b Department of Chemistry, Zhejiang Normal University, Jinhua 321004, China
Benzoxazinones are an important class of fused heterocycles that are widely present in diverse natural products and pharmaceuticals [1], such as TEI-5624 [2], Cetilistat [3], and AX-9657 (Fig. 1) [4]. In addition, they are also valuable intermediates in organic synthesis [5]. For example, 2-substituted benzoxazinones could be converted to bioactive quinazolinone derivatives. As a result, developing efficient protocols for the synthesis of benzoxazinones has been an important research topic in organic synthesis [6-9]. Conventionally, benzoxazinones can be synthesized through the annulation of N-acyl anthranilic acid or anthranilic acid [7]. Furthermore, benzoxazinones can also be prepared via Pd-catalyzed carbonylation of ortho-haloanilines [8] and oxidation of indoles [9]. Despite all these progress, most of these approaches tend to suffer from either requiring prefunctionalized substrates or harsh reaction conditions. Recently, transition-metal-catalyzed C–H activation has become a powerful synthetic tool in contemporary organic synthesis due to its high atom/step economy [10]. Hence, developing direct and efficient methods for the construction of benzoxazinones via C–H activation mode can be of great significance.

|
Download:
|
| Fig. 1. Biologically active benzoxazinones. | |
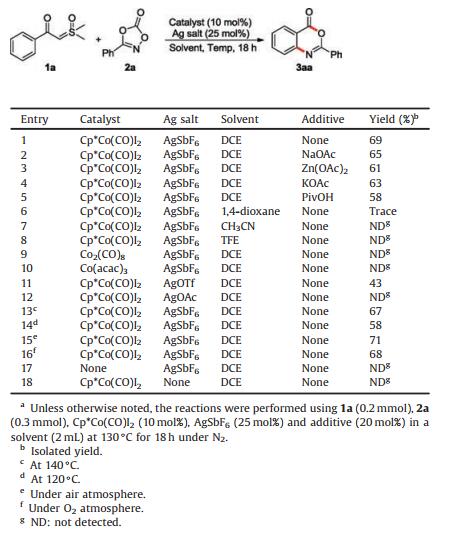
Indeed, the preparation of benzoxazinones through transition-metal-catalyzed C–H activation has been reported (Scheme 1) [11]. Lloyd-Jones, Booker-Milburn [11a] and Yu [11b] reported that benzoxazinones can be synthesizedvia Pd(Ⅱ)-catalyzed C–H carbonylation of aniline derivatives (Scheme 1a). It should be noted that these reactions require the use of toxic carbon monoxide gas. Kim [11c], Wang [11d] and Dong [11e] reported Rh(Ⅲ) or Ir(Ⅲ)-catalyzed C–H amidation of aldehydes or their equivalents to deliver 2-aminobenzaldehydes, which could further undergo intramolecular oxidative cyclization to give benzoxazinones (Scheme 1b). In these cases, the additional oxidation step was essential for the synthesis of benzoxazinones. Very recently, Zou reported a Rh(Ⅲ)-catalyzed cascade reaction of benzoic acids with dioxazolones for the construction of 2, 5-substituted benzoxazinones (Scheme 1c) [11g]. Though highly efficient, due to the cost issues associated with the use of palladium, iridium and rhodium metals, it would be highly desirable if similar transformations can be accomplished by employing cheaper catalysts. Recent studies have shown that Cp*Co(Ⅲ) catalysts are not only promising alternatives to Cp*Rh(Ⅲ) catalysts, they also show unique catalytic reactivity in C–H activation [12]. It is worth noting that enormous advances in this area have been accomplished by the groups of Cheng [13], Kanai [14], Matsunaga [15], Chang [16], Ackermann [17], Li [18], Glorius [19], Shi [20] and others [21]. Inspired by these studies and in continuation of our research on C–H bond functionalizations [22], we herein present a direct and practical method for the synthesis of benzoxazinones via Cp*Co(Ⅲ)-catalyzed C–H activation and [3+3] annulation reaction of sulfoxonium ylides [23] with dioxazolones (Scheme 1d). It should be mentioned that during the course of our investigation, Li reported a similar cobalt(Ⅲ)-catalyzed C–H functionalization of sulfoxonium ylides with dioxazolones under different reaction conditions and only amidation products were obtained (Scheme 1e) [24].
|
Download:
|
| Scheme 1. Comparison of previous works with this work. | |
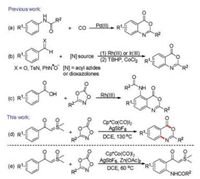
Our initial plan was to realize Cp*Co(Ⅲ)-catalyzed C(aryl)–H amidation of sulfoxonium ylides with dioxazolones as the amidating reagents. Much to our surprise, when we treated sulfoxonium ylide 1a (0.2 mmol) with dioxazolone 2a (0.3 mmol) in the presence of Cp*Co(CO)I2 (0.02 mmol), and AgSbF6 (0.05 mmol) in DCE (2 mL) under N2 at 130 ℃ for 18h, benzoxazinone derivative 3aa was obtained in 69% yield (Table 1, entry 1). Encouraged by this result, we further studied other reaction conditions for the transformation. It was found that introduction of additives such as NaOAc, Zn(OAc)2, KOAc and PivOH could not improve the yield of 3aa (entries 2–5). Subsequent screening of solvents suggested that DCE was the most efficient medium for the transformation, while other solvents such as 1, 4-dioxane, CH3CN, and TFE were totally ineffective (entries 6–8). Other cobalt catalysts such as Co2(CO)8 and Co(acac)3 were also examined and found to be ineffective for this reaction (entries 9 and 10). It was observed that the choice of Ag salt had an important effect on the reaction. When AgOTf was used in place of AgSbF6, 3aa could be isolated in 43% yield (entry 11). In sharp contrast, no desired product was detected when AgOAc was employed (entry 12). The yield of 3aa was not improved under elevated (67%, 140 ℃) or lower (58%, 120 ℃) reaction temperatures (entries 13 and 14). It is worthwhile to note that when the reaction temperature was lowered to 70 ℃, onlyortho C–H amidation product of sulfoxonium ylide was obtained, and no cyclization product 3aa was detected. When the reaction was carried out under an air atmosphere, the yield of 3aa could be improved to 71% (entry 15). Control experiments revealed that both Cp*Co(CO)I2 and AgSbF6 were necessary for the formation of 3aa (entries 17 and 18). It should be mentioned that when another amidating reagent 5-phenyl-1, 3, 2, 4-dioxathiazole 2-oxide was employed in place of 2a, no desired product was detected (not shown in Table 1). Therefore, we decided to set reacting 1a with 1.5 equiv. of 2a in the presence of 10 mol% of Cp*Co(CO)I2 and 25 mol% of AgSbF6 in DCE under air at 130 ℃ for 18h as our optimized reaction conditions. It should be noted that only 5 mol% of cobalt catalyst is enough to obtain similar yields when the reaction is run at larger scale, details see Supporting information.
|
|
Table 1 Optimization of the reaction conditions.a |
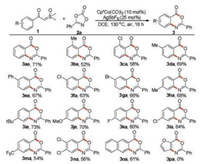
We next examined the substrate scope with respect to the sulfoxonium ylides under the optimized conditions, and the results are summarized in Scheme 2. It was observed that sulfoxonium ylides substituted with electron-donating or electron-withdrawing groups on the phenyl rings all reacted smoothly with dioxazolone 2a to provide the corresponding benzoxazinones in moderate to good yields (3aa-3oa). From the table, we can see that when ortho-substituted sulfoxonium ylides were used as the substrates, the corresponding products were obtained with lower yields (3ba and 3ca), which is perhaps due to steric hindrance. As for the meta-substituted sulfoxonium ylides, the C–H activation occurred exclusively on the less hindered site (3da-3ga). It should be mentioned that 3, 4-disubstituted sulfoxonium ylide 1n was found to be a viable substrate, affording the desired product 3na in 56% yield. Furthermore, sulfoxonium ylide with a naphthalene ring could also participate in the reaction to give the desired product 3oa in 61% yield. Unfortunately, heterocyclic sulfoxonium ylides such as α-2-furoyl sulfoxonium ylide were found to be incompatible for the system (Scheme 2, 3pa).
|
Download:
|
| Scheme 2. Substrate scope of sulfoxonium ylides. Reaction conditions: 1 (0.2 mmol), 2a (0.3 mmol), Cp*Co(CO)I2 (10 mol%), AgSbF6 (25 mol%), DCE (2 mL), 130 ℃, air, 18 h. Isolated yield. | |
Subsequently, the scope of dioxazolones was explored as well (Scheme 3). From the scheme, we can see that aryl-substituted dioxazolones bearing either electron-donating (methyl and methoxy) or electron-withdrawing (fluoro, chloro, bromo, and nitro) groups are all viable in this system, delivering the corresponding products in moderate to good yields (3ab−3al). However, 2-methylphenyl and 2-fluorophenyl-substituted dioxazolones (2b and 2c) were less reactive possibly due to steric effect. It should be noted that naphthalene ring and heterocycle substituted dioxazolones also reacted smoothly with 1a to deliver 3am and 3an in yields of 72% and 32%, respectively. We were also delighted to find that alkyl-substituted dioxazolone 2o and 2p also proved to be viable substrates, giving 3ao and 3ap in 38% and 53% yield, respectively. It is worthwhile to point out that compound 3gq, a high-density lipoprotein elevator [25], could also be synthesized in 63% yield under our reaction conditions.

|
Download:
|
| Scheme 3. Substrate scope of dioxazolones. Reaction conditions: 1 (0.2 mmol), 2 (0.3 mmol), Cp*Co(CO)I2 (10 mol%), AgSbF6 (25 mol%), DCE (2 mL), 130 ℃, air, 18 h. Isolated yield. | |
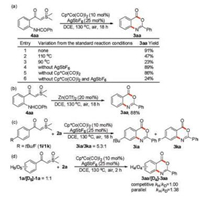
To elucidate the reaction mechanism, several mechanistic experiments were conducted (Scheme 4). At first, to probe the annulation process, NH-benzoyl-substituted sulfoxonium ylide 4aa was prepared [24]. Treatment of the compound 4aa with 10 mol% of Cp*Co(CO)I2 and 25 mol% of AgSbF6 in DCE at 130 ℃ afforded product 3aa in 91% yield, indicating that 4aa is an intermediate in the reaction (Scheme 4a, entry 1). Lowering the reaction temperature significantly decreased the yield of 3aa (Scheme 4a, entries 2 and 3), suggesting that high temperature is necessary for the cyclization process to proceed. It is worthwhile to note that in the presence of Cp*Co(CO)I2 or AgSbF6, 3aa could be obtained in excellent yield (Scheme 4a, entries 1, 4 and 5). In sharp contrast, when both Cp*Co(CO)I2 and AgSbF6 are omitted, 3aa could only be obtained in 24% yield (Scheme 4a, entry 6). These results revealed that Cp*Co(CO)I2 and AgSbF6 may play an important role in the cyclization process. We suspected that Cp*Co(CO)I2 and AgSbF6 may serve as Lewis acid to activate the carbonyl of sulfoxonium ylides. To verify this conjecture, we performed a control experiment with Lewis acid Zn(OTf)2 (Scheme 4b). Gratifyingly, treatment of 4aa with 20mol% of Zn(OTf)2 delivered 3aa in 88% yield, showing the importance of Lewis acid participation. Then, an intermolecular competition reaction between electronically different sulfoxonium ylides 1i and 1k were conducted (Scheme 4c). The result suggested that more electron-rich substrate 1i was favored for the reaction. Furthermore, we carried out several experiments to study the kinetic isotopic effect (KIE). Both intermolecular competition reaction (kH/kD=1.00) and parallel reaction (kH/kD=1.38) gave relatively small KIE values, revealing that C–H activation may not be involved in the turnover-limiting step (Scheme 4d). Combined with Li's work [24], we suspected that the cyclization of intermediate 4 may be the rate-limiting step of the whole reaction process.
|
Download:
|
| Scheme 4. Mechanistic studies. | |
On the basis of mechanistic experiments and previous literatures [22f, 26], a plausible mechanism for the reaction is proposed in Scheme 5. First, the active cationic Co(Ⅲ) complex A is generated upon treatment of Cp*Co(CO)I2 with AgSbF6. Then, A coordinates to the carbonyl oxygen of 1a and activates the ortho C–H bond to give a five-membered cobaltacyclic intermediate B. Subsequent coordination of the nitrogen of dioxazolone 2a to the Co center delivers the intermediate C, which subsequently is converted to intermediate D via extrusion of a molecule of CO2 and migratory insertion process. Next, protonolysis of D regenerates the Co(Ⅲ) catalyst for a new catalytic cycle and releases the key intermediate 4aa. Finally, intramolecular nucleophilic attack of the amide carbonyl oxygen on the sulfoxonium ylide carbonyl group with the assistance of Cp*Co(CO)I2 or AgSbF6 gives the product 3aa via the loss of a molecule of Corey's ylide (please see the Supporting information for its trapping experiment) [26d].
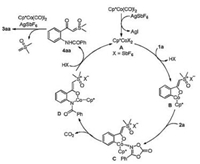
|
Download:
|
| Scheme 5. Possible mechanism. | |
In conclusion, we have developed a highly novel and direct route for the synthesis of benzoxazinones via Cp*Co(Ⅲ)-catalyzed C–H activation and [3+3] annulation of sulfoxonium ylides with dioxazolones. This strategy employs cost-effective and air-stable Cp*Co(CO)I2 as the catalyst and features operational simplicity. Another remarkable feature is that the reaction is conducted under base-free conditions. Starting from diversely substituted sulfoxonium ylides and dioxazolones, a variety of benzoxazinones could be prepared in one step in moderate to good yields. Further mechanistic investigation and application of this protocol are in progress, and the results will be forthcoming.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsWe acknowledge the financial support from the Hunan Provincial Innovation Foundation for Postgraduate (No. CX20190267), Natural Science Foundation of Hunan Province (No. 2020JJ4171).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.09.020.
| [1] |
A. Krantz, R.W. Spencer, T.F. Tam, et al., J. Med. Chem. 33 (1990) 464-479. DOI:10.1021/jm00164a002 |
| [2] |
Y. Uejima, M. Kokubo, J. Oshida, et al., J. Pharmacol. Exp. Ther. 265 (1993) 516-523. |
| [3] |
Y. Yamada, T. Kato, H. Ogino, et al., Horm. Metab. Res. 40 (2008) 539-543. DOI:10.1055/s-2008-1076699 |
| [4] |
L. Wang, Y.B. Xie, N.Y. Huang, et al., ACS Catal. 6 (2016) 4010-4016. DOI:10.1021/acscatal.6b00165 |
| [5] |
K.C. Prousis, A. Tzani, N. Avlonitis, J. Heterocycl. Chem. 50 (2013) 1313-1321. DOI:10.1002/jhet.1869 |
| [6] |
G.M. Coppola, J. Heterocycl. Chem. 37 (2000) 1369-1388. DOI:10.1002/jhet.5570370601 |
| [7] |
E. Manivannan, S.C. Chaturvedi, Bioorg. Med. Chem. 19 (2011) 4520-4528. DOI:10.1016/j.bmc.2011.06.019 |
| [8] |
X.F. Wu, H. Neumann, M. Beller, Chem. Eur. J. 18 (2012) 12599-12602. DOI:10.1002/chem.201202142 |
| [9] |
X.L. Lian, H. Lei, Z.H. Guan, et al., Chem. Commun. 49 (2013) 8196-8198. DOI:10.1039/c3cc44215b |
| [10] |
(a) R.K. Chinnagolla, S. Pimparkar, M. Jeganmohan, Chem. Commun. 49 (2013) 3703-3705; (b) J. Peng, M. Chen, Q. Zhu, et al., Org. Chem. Front. 1 (2014) 777-781; (c) Z. Chen, B. Wang, Y. Zhang, et al., Org. Chem. Front. 2 (2015) 1107-1295; (d) W.H. Rao, B.F. Shi, Org. Chem. Front. 3 (2016) 1028-1047; (e) T. Liu, W. Zhou, J. Wu, Org. Lett. 19 (2017) 6638-6641; (f) P. Gandeepan, T. Müller, L. Ackermann, et al., Chem. Rev. 119 (2019) 2192-2452; (g) Q. Zhang, B.F. Shi, Chin. J. Chem. 37 (2019) 647-656; (h) Y. Feng, Z. Zhang, X. Cui, et al., Chin. Chem. Lett. 31(2020) 58-60. |
| [11] |
(a) C.E. Houlden, G.C. Lloyd-Jones, K.I. Booker-Milburn, et al., Angew. Chem. Int. Ed. 48 (2009) 1830-1833; (b) R. Giri, J.K. Lam, J.Q. Yu, J. Am. Chem. Soc. 132 (2010) 686-693; (c) S. Kim, P. Chakrasali, I.S. Kim, et al., J. Org. Chem. 82 (2017) 7555-7563; (d) H. Deng, H. Li, L. Wang, et al., Chem. Commun. 53 (2017) 10322-10325; (e) C.F. Liu, M. Liu, L. Dong, et al., Org. Chem. Front. 5 (2018) 2115-2119; (f) I. Tanimoto, K. Kawai, S. Matsunaga, et al., Heterocycles 99 (2019) 118-125; (g) J. Li, S. Zhang, H. Zou, et al., Chem. Commun. 55 (2019) 11203-11206. |
| [12] |
(a) P.G. Chirila, C.J. Whiteoak, Dalton Trans. 46 (2017) 9721-9739; (b) J. Ghorai, P. Anbarasan, Asian J. Org. Chem. 8 (2019) 430-455. |
| [13] |
R. Kuppusamy, R. Santhoshkumar, C.H. Cheng, et al., ACS Catal. 8 (2018) 1880-1883. DOI:10.1021/acscatal.7b04087 |
| [14] |
B. Sun, M. Kanai, S. Matsunaga, et al., Angew. Chem. Int. Ed. 54 (2015) 12968-12972. DOI:10.1002/anie.201507744 |
| [15] |
S. Fukagawa, Y. Kato, S. Matsunaga, et al., Angew. Chem. Int. Ed. 58 (2019) 1153-1157. DOI:10.1002/anie.201812215 |
| [16] |
P. Patel, S. Chang, ACS Catal. 5 (2015) 853-858. DOI:10.1021/cs501860b |
| [17] |
S. Nakanowatari, R. Mei, L. Ackermann, et al., ACS Catal. 7 (2017) 2511-2515. DOI:10.1021/acscatal.7b00207 |
| [18] |
(a) L. Kong, X. Yang, X. Li, et al., Org. Chem. Front. 3 (2016) 813-816; (b) X. Zhou, Y. Luo, X. Li, et al., ACS Catal. 7 (2017) 7296-7304; (c) X. Zhou, Y. Pan, X. Li, Angew. Chem. Int. Ed. 56 (2017) 8163-8167. |
| [19] |
J.H. Kim, S. Greßies, F. Glorius, Angew. Chem. Int. Ed. 55 (2016) 5577-5581. DOI:10.1002/anie.201601003 |
| [20] |
(a) Z.Z. Zhang, B. Liu, B.F. Shi, et al., Org. Lett. 18 (2016) 1776-1779; (b) S.Y. Yan, P.X. Ling, B.F. Shi, et al., Adv. Synth. Catal. 359 (2017) 2912-2917; (c) D.Y. Huang, Q.J. Yao, B.F. Shi, et al., Org. Lett. 21 (2019) 951-954; (d) Y.H. Liu, P.X. Li, B.F. Shi, et al., Org. Lett. 21 (2019) 1895-1899. |
| [21] |
(a) J.R. Hummel, J.A. Ellman, J. Am. Chem. Soc. 137 (2015) 490-498; (b) G. Sivakumar, A. Vijeta, M. Jeganmohan, Chem. Eur. J. 22 (2016) 5899-5903; (c) X.G. Liu, H. Gao, H. Wang, et al., ACS Catal. 7 (2017) 5078-5086; (d) P.W. Tan, A.M. Mak, D.J. Dixon, et al., Angew. Chem. Int. Ed. 56 (2017) 16550-16554; (e) X. Hu, X. Chen, W. Zeng, et al., ACS Catal. 8 (2018) 1308-1312; (f) J.A. Boerth, S. Maity, J.A. Ellman, et al., Nat. Catal. 1 (2018) 673-679; (g) S. Ji, K. Yan, B. Wang, et al., Org. Lett. 20 (2018) 5981-5984; (h) Q. Li, Y. Wang, B. Wang, et al., Org. Lett. 20 (2018) 7884-7887; (i) Y. Huang, C. Pi, X. Cui, et al., Chin. Chem. Lett. 31 (2020) 3237-3240. |
| [22] |
(a) L. Hu, X. Chen, Z. Tan, et al., Org. Chem. Front. 5 (2018) 216-221; (b) L. Yu, X. Chen, Z. Tan, et al., Adv. Synth. Catal. 360 (2018) 1346-1351; (c) L. Yu, X. Chen, Z. Tan, et al., Org. Lett. 20 (2018) 3206-3210; (d) Y. Yu, Q. Wu, Z. Tan, et al., J. Org, Chem. 84 (2019) 7449-7458; (e) L. Yu, C. Yang, Z. Tan, et al., Org. Lett. 21 (2019) 5634-5638; (f) Y. Yu, Q. Wu, Z. Tan, et al., Org. Chem. Front. 6 (2019) 3868-3873. |
| [23] |
(a) Y. Xu, X. Yang, X. Li, et al., Org. Lett. 19 (2017) 4307-4310; (b) M. Barday, C. Janot, C. Aissa, et al., Angew. Chem. Int. Ed. 56 (2017) 13117-13121; (c) J.D. Neuhaus, R. Oost, N. Maulide, et al., Top. Curr. Chem. 376 (2018) 15; (d) J. Vaitla, A. Bayer, Synthesis 51 (2019) 612-628; (e) X. Wu, S. Sun, J. Cheng, et al., Synlett 30 (2019) 21-29; (f) X. Chen, M. Wang, X. Fan, et al., Org. Lett. 21 (2019) 2541-2545; (g) V. Hanchate, A. Kumar, K.R. Prabhu, et al., Org. Lett. 21 (2019) 8424-8428. |
| [24] |
Q. Jia, L. Kong, X. Li, Org. Chem. Front. 6 (2019) 741-745. DOI:10.1039/C8QO01270A |
| [25] |
G. Fenton, C.G. Newton, B.M. Wyman, et al., J. Med. Chem. 32 (1989) 265-272. DOI:10.1021/jm00121a047 |
| [26] |
(a) F. Wang, H. Wang, X. Li, et al., Org. Lett. 18 (2016) 1306-1309; (b) P. Shi, L. Wang, J. Zhu, et al., Org. Lett. 19 (2017) 2418-2421; (c) S.S. Bera, M.R. Sk, M.S. Maji, Chem. Eur. J. 25 (2019) 1806-1811; (d) M.D. Zhou, Z. Peng, L. Li, et al., Adv. Synth. Catal. 361 (2019) 5191-5197. |
 2021, Vol. 32
2021, Vol. 32