspects, such as cellular metabolism, structure, assembly and identification, and are information carriers through biological interaction. In the study of the structure of these compounds, it is important to analyze the composition of the carbohydrate moiety and ensure its physical and chemical properties [91]. WHO provides excellent guidance for key quality control tests at each stage of Hib conjugate vaccine, meningococcal conjugate vaccine and pneumococcal conjugate vaccine. For example, activated polysaccharides should be tested for their degree of activation and size, carrier proteins should be tested for purity, and polysaccharide conjugate vaccines should be tested for stability, identity and etc. [30]. Therefore, after the successful coupling of polysaccharides and proteins, various tests are required to ensure its stability, safety and effectiveness. This section briefly introduces the instruments and methods commonly used to detect polysaccharide conjugate vaccines.
5.1. Size exclusion chromatography (SEC)When polysaccharide is used as an antigen, its immunogenicity is directly related to its molecular weight. Therefore, the distribution coefficient of SEC can be a standard and also the requirement that the tested vaccine needs to meet. SEC is a method with rapid and reasonable reproducibility [92], which was initially performed on a cross-linked agarose gel (Sepharose CL-4B). In 1989, Newland et al. reported high-performance SEC (HPSEC) on a 2-column set packed with TSK G5000 PW and TSK G2500 PW (Toyo Soda, Tokyo, Japan) in series, with 0.2 mol/L aqueous ammonium acetate as eluent, which can obtain comparable results in a relatively short time, the demand for samples is small, and the molecular weight caused by depolymerization during vaccine storage distribution changes can be easily detected by HPSEC technology [93]. In order to confirm molecular weight, SEC must be used in conjunction with a detector that provides molecular weight information. The combination of SEC and light scattering detectors can determine and/or confirm molecular weight in a better way. Size exclusion chromatography coupled with multi-laser light scattering (SEC-MALS) can provide information about sample size, shape and concentration [94]. At the same time, the protein in the polysaccharide conjugate vaccine also needs to be detected. When evaluating the formulation and stability of therapeutic proteins, it is important to detect aggregates (dimers or multimers of the protein), because the presence of certain aggregates can significantly to change the characteristics of therapeutic proteins, such as efficacy and immunogenicity, and SEC can be used to detect the aggregates [92], However, if the protein is non-spherical (such as highly-coiled proteins) and its stokes radius is greater than the radius of the globular protein, then the size of the protein or its aggregates may be overestimated. In addition, if the protein contains carbohydrates or interacts with the chromatographic column, the protein elution profile may have a change, resulting in an erroneous estimation of its molecular weight. To solve these problems, SEC-HPLC could be used in conjunction with both light scattering and refractive index detectors to determine the size of protein or its aggregates [95]. SEC has been used to detect the molecular weight and aggregation of polysaccharides in polysaccharide vaccines. In addition, according to the research of Tingting Zhang et al., SEC can also be used for the purification of polysaccharide protein conjugates [96].
5.2. High performance anion-exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD)The presence of free polysaccharides in vaccine preparations can reduce the immune activity of conjugate vaccine. Studies have shown that when the amount of free polysaccharide is higher than 10% of conjugated polysaccharide, it will inhibit type-specific immune [97], therefore, the polysaccharide content of the combined vaccine needs to be tested. HPAEC-PAD is used to estimate free and total saccharide content for free and total saccharide contents estimation. HPAEC (also known as ion chromatography (IC)), combined with PAD provides an effective platform for the separation and quantification of carbohydrates, with the characteristics of high selectivity, sensitivity and accuracy [98], it was developed in 1990 and can be used for detection without relying on fluorescence or absorption labels. It can track the fate of substrates and products, so analysts can judge the success of the reaction [99]. HPAEC-PAD is a good method to separate neutral sugar, acid sugar and reducing sugar [100]. HPAE-PAD can detect natural oligosaccharides, although PAD is not as sensitive as fluorescence detection, the lack of labeling-induced bias provides a more reliable characterization of components in the released glycan pool [101]. HPAE-PAD has been used to quantify polysaccharides, determine the amount of conjugated and free polysaccharides in glycoconjugate vaccine, measure vaccine stability, and determine polysaccharide impurities. It has been used for Hib, Streptococcus pneumoniae, Neisseria meningitis vaccine, and etc. [102]. Some polysaccharides contain sialic acid (such as Neisseria meningitidis polysaccharides). Sialic acid is a family of negatively charged monosaccharides and is a monosaccharide family sharing a common 9-carbon bone structure. In most cases, they bind to the C3 or C6 of the former sugar with its C2, and usually exist at the end of the oligosaccharide. The amine group on C5 of sialic acid is mainly modified to N-acetyl or N-hydroxyacetyl to form Neu5Ac and Neu5Gc, respectively. The most common sialic acid is N-actetylneuraminic acid (abbreviated as NeuAc, Neu5Ac or NANA) [103, 104], mild hydrolysis conditions (weak acid/lower temperature) are required to depolymerize the polysaccharide to release sialic acid without causing too much chemical damage (degradation) in order to quantify by subsequent HPAEC-PAD analysis [105], M.M. Ho et al., have already explained this in detail [106]. In simple terms, the steps of the operation are to hydrolyze glycoproteins or other glycoconjugates in volatile acids (such as TFA) to release monosaccharides, remove the acid by evaporation, reconstitute the sample in deionized water, and then inject HPAE-PAD system analysis. Subsequently, the monosaccharide was separated on the Dionex CarboPac PA1 column of 16 mmol/L sodium hydroxide mobile phase, and then detected by PAD [107].
5.3. Nuclear magnetic resonance (NMR)NMR spectroscopy is used for advanced characterization and quality control of existing and novel products. It can be used for structural determination and identity/integrity detection of carbohydrate composition, as well as absolute quantification of PS [108]. For polysaccharide conjugate vaccines, the identity of glycan chains needs to be determined, which should include an assessment of the degree of O-acetylation (studies have shown that the presence of O-acetyl groups can affect the immunogenicity induced by polysaccharides. The results of studies conducted by David S. Berry et al. showed that the O-acetyl groups of polysaccharides from meningococcal group A affect their immunogenicity. O-Acetylation is obviously an important parameter that is maintained during PS protein binding [109]), as labile substituents may be lost in the manufacturing process [110]. NMR spectroscopy is a powerful analytical technique that can be used to determine the composition and structure of repeating units in bacterial polysaccharides, reveal all structural characteristics of bacterial polysaccharides, monitor the entire coupling process, study intermediates in the conjugation process, and also identify and quantify the residual reagents and impurities generated during this process [111, 112]. NMR spectroscopy is used to analyze glycosidic linkages, ring configuration and anomeric configuration of monomeric units. 2D NMR (nuclear overhauser effect spectroscopy (NOESY), total correlation spectroscopy (TOCSY), correlation spectroscopy (COSY)) is the main method for analyzing the chemical composition of exopolysaccharides [113]. NMR spectroscopy can check the chemical integrity of industrially produced sugar vaccine products, which provides a conventional release test for identifying the identity and consistency of bulk monovalent, blended, activated and conjugated polysaccharide components in conjugates. Circular dichroism spectroscopy (CD) is a complementary method of NMR, which can monitor the secondary structure changes of carrier protein before and after the binding process [24]. In short, NMR could be applicated to verify the purity, structure and identity of PS, and has been used for the systematic physical and chemical characterization of meningococcal vaccines [114].
5.4. Enzyme-linked immunosorbent assay (ELISA)Since the introduction of ELISA in 1971, it has become the first choice for the determination of soluble antigens and antibodies. ELISA shows the antigen-antibody reactions through the color change produced by enzyme-linked conjugate and enzyme substrate and is used to identify the presence and concentration of molecules in biological fluids [115, 116]. Enzyme-linked immunosorbent assay (ELISA) is widely used for quantification of various polysaccharides and antibody reaction after vaccination with polysaccharide conjugate vaccine. To determine the anti-Hib antibody titer in human serum, a commercial enzyme-linked immunosorbent assay kit can be used [117]. Because the structure and characteristics of the tested substances are not always the same, a variety of ELISA types have been developed to improve the specificity of the measurement, such as direct ELISA, indirect ELISA, sandwich ELISA, competitive ELISA [115]. According to refs. [118-120], ELISA has been widely used to detect the corresponding antibody titers of meningococcal polysaccharide conjugate vaccine, pneumonia polysaccharide conjugate vaccine and Hib polysaccharide conjugate vaccine. ELISA is now widely used to detect antibodies against protein antigens. Because these substances have many acidic groups, usually with a net negative charge, and have poor adsorption to plastics and other support materials, it is difficult to apply this technique for antibody measurement to bacterial polysaccharides, Barry M. Gray et al. solved this problem by first covalently binding the polysaccharide to the protein and then adsorbing it on the plastic, thereby fixing the polysaccharide antigen. The coupling method is simple, fast and uses a small amount of antigen. This makes the application of ELISA technology suitable for quantitative antibody determination possible [121]. Fátima Reyes et al. established four sandwich ELISAs for quantifying capsular polysaccharide (CP) in conjugated and plain meningococcal CP-based vaccines. ELISA shows good precision and high sensitivity. The results show that these assays are suitable for screening multiple vaccine samples and can be used for batch-to-batch consistency and stability analysis [122]. Although NMR can be used to identify the identity of PS, building the NMR laboratory requires a lot of investment, which increases the cost of the vaccine. Therefore, WHO recommends that ELISA can be used as an alternative test for PS identify [123].
5.5. OthersIn addition to the several commonly used instruments described above, there are many other methods for detecting the quality of polysaccharide conjugate vaccines. After the polysaccharide and protein are coupled together, it is necessary to determine the success of the coupling. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) has been used for protein separation and molecular weight determination for decades, and can be used to detect whether the protein and polysaccharide in the polysaccharide conjugate vaccine are successfully coupled [124]. For conjugates that are successfully coupled, we need to perform quality control on their proteins, polysaccharides, and some of the main reagents used in the synthesis process. Linker-ADH used in the synthesis process, the residual amount needs to be detected. After derivatizing the polysaccharide conjugate vaccine with PBA, high performance liquid chromatography-reversed phase (HPLCRP) was used to analyze the residual amount of ADH [125]. The conjugate needs to be purified, currently available methods for purifying polysaccharide conjugate protein vaccines include size-exclusion chromatography, hydrophobic interaction chromatography, reverse-phase chromatography, ammonium sulfate precipitation and ultrafiltration and etc. [126]. Parinaz Emami et al., apply the results of Hadidi et al., and Emami et al., to improve the performance of ultrafiltration to purify the conjugated vaccine (which is highly charged vaccine conjugate made by coupling a CPS from S. pneumoniae to CRM197). After this team's research, their method can remove more than 98% of the free polysaccharides, which is a major improvement compared to the previously reported purification results for such conjugate vaccines. These results demonstrate the potential of using ultrafiltration/diafiltration to purify the conjugate vaccine product [126]. The use of HPAEC-PAD to quantify the amount of polysaccharide in the vaccine is described above. In addition, the total polysaccharide in the purified conjugate can be estimated by the resorcinol assay. The amount of free sugar is measured after combining the sample with sodium deoxycholate. In addition, PS content can also be measured by the phosphorus method [96, 127]. Some polysaccharides are O-acetylated, such as meningococcal group C capsular polysaccharides, for this class of polysaccharides, the O-acetyl content should be monitored [128], the O-acetyl content of PS can be determined by colorimetric assay [129]. Protein concentration can be measured by Lowry method [130]. The quality control of polysaccharide conjugate vaccine is very important. When preparing polysaccharide conjugate vaccines, the quality of polysaccharide conjugate vaccines should be strictly controlled to meet WHO's various quality control requirements for vaccines to ensure their safety, stability and effectiveness. We have briefly summarized the test contents and test methods of the polysaccharide conjugate vaccine mentioned in this review (Table 2).
|
|
Table 2 Materials or methods usually required to prepare polysaccharide conjugate vaccine. |
This review introduces the development, preparation, and equipment currently used for quality control of polysaccharide conjugate vaccines. Recent years, the development of polysaccharide conjugate vaccines is rapid, but the marketed polysaccharide conjugate vaccines are still few and cannot fully meet the needs. Especially for developing countries, the cost and technical shortage of preparing polysaccharide conjugate vaccines have slowed the development of polysaccharide conjugate vaccines. There are many factors can affect the immunogenicity of glycoconjugates vaccines, such as the length of the sugar, the amount of incorporated sugar, the type of sugar or charge on the nonreducing terminal end, coupling chemistry and the linker used for coupling, the carbohydrate chain is still a controversial issue, and it is reported that different vaccine candidates require different lengths to produce the best immune response. At the same time, the size of sugar must be sufficiently large to represent of the native antigen epitopes (antigenic epitopes are a dangerous signal to stimulate and mature APC, and then activate T cells) [88, 127, 131]. Therefore, in the process of developing vaccines, we need to take these factors into consideration and take certain measures to control their quality. Although the carriers currently used in polysaccharide conjugate vaccines, such as DT, TT, CRM197, have good effects and are widely used, the observed effect of CIES is still a problem that cannot be ignored. We should find the mechanism that causes CIES and propose possible solutions. Studies have shown that PEG coupling can significantly reduce TT-specific IgG titers caused by PS-TT, thereby enhancing PS-TT-induced PS-specific IgG titers, thereby enhancing PS-TT-induced PS-specific IgG titers degree [132]. At the same time, we should develop more protein carriers that can be used to prepare vaccines to promote the development of polysaccharide conjugate vaccines. As Fikri Avci et al. put forward, we should: ⅰ) understand the effect of the size of the polysaccharide or the size of the conjugate on the immune response, ⅱ) evaluate the immune mechanism of the carrier protein to change the immune response, ⅲ) conduct more research to verify the impact of CIES, ⅳ) understand the correlations leading to the competition of polysaccharide-antigen Basic immunological mechanisms, ⅴ) discover and applicate more useful analytical tools to analyze various characterizations of polysaccharide conjugate vaccines, ⅵ) study the effect of coupling chemistry on the immunogenicity of polysaccharides, etc. [133]. Vaccine preparations as biological products are a special class of drugs, and their quality control requirements are particularly strict. WHO has different requirements for the quality control of different vaccines. Each country also has its own requirements, although there are many instruments and methods that can be used to detect vaccines and are constantly being improved and supplemented, we should find more advanced instruments or more rigorous detection methods to quantify the substance of polysaccharide conjugate vaccine and verify its immunogenicity. In addition, adjuvants can be added to the polysaccharide conjugate vaccine to enhance immunogenicity. Adjuvants have been widely used in vaccine development in recent years. Adjuvants can be used to enhance specific immune responses against antigens. The development of combination vaccines also requires consideration of appropriate delivery routes and adjuvants. Some researchers have suggested that Toll-like receptors (TLR) can be used as a built-in adjuvant to participate in the development of vaccines during the vaccine development process, which can have a better immune effect [7]. Combining the conjugated vaccine and adjuvant in the same delivery system is considered to be more effective than co-administration in increasing the activity of the irritating adjuvant. And studies have shown that the use of β-glucan and Toll-like receptor 7 agonist (TLR7a) in combination with meningococcal conjugate vaccine can significantly improve PS-specific immunogenicity [132]. Drug delivery vehicles can effectively encapsulate drugs and deliver them to target tissues at the desired level for treatment without breaking down the therapeutic agent. Several drug carrier systems have recently emerged, such as nanoparticles, hydrogels, microspheres, liposomes, and polymer micelles, which adsorb, encapsulate and covalently bond drugs through complex methods [134]. We can apply the carrier concept to polysaccharide conjugate vaccines to improve their immunogenicity. Junfeng Liu et al. designed a simple and competent nanocarrier of mannose modified PEGylated branched PEI25k (PEI-PEG-Man), the research results show that PEI-PEG-Man nanoparticles encapsulate CpG (PEI-PEG-Man@CpG) may be used as a powerful carrier for in vitro engineering to promote DC targeting and maturation, thereby improving the efficiency of vaccines against cancer or infectious diseases [135]. Therefore, in the future development, we should consider in many ways, and strive to improve its effectiveness while ensuring its safety and stability.
In short, we can see that the polysaccharide conjugate vaccine is a kind of vaccine with great development potential and can be a sharp edge for us to prevent diseases. At the same time, we can also try to expand its application fields to other fields, such as tumors, tuberculosis, influenza, which may have unexpected results.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work is funded by National Natural Science Foundation of China (No. 82073311), Key Research Development Program of Science and Technology Department of Sichuan Provincial (No. 2019YFS0514), National Natural Science Foundation of China (No. 81741100), the Natural Science Foundation of Beijing, China (No. 6181001), National Key Research and Development Program of China (No. 2020YFC2005500), Key Research and Development Program of Science and Technology Department of Sichuan Province (No. 2019YFS0514) and The Major Science and Technology R & G Program of Jiangxi Province (No. 20194ABC28007).
| [1] |
M. Vella, D. Pace, Expert Opin. Biol. Ther. 15 (2015) 529-546. DOI:10.1517/14712598.2015.993375 |
| [2] |
A.E. Zarei, H.A. Almehdar, E.M. Redwan, J. Immunol. Res. 2016 (2016) 7203587. |
| [3] |
C.M. Wilfert, Pediatrics 85 (1990) 631-635. |
| [4] |
F.E. Andre, R. Booy, H.L. Bock, et al., Bull. World Health Organ. 86 (2008) 140-146. DOI:10.2471/BLT.07.040089 |
| [5] |
C.P. Gross, K.A. Sepkowitz, Int. J. Infect. Dis. 3 (1998) 54-60. DOI:10.1016/S1201-9712(98)90096-0 |
| [6] |
M. Gou, M. Dai, X. Li, et al., Colloids Surf. B: Biointerfaces 64 (2008) 135-139. DOI:10.1016/j.colsurfb.2007.12.014 |
| [7] |
Z. Zhou, H. Lin, C. Li, Z. Wu, Chin. Chem. Lett. 29 (2018) 19-26. DOI:10.1016/j.cclet.2017.09.047 |
| [8] |
W. Song, J. Cai, X. Zou, X. Wang, J. Hu, J. Yin, Chin. Chem. Lett. 29 (2018) 27-34. DOI:10.1016/j.cclet.2017.09.061 |
| [9] |
F. Micoli, P. Costantino, R. Adamo, FEMS Microbiol. Rev. 42 (2018) 388-423. DOI:10.1093/femsre/fuy011 |
| [10] |
M. Sun, H. Hu, L. Sun, Z. Fan, Chin. Chem. Lett. 31 (2020) 1729-1736. DOI:10.1016/j.cclet.2020.02.035 |
| [11] |
A.H. Lucas, M.A. Apicella, C.E. Taylor, Clin. Infect. Dis. 41 (2005) 705-712. DOI:10.1086/432582 |
| [12] |
J. Hutter, B. Lepenie, Methods Mol. Biol. 1331 (2015) 1-10. |
| [13] |
M. Broker, F. Berti, J. Schneider, I. Vojtek, Vaccine 35 (2017) 3286-3294. DOI:10.1016/j.vaccine.2017.04.078 |
| [14] |
J.A. Herbert, E.J. Kay, S.E. Faustini, et al., Vaccine 36 (2018) 3809-3819. DOI:10.1016/j.vaccine.2018.05.036 |
| [15] |
Centers for Disease Control and Prevention (CDC), MMWR Morb. Mortal. Wkly. Rep. 51 (2002) 234-237. |
| [16] |
I.T. Burns, R.K. Zimmerman, J. Fam. Pract. 49 (2000) S7-S14. |
| [17] |
J.S. Kim, Y.T. Jang, J.D. Kim, Vaccine 22 (2004) 3952-3962. DOI:10.1016/j.vaccine.2004.04.003 |
| [18] |
R.E. Rayner, J. Savill, L.M. Hafner, F. Huygen, Future Microbiol. 10 (2015) 653-664. DOI:10.2217/fmb.14.153 |
| [19] |
R. Cherazard, M. Epstein, T.L. Doan, et al., Am. J. Ther. 24 (2017) e361-e369. DOI:10.1097/MJT.0000000000000551 |
| [20] |
D.H. Engholm, M. Kilian, D.S. Goodsell, E.S. Andersen, R.S. Kjærgaard, FEMS Microbiol. Rev. 41 (2017) 854-879. DOI:10.1093/femsre/fux037 |
| [21] |
S. Hollingshead, C.M. Tang, Methods Mol. Biol. 1969 (2019) 1-16. |
| [22] |
N.G. Rouphael, D.S. Stephens, Methods Mol. Biol. 799 (2012) 1-20. |
| [23] |
P.V. Scaria, B.B. Chen, C.G. Rowe, et al., Vaccine 38 (2020) 5480-5489. DOI:10.1016/j.vaccine.2020.06.018 |
| [24] |
T.E. MacCalman, M.K. Phillips-Jones, S.E. Harding, Biotechnol. Genet. Eng. Rev. 35 (2019) 93-125. DOI:10.1080/02648725.2019.1703614 |
| [25] |
X. Sun, G. Stefanetti, F. Berti, D.L. Kasper, Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 193-198. DOI:10.1073/pnas.1816401115 |
| [26] |
F.Y. Avci, D.L. Kasper, Annu. Rev. Immunol. 28 (2010) 107-130. DOI:10.1146/annurev-immunol-030409-101159 |
| [27] |
F.Y. Avci, X. Li, M. Tsuji, D.L. Kasper, Nat. Med. 17 (2011) 1602-1609. DOI:10.1038/nm.2535 |
| [28] |
F. Thiele, S. Tao, Y. Zhang, et al., J. Virol. 89 (2014) 2698-2709. |
| [29] |
M. Cavallari, G. De Libero, Vaccines 5 (2017) 4. DOI:10.3390/vaccines5010004 |
| [30] |
C.E. Frasch, Vaccine 27 (2009) 6468-6470. DOI:10.1016/j.vaccine.2009.06.013 |
| [31] |
E.C. Briere, MMWR Morb. Mortal. Wkly. Rep. 65 (2016) 418-419. DOI:10.15585/mmwr.mm6516a3 |
| [32] |
Centers for Disease Control and Prevention (CDC), MMWR Morb. Mortal. Wkly. Rep. 58 (2009) 1008-1009. |
| [33] |
S.T. Duggan, Drugs 70 (2010) 1973-1986. DOI:10.2165/11205110-000000000-00000 |
| [34] |
A.C. Berical, D. Harris, C.S. Dela Cruz, J.D. Possick, Ann. Am. Thorac. Soc. 13 (2016) 933-944. DOI:10.1513/AnnalsATS.201511-778FR |
| [35] |
S. Tomczyk, N.M. Bennett, C. Stoecker, et al., MMWR Morb. Mortal. Wkly. Rep. 63 (2014) 822-825. |
| [36] |
A. Badahdah, H. Rashid, A. Khatami, Expert Rev. Vaccines 15 (2016) 9-29. DOI:10.1586/14760584.2016.1115726 |
| [37] |
N. Ilyina, S. Kharit, L. Namazova-Baranova, et al., Hum. Vaccin. Immunother. 10 (2014) 2471-2481. DOI:10.4161/hv.29571 |
| [38] |
D. Greenberg, P.A. Hoover, T. Vesikari, et al., Vaccine 36 (2018) 6883-6891. DOI:10.1016/j.vaccine.2018.02.113 |
| [39] |
J.T. Peterson, H.L. Stacey, J.E. MacNair, et al., Hum. Vaccin. Immunother. 15 (2019) 540-548. DOI:10.1080/21645515.2018.1532250 |
| [40] |
C.A. Lexau, R. Lynfield, R. Danila, et al., JAMA 294 (2005) 2043-2051. DOI:10.1001/jama.294.16.2043 |
| [41] |
C.G. Whitney, M.M. Farley, J. Hadler, et al., N. Engl. J. Med. 348 (2003) 1737-1746. DOI:10.1056/NEJMoa022823 |
| [42] |
W.T. Sime, A. Aseffa, Y. Woldeamanuel, et al., BMC Infect. Dis. 19 (2019) 409. DOI:10.1186/s12879-019-4024-1 |
| [43] |
E.D. McCollum, S. Ahmed, A.D. Roy, et al., Vaccine 38 (2020) 6508-6516. DOI:10.1016/j.vaccine.2020.08.035 |
| [44] |
A. Matanock, G. Lee, R. Gierke, et al., MMWR Morb. Mortal. Wkly. Rep. 68 (2019) 1069-1075. DOI:10.15585/mmwr.mm6846a5 |
| [45] |
I. Caro-Aguilar, L. Indrawati, R.M. Kaufhold, et al., Vaccine 35 (2017) 865-872. DOI:10.1016/j.vaccine.2016.12.055 |
| [46] |
D. Hurley, C. Griffin, M. Young, et al., Clin. Infect. Dis. (2020) ciaa1045. DOI:10.1093/cid/ciaa1045 |
| [47] |
R. Borrow, R. Abad, C. Trotter, F.R. van der Klis, J.A. Vazquez, Vaccine 31 (2013) 4477-4486. DOI:10.1016/j.vaccine.2013.07.083 |
| [48] |
J. Southern, R. Borrow, N. Andrews, et al., Clin. Vaccin. Immunol. 16 (2009) 194-199. DOI:10.1128/CVI.00420-08 |
| [49] |
P. Richmond, R. Borrow, E. Miller, et al., J. Infect. Dis. 179 (1999) 1569-1572. DOI:10.1086/314753 |
| [50] |
T. Vesikari, R. Borrow, A. Forsten, et al., Hum. Vaccin. Immunother. 16 (2020) 1306-1312. DOI:10.1080/21645515.2020.1733869 |
| [51] |
M. Broker, Hum. Vaccin. Immunother. 12 (2016) 664-667. DOI:10.1080/21645515.2015.1086048 |
| [52] |
F. Micoli, R. Adamo, P. Costantino, Molecules 23 (2018) 1451. DOI:10.3390/molecules23061451 |
| [53] |
T. Uchida, A.M. Pappenheimer Jr., R. Greany, J. Biol. Chem. 248 (1973) 3838-3844. DOI:10.1016/S0021-9258(19)43810-6 |
| [54] |
K. Wang, L. Zhou, X. Zhang, et al., Antiviral Res. 164 (2019) 154-161. DOI:10.1016/j.antiviral.2019.02.013 |
| [55] |
T. Uchida, Pharmacol. Ther. 19 (1982) 107-122. DOI:10.1016/0163-7258(82)90043-2 |
| [56] |
F. Najafzadeh, R. Shapouri, M. Rahnema, et al., Jundishapur J. Microbiol. 8 (2015)) e17712. |
| [57] |
M.R. Capeding, S. Teshome, T. Saluja, K.A. Syed, Vaccine 36 (2018) 3794-3801. DOI:10.1016/j.vaccine.2018.05.038 |
| [58] |
C. Bayart, S. Peronin, E. Jean, et al., J. Chromatogr. B: Analyt. Technol. Biomed. Life Sci. 1054 (2017) 80-92. DOI:10.1016/j.jchromb.2017.04.009 |
| [59] |
T. Vesikari, R. Borrow, A. Forsten, et al., Hum. Vaccin. Immunother. 16 (2020) 1306-1312. DOI:10.1080/21645515.2020.1733869 |
| [60] |
G. Áñez, J. Hedrick, M.W. Simon, et al., Hum. Vaccin. Immunother. 16 (2020) 1292-1298. DOI:10.1080/21645515.2020.1733867 |
| [61] |
J.M. Hickey, V.M. Toprani, K. Kaur, et al., J. Pharm. Sci. 107 (2018) 1806-1819. DOI:10.1016/j.xphs.2018.03.002 |
| [62] |
R.P.N. Mishra, R.S.P. Yadav, C. Jones, et al., Biosci. Rep. 38 (2018) BSR20180238. DOI:10.1042/BSR20180238 |
| [63] |
M. Tontini, F. Berti, M.R. Romano, et al., Vaccine 31 (2013) 4827-4833. DOI:10.1016/j.vaccine.2013.07.078 |
| [64] |
R. Rappuoli, Appl. Environ. Microbiol. 46 (1983) 560-564. DOI:10.1128/aem.46.3.560-564.1983 |
| [65] |
H.R. Shinefield, Vaccine 28 (2010) 4335-4339. DOI:10.1016/j.vaccine.2010.04.072 |
| [66] |
G. Giannini, R. Rappuoli, G. Ratti, Nucleic Acids Res. 12 (1984) 4063-4069. DOI:10.1093/nar/12.10.4063 |
| [67] |
M. Bröker, P. Costantino, L. DeTora, et al., Biologicals 39 (2011) 195-204. DOI:10.1016/j.biologicals.2011.05.004 |
| [68] |
A. Stefan, M. Conti, D. Rubboli, et al., J. Biotechnol. 156 (2011) 245-252. DOI:10.1016/j.jbiotec.2011.08.024 |
| [69] |
R. Dagan, J. Poolman, C.A. Siegrist, Vaccine 28 (2010) 5513-5523. DOI:10.1016/j.vaccine.2010.06.026 |
| [70] |
Y. Chang, X. Meng, Y. Li, et al., MedChemComm 10 (2019) 543-553. DOI:10.1039/C8MD00546J |
| [71] |
S. Pecetta, B. Vijayakrishnan, M.R. Romano, et al., Vaccine 34 (2016) 1405-1411. DOI:10.1016/j.vaccine.2016.01.040 |
| [72] |
S. Pecetta, M. Tontini, E. Faenzi, et al., Vaccine 34 (2016) 2334-2341. DOI:10.1016/j.vaccine.2016.03.055 |
| [73] |
G. Yin, J. Wei, Y. Shao, et al., Chin. Chem. Lett. 32 (2021) 353-356. DOI:10.1016/j.cclet.2020.04.034 |
| [74] |
C.C. Peeters, P.R. Lagerman, O. de Weers, et al., Methods Mol. Med. 87 (2003) 153-174. |
| [75] |
K. Jiang, H. Zhu, C. Xiao, et al., Anal. Chim. Acta 962 (2017) 32-40. DOI:10.1016/j.aca.2017.01.049 |
| [76] |
H. Alinezhad, M. Tajbakhsh, N. Hamidi, Chin. Chem. Lett. 21 (2010) 47-50. DOI:10.1016/j.cclet.2009.07.018 |
| [77] |
A.E.B. Turner, J.E. Gerson, H.Y. So, et al., Synth. Syst. Biotechnol. 2 (2017) 49-58. DOI:10.1016/j.synbio.2016.12.002 |
| [78] |
A. Pawlowski, G. Källenius, S.B. Svenson, Vaccine 17 (1999) 1474-1483. DOI:10.1016/S0264-410X(98)00385-5 |
| [79] |
L. Lin, M. Qiao, X. Zhang, R.J. Linhardt, Carbohydr. Polym. 14 (1996) 190-198. |
| [80] |
A. Lees, B.L. Nelson, J.J. Mond, Vaccine 14 (1996) 190-198. DOI:10.1016/0264-410X(95)00195-7 |
| [81] |
D.E. Shafer, B. Toll, R.F. Schuman, et al., Vaccine 18 (2000) 1273-1281. DOI:10.1016/S0264-410X(99)00370-9 |
| [82] |
Z. Jin, C. Chu, J.B. Robbins, R. Schneerson, Infect. Immun. 71 (2003) 5115-5120. DOI:10.1128/IAI.71.9.5115-5120.2003 |
| [83] |
D. Kim, H. Yoon, S. Kim, et al., J. Microbiol. Biotechnol. 28 (2018) 2113-2120. DOI:10.4014/jmb.1810.10009 |
| [84] |
I.A. Silveira, R.C. Bastos, M.S. Neto, et al., Vaccine 25 (2007) 7261-7270. DOI:10.1016/j.vaccine.2007.07.037 |
| [85] |
C.H. Lee, W.C. Kuo, S. Beri, et al., Vaccine 27 (2009) 726-732. DOI:10.1016/j.vaccine.2008.11.065 |
| [86] |
K. Ahmadi, M.M. Aslani, G. Pouladfar, et al., IUBMB Life 72 (2020) 226-236. DOI:10.1002/iub.2159 |
| [87] |
T. Li, M. Huang, Z. Song, H. Zhang, C. Chen, Can. J. Vet. Res. 82 (2018) 48-54. |
| [88] |
Q. Huang, D. Li, A. Kang, et al., J. Control. Release 172 (2013) 382-389. DOI:10.1016/j.jconrel.2013.03.008 |
| [89] |
Q. Wang, J. Xu, H. Jin, et al., Chin. Chem. Lett. 28 (2017) 1801-1807. DOI:10.1016/j.cclet.2017.07.011 |
| [90] |
H. Kuang, S. Yang, Y. Wang, et al., J. Biomed. Nanotechnol. 15 (2019) 77-84. DOI:10.1166/jbn.2019.2666 |
| [91] |
T.R. Cataldi, C. Campa, G.E. De Benedetto, Fresenius J. Anal. Chem. 368 (2000) 739-758. DOI:10.1007/s002160000588 |
| [92] |
A. Planinc, J. Bones, B. Dejaegher, P. van Antwerpen, C. Delporte, Anal. Chim. Acta 921 (2016) 13-27. DOI:10.1016/j.aca.2016.03.049 |
| [93] |
S.C. Churms, J. Chromatogr. A 720 (1996) 151-166. DOI:10.1016/0021-9673(95)00305-3 |
| [94] |
P. Hong, S. Koza, E.S. Bouvier, J. Liq. Chromatogr. Relat. Technol. 35 (2012) 2923-2950. DOI:10.1080/10826076.2012.743724 |
| [95] |
W. Wang, Int. J. Pharm. 185 (1999) 129-188. DOI:10.1016/S0378-5173(99)00152-0 |
| [96] |
T. Zhang, W. Yu, Y. Wang, T. Hu, Vaccine 33 (2015) 3208-3214. DOI:10.1016/j.vaccine.2015.04.094 |
| [97] |
M.E. Rodriguez, G.P. van den Dobbelsteen, L.A. Oomen, et al., Vaccine 16 (1998) 1941-1949. DOI:10.1016/S0264-410X(98)00129-7 |
| [98] |
L. Yi, Y. Ouyang, X. Sun, et al., J. Chromatogr. A 1423 (2015) 79-85. DOI:10.1016/j.chroma.2015.10.064 |
| [99] |
Z. Zhang, N.M. Khan, K.M. Nunez, E.K. Chess, C.M. Szabo, Anal. Chem. 84 (2012) 4104-4110. DOI:10.1021/ac300176z |
| [100] |
S. Ricci, A. Bardotti, S. D'Ascenzi, N. Ravenscroft, Vaccine 19 (2001) 1989-1997. DOI:10.1016/S0264-410X(00)00427-8 |
| [101] |
Z. Szabo, J.R. Thayer, Y. Agroskin, et al., Anal. Bioanal. Chem. 409 (2017) 3089-3101. DOI:10.1007/s00216-017-0248-3 |
| [102] |
J.S. Rohrer, SLAS Technol. 25 (2019) 320-328. |
| [103] |
E.J.G.J. Higgins, Glycoconj. J. 27 (2010) 211-225. DOI:10.1007/s10719-009-9261-x |
| [104] |
X. Zhou, S. Yang, G. Yang, Z. Tan, F. Guan, Chin. Chem. Lett. 30 (2019) 676-680. DOI:10.1016/j.cclet.2018.12.016 |
| [105] |
S.K. Gudlavalleti, E.N. Crawford, J.D. Harder, J.R. Reddy, Anal. Chem. 86 (2014) 5383-5390. DOI:10.1021/ac5003933 |
| [106] |
M.M. Ho, B. Bolgiano, M.J. Corbel, Vaccine 19 (2000) 716-725. DOI:10.1016/S0264-410X(00)00261-9 |
| [107] |
J.S. Rohrer, L. Basumallick, D. Hurum, Biochemistry (Mosc.) 78 (2013) 697-709. DOI:10.1134/S000629791307002X |
| [108] |
S. Martini, M. Aggravi, S. Cianetti, et al., ACS Omega 4 (2019) 12827-12832. DOI:10.1021/acsomega.9b01678 |
| [109] |
D.S. Berry, F. Lynn, C.H. Lee, C.E. Frasch, M.C. Bash, Infect. Immun. 70 (2002) 3707-3713. DOI:10.1128/IAI.70.7.3707-3713.2002 |
| [110] |
C. Jones, J. Pharm. Biomed. Anal. 38 (2005) 840-850. DOI:10.1016/j.jpba.2005.01.044 |
| [111] |
C. Abeygunawardana, T.C. Williams, J.S. Sumner, J.P. Hennessey Jr, Anal. Biochem. 279 (2000) 226-240. DOI:10.1006/abio.1999.4470 |
| [112] |
S. Beri, D. Gandhi, N. Ravenscroft, Biologicals 62 (2019) 102-106. DOI:10.1016/j.biologicals.2019.10.005 |
| [113] |
S. Rana, L.S.B. Upadhyay, Int. J. Biol. Macromol. 157 (2020) 577-583. DOI:10.1016/j.ijbiomac.2020.04.084 |
| [114] |
F.Y. Avci, D.L. Kasper, Annu. Rev. Immunol. 28 (2010) 107-130. DOI:10.1146/annurev-immunol-030409-101159 |
| [115] |
S. Aydin, Peptides 72 (2015) 4-15. DOI:10.1016/j.peptides.2015.04.012 |
| [116] |
P.V. Hornbeck, Curr. Protoc. Immunol. 110 (2015) 2.1.1-2.1.23.. |
| [117] |
A. Hamidi, H. Kreeftenberg, Hum. Vaccin. Immunother. 10 (2014) 2697-2703. DOI:10.4161/hv.29300 |
| [118] |
K. Mimura, S. Kimura, C. Kajiwara, et al., Microbes Infect. 22 (2020) 312-321. DOI:10.1016/j.micinf.2019.12.005 |
| [119] |
P.W. Whitby, D.J. Morton, H.J. Mussa, L. Mirea, T.L. Stull, Vaccine 38 (2020) 2960-2970. DOI:10.1016/j.vaccine.2020.02.054 |
| [120] |
N.J. Beresford, A. Martino, I.M. Feavers, et al., Vaccine 35 (2017) 3598-3606. DOI:10.1016/j.vaccine.2017.03.066 |
| [121] |
B.M. Gray, J. Immunol, Methods 28 (1979) 187-192. |
| [122] |
F. Reyes, O. Otero, M. Cuello, et al., J. Immunol. Methods 407 (2014) 58-62. DOI:10.1016/j.jim.2014.03.020 |
| [123] |
N. Sharma, S. Hanif, D. Upadhyay, M.K. Chhikara, J. Immunol, Methods 473 (2019) 112634. |
| [124] |
I. Holková, D. Rauová, M. Mergová, L. Bezáková, P. Mikuš, Molecules 24 (2019) 4268. DOI:10.3390/molecules24234268 |
| [125] |
F. Micoli, S. Rondini, I. Pisoni, et al., Vaccine 30 (2012) 853-861. DOI:10.1016/j.vaccine.2011.11.108 |
| [126] |
P. Emami, S.P. Motevalian, E. Pepin, A.L. Zydney, Biotechnol. Bioeng. 116 (2019) 591-597. DOI:10.1002/bit.26867 |
| [127] |
J. Dalal, R. Rana, K. Harale, et al., Vaccine 37 (2019) 5297-5306. DOI:10.1016/j.vaccine.2019.07.053 |
| [128] |
L. Jódar, E. Griffiths, I. Feavers, Vaccine 22 (2004) 1047-1053. DOI:10.1016/j.vaccine.2003.08.040 |
| [129] |
M. Xu, X. Xing, Z. Wu, Y. Du, T. Hu, Vaccine 33 (2015) 5815-5821. DOI:10.1016/j.vaccine.2015.09.021 |
| [130] |
S.M.F. Albani, M.R. da Silva, M. Takagi, J. Cabrera-Crespo, Appl. Biochem. Biotechnol. 167 (2012) 2068-2075. DOI:10.1007/s12010-012-9750-4 |
| [131] |
A. Nilo, I. Passalacqua, M. Fabbrini, M. Allan, et al., Bioconjug. Chem. 26 (2015) 1839-1849. DOI:10.1021/acs.bioconjchem.5b00365 |
| [132] |
X. Chang, W. Yu, S. Ji, et al., Vaccine 35 (2017) 1698-1704. DOI:10.1016/j.vaccine.2017.02.027 |
| [133] |
F. Avci, F. Berti, P. Dull, et al., mSphere 4 (2019) e00520-19. |
| [134] |
R. Ahmad, Y. Deng, R. Singh, et al., J. Biomed. Nanotechnol. 14 (2018) 20-43. DOI:10.1166/jbn.2018.2476 |
| [135] |
J. Liu, J. Wang, Q. Zhu, et al., J. Biomed. Nanotechnol. 15 (2019) 1454-1467. DOI:10.1166/jbn.2019.2790 |
 2021, Vol. 32
2021, Vol. 32